Male Infertility: Current Knowledge and Future Perspectives
A topical collection in Life (ISSN 2075-1729). This collection belongs to the section "Reproductive and Developmental Biology".
Viewed by 82250Editors
Interests: environmental and lifestyle factors and male fertility; diagnosis of male infertility; sperm function and quality; human spermatogenesis
Interests: male fertility; male aging; metabolic syndrome; benign prostate hyperplasia; immunosupressants; public health; apoptosis; prostate
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
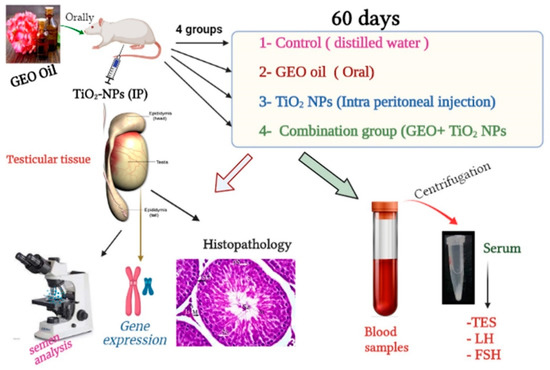
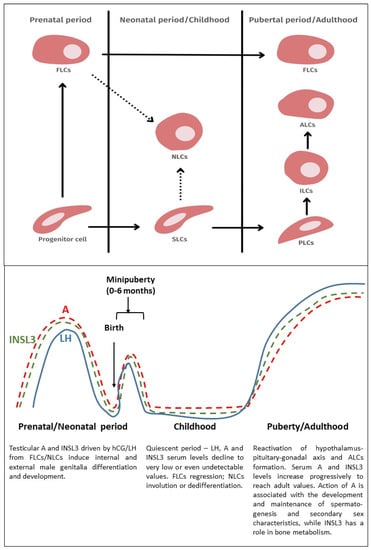
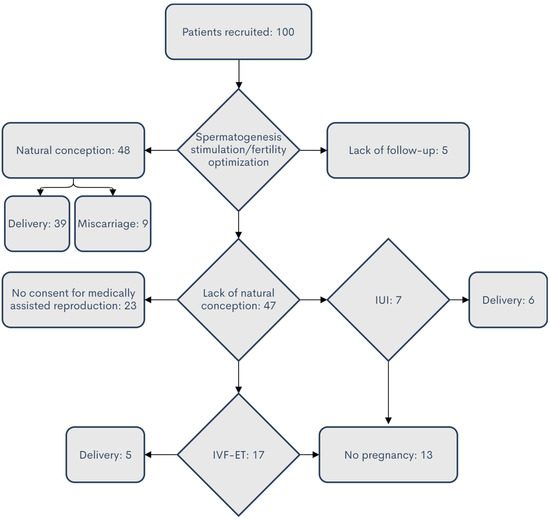
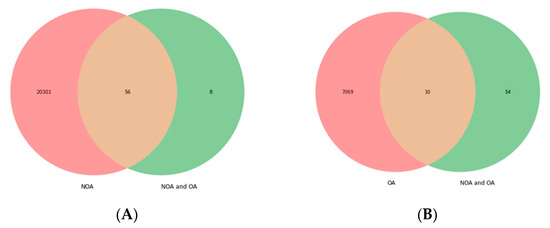
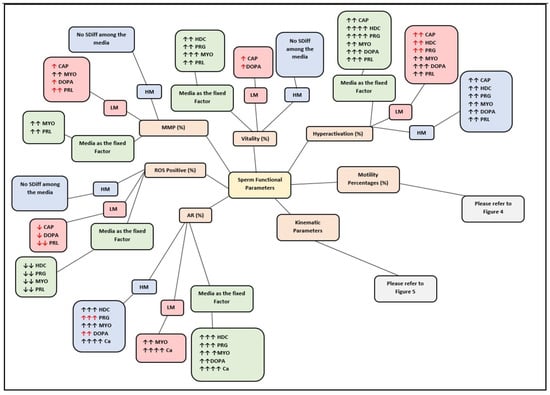
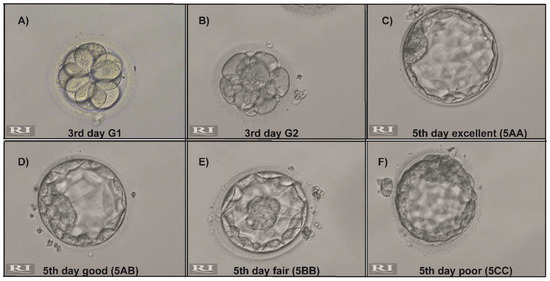
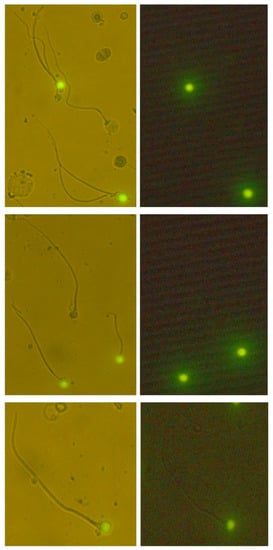
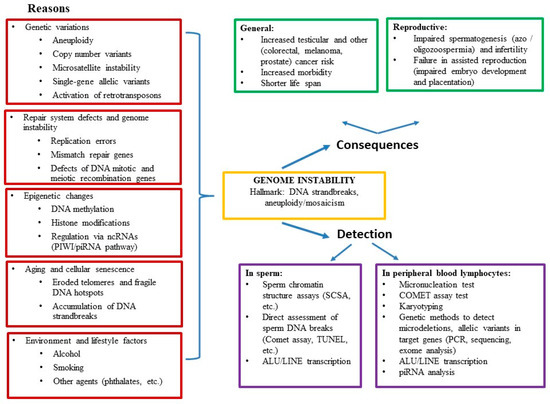
Infertility is an important and growing health problem affecting around 15% of couples worldwide. It is estimated that male factors contribute to 20–70% of the cases depending on the latitude. Male infertility can be related to a variety of congenital or acquired, well-defined causes that lead to a decline in semen quality, manifesting as decreased sperm counts (even the complete absence of sperm in the ejaculate), and abnormal sperm quality and function due to the impairment of spermatogenesis in the gonads and/or the dysfunction of the accessory glands. It should be emphasized that current andrological diagnostics do not allow establishing the etiological agent in almost 50% of men. The recent advances in the understanding of male infertility point out the involvement of environmental and lifestyle factors as well as advanced paternal age as the main etiological factors. In this context, the rate of the observed changes in sperm quality suggests that oxidative stress and epigenetic modifications may be involved in the etiology of nuclear chromatin integrity disorders of ejaculated sperm. A high quality of sperm DNA is essential not only for fertilization but also for zygote development and the health of the offspring. Although basic semen analysis is still a cornerstone of initial diagnosis in male partner management, it has limited accuracy for determining male fertility potential and provides little qualitative and functional information about the sperm. There is no doubt that modern andrology needs new diagnostic options to complement well-established endocrine, genetic, imaging and microbial diagnosis that can aid in understanding the underlying physiopathology of impaired fertility in men.
Therefore, this Topical Collection is open to original articles about basic and clinical studies investigating novel approaches to diagnostic tools, treatment options directed towards personalized medicine and preventive strategies in male infertility and extensive reviews concerning this important men’s health problem.
Dr. Renata Walczak-Jędrzejowska
Prof. Dr. Małgorzata Piasecka
Prof. Dr. Jolanta Słowikowska-Hilczer
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Life is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2600 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- male infertility
- diagnosis
- treatment
- seminal biomarkers
- idiopathic infertility
- environmental and lifestyle factors
- azoospermia
- inflammation/infection
- oxidative stress
- sperm epigenetics
- sperm DNA damage
- assisted reproductive techniques