Micronutrient Malnutrition, Infection, and Immunity in Children
A special issue of Nutrients (ISSN 2072-6643). This special issue belongs to the section "Micronutrients and Human Health".
Deadline for manuscript submissions: closed (25 April 2023) | Viewed by 16144
Special Issue Editors
Interests: vitamin A; diarrhea; malnutrition; infectious disease epidemiology; child nutrition; childhood/pediatric obesity; zinc
Interests: statistical methods; analysis; regression; public health; data management; Bayesian methods; spatial correlation; spatial misalignment; confounding
2. School of Occupational and Public Health, Ryerson University, 350 Victoria St, Toronto, ON M5B 2K3, Canada
Interests: epidemiology; enteric infection; public health; WASH; child growth; maternal & child health; global health
Special Issue Information
Dear Colleagues,
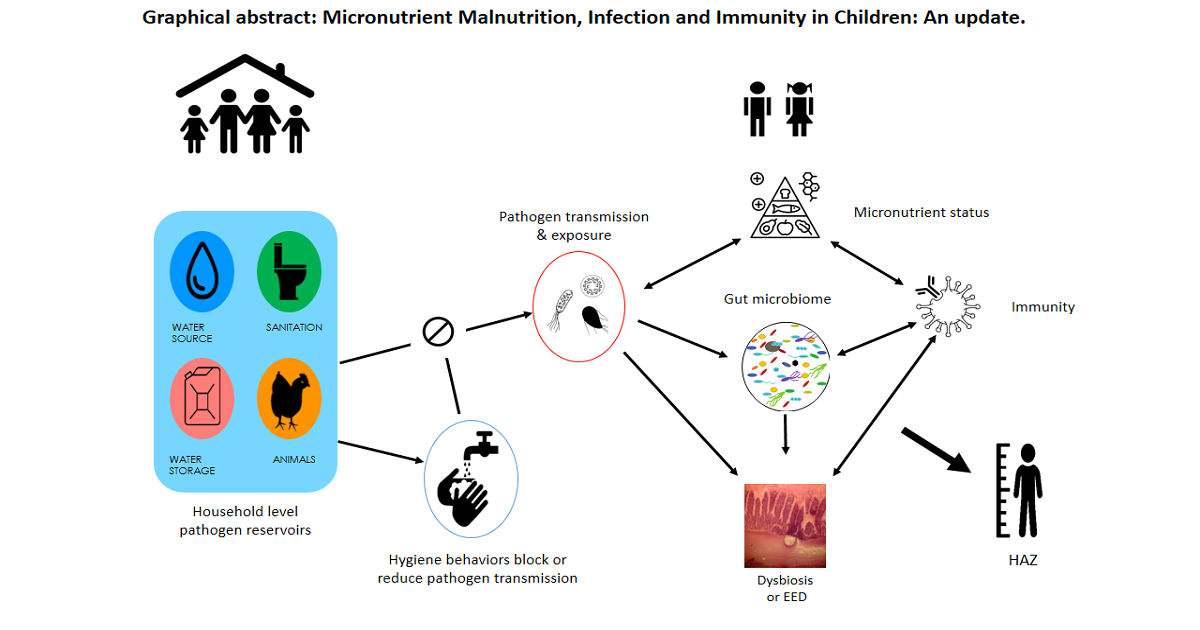
The synergism between nutrition and infectious diseases substantially contributes to the childhood burden of disease in low- and middle-income countries. Clinical and population studies have addressed the spectrum of nutritional deficiencies and the multitude of infectious agents that can comprise this synergism. The papers to be included in the proposed book will examine these relationships at different scales and apply innovative analytical techniques to provide a better understanding of the causal pathways linking nutritional status and infectious disease. The first chapters will be concerned with how nutritional status either through diet or through nutritional interventions affects the gut microbiome and enteric infections among young children. The impact of children’s nutritional status on the immune response that underlies these relationships will also be addressed in these chapters. Causal associations between childhood stunting and specific enteric infections in the household environment will be examined in the next chapter using structural linear models. The association of soil-transmitted helminths and childhood malnutrition will then be explored at regional levels in Sub-Saharan Africa through spatial modeling techniques. These studies presented in these chapters will provide an overview and further understanding of the causal relationships between malnutrition and infectious diseases that can inform efforts to more systematically reduce childhood disease burden.
Dr. Kurt Long
Dr. Helen Powell
Dr. Johanna Sanchez
Guest Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the special issue website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Nutrients is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2900 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- micronutrient malnutrition
- nutrition deficiencies
- infection and infectious disease
- children immunity
- COVID-19
- gut microbiome
- diet intervention
- epidemic






