Understanding Aging Mechanisms to Prevent Age-Related Diseases
A special issue of Cells (ISSN 2073-4409). This special issue belongs to the section "Cellular Aging".
Deadline for manuscript submissions: 31 December 2024 | Viewed by 7033
Special Issue Editors
Interests: Alzheimer’s disease; frailty; ageing process; epidemiology; successful aging
Special Issues, Collections and Topics in MDPI journals
Interests: aging; geriatrics; frailty; sarcopenia; Alzheimer’s disease; nutrition
Special Issues, Collections and Topics in MDPI journals
Interests: lipids; oxysterols; fatty acids; polyphenols; oils; oxidation; inflammation; mitochondria; peroxisomes; lysosomes; apoptosis; autophagy; natural products; synthethic molecules; biomarkers; neurodegeneration; neurodegenerative diseases; aging; age-related diseases; nanoparticles; targeted therapy
Special Issues, Collections and Topics in MDPI journals
Interests: oxysterols; very-long-chain fatty acids; lipid metabolism; diet, peroxisomes; biotherapies; inflammation; cancer; cell cycle and apoptosis; autophagy; biological membranes; oxidative damage; biomarkers; neurodegenerative diseases
Special Issues, Collections and Topics in MDPI journals
Special Issue Information
Dear Colleagues,
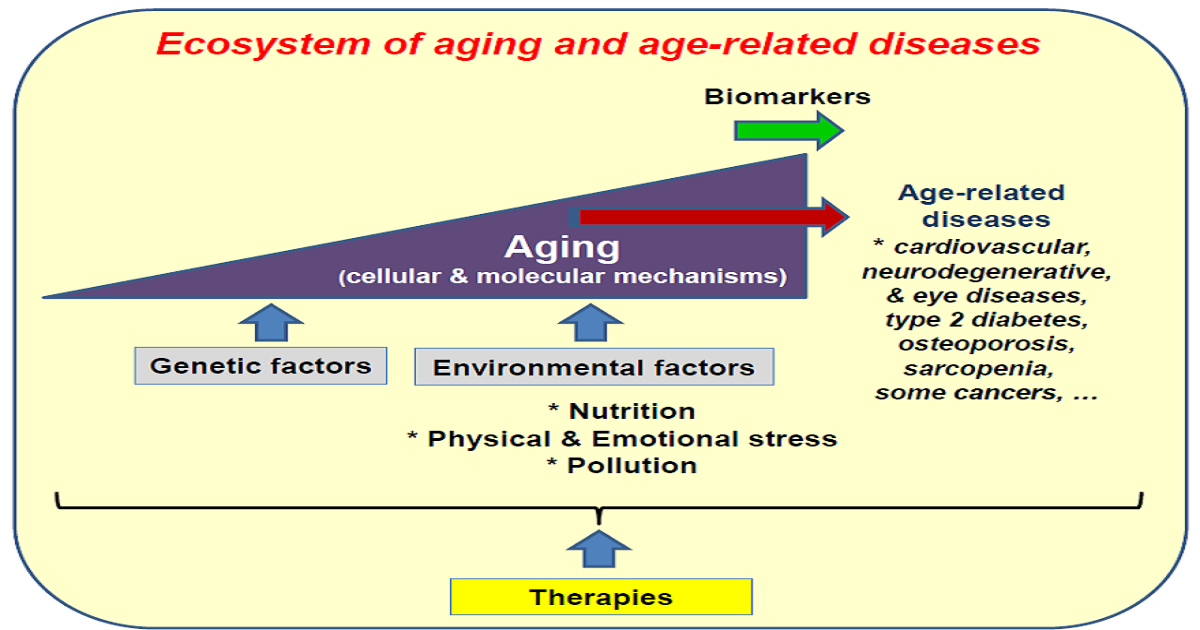
During recent decades, the percentage of people over 65, as well as the lifespan, has increased considerably and is expected to increase further in the coming years. Aging in good health has therefore become a societal and economic issue. Therefore, understanding the mechanisms of aging and preventing it by opposing the onset of age-related diseases is a public health issue. In this context, it is important to identify the genetic and epigenetic factors that can influence aging and to determine the associated mechanisms. It is now crucial to determine strategies to slow aging in order to oppose the onset of age-related diseases. Identifying predictive biomarkers of age-related diseases is a major challenge, as is the discovery of molecules that can oppose aging and the occurrence of associated diseases. This Special Issue of Cells entitled 'Understanding Aging Mechanisms to Prevent Age-Related Diseases' aims to bring together relevant research works dealing with cellular and molecular, pharmacological and nutritional aspects of aging and age-related diseases.
Prof. Dr. Pierre Jouanny
Prof. Dr. Sonia Hammami
Dr. Gérard Lizard
Dr. Anne Vejux
Guest Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the special issue website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.









