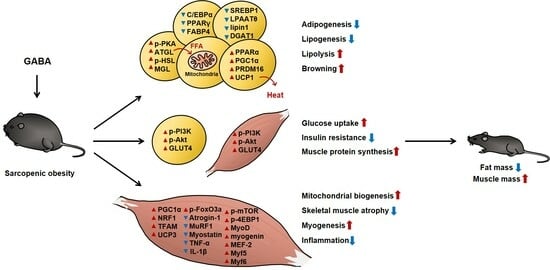
GABA Prevents Age-Related Sarcopenic Obesity in Mice with High-Fat-Diet-Induced Obesity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of GABA
2.2. Experimental Animals and Treatments
2.3. Body Mass Measurement
2.4. Rectal Temperature Measurement
2.5. Fasting Blood Glucose Measurement
2.6. Oral Glucose Tolerance Testing
2.7. Grip Strength Measurement
2.8. Dual-Energy X-ray Absorptiometry
2.9. Biochemical Analysis
2.10. Histological Analysis
2.11. Immunofluorescence
2.12. Western Blot Analysis
2.13. Statistical Analysis
3. Results
3.1. Effect of HFD-Induced Obesity and Aging on the Body Composition of Young and Aged Mice
3.2. GABA Prevents HFD-Induced Visceral Obesity in Aged Mice
3.3. GABA Decreased Adipocyte Size and Inhibits Lipid Accumulation in Aged Mice
3.4. GABA Increased Body Temperature and Energy Expenditure in Aged Mice
3.5. GABA Stimulation of Skeletal Muscle Mitochondrial Biogenesis in the Skeletal Muscle of Aged Mice
3.6. GABA Alleviation of Dyslipidemia, Glucose Tolerance, and Insulin Resistance in Aged Mice
3.7. GABA Prevented the Loss of Skeletal Muscle Mass and Strength in Aged Mice
3.8. GABA Decreased Sarcopenic Muscle Protein Degradation in Aged Mice
3.9. GABA Increased Muscle Fiber Size and Myogenesis in Aged Mice
3.10. GABA Decreases Pro-Inflammatory Cytokines and Increases Anabolic Hormones in Aged Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, X.; Li, H. Obesity: Epidemiology, pathophysiology, and therapeutics. Front. Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Li, Y.; Ma, L. Sarcopenic obesity: An emerging public health problem. Aging Dis. 2022, 13, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.N.; Choi, K.M. Sarcopenia: Definition, epidemiology, and pathophysiology. J. Bone Metab. 2013, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bilski, J.; Pierzchalski, P.; Szczepanik, M.; Bonior, J.; Zoladz, J.A. Multifactorial mechanism of sarcopenia and sarcopenic obesity. Role of physical exercise, microbiota and myokines. Cells 2022, 11, 160. [Google Scholar] [CrossRef] [PubMed]
- Roh, E.; Choi, K.M. Health consequences of sarcopenic obesity: A narrative review. Front. Endocrinol. 2020, 11, 332. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.M. Sarcopenia and sarcopenic obesity. Korean J. Intern. Med. 2016, 31, 1054–1060. [Google Scholar] [CrossRef]
- Kuk, J.L.; Saunders, T.J.; Davidson, L.E.; Ross, R. Age-related changes in total and regional fat distribution. Ageing Res. Rev. 2009, 8, 339–348. [Google Scholar] [CrossRef]
- Li, C.W.; Yu, K.; Shyh-Chang, N.; Jiang, Z.; Liu, T.; Ma, S.; Luo, L.; Guang, L.; Liang, K.; Ma, W.; et al. Pathogenesis of sarcopenia and the relationship with fat mass: Descriptive review. J. Cachexia Sarcopenia Muscle 2022, 13, 781–794. [Google Scholar] [CrossRef]
- Foster, M.T.; Pagliassotti, M.J. Metabolic alterations following visceral fat removal and expansion: Beyond anatomic location. Adipocyte 2012, 1, 192–199. [Google Scholar] [CrossRef]
- Jin, H.; Oh, H.-J.; Cho, S.; Lee, O.-H.; Lee, B.-Y. Okra (abelmoschus esculentus l. Moench) prevents obesity by reducing lipid accumulation and increasing white adipose browning in high-fat diet-fed mice. Food Funct. 2022, 13, 11840–11852. [Google Scholar] [CrossRef]
- Kelley, D.E.; Goodpaster, B.; Wing, R.R.; Simoneau, J.-A. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am. J. Physiol. -Endocrinol. Metab. 1999, 277, E1130–E1141. [Google Scholar] [CrossRef]
- Amorim, J.A.; Coppotelli, G.; Rolo, A.P.; Palmeira, C.M.; Ross, J.M.; Sinclair, D.A. Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat. Reviews. Endocrinol. 2022, 18, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Busiello, R.A.; Savarese, S.; Lombardi, A. Mitochondrial uncoupling proteins and energy metabolism. Front. Physiol. 2015, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Lee, K.; Chei, S.; Oh, H.J.; Lee, K.P.; Lee, B.Y. Ecklonia stolonifera extract suppresses lipid accumulation by promoting lipolysis and adipose browning in high-fat diet-induced obese male mice. Cells 2020, 9, 871. [Google Scholar] [CrossRef] [PubMed]
- Fazakerley, D.J.; Krycer, J.R.; Kearney, A.L.; Hocking, S.L.; James, D.E. Muscle and adipose tissue insulin resistance: Malady without mechanism? J. Lipid Res. 2019, 60, 1720–1732. [Google Scholar] [CrossRef]
- Hong, S.-h.; Choi, K.M. Sarcopenic obesity, insulin resistance, and their implications in cardiovascular and metabolic consequences. Int. J. Mol. Sci. 2020, 21, 494. [Google Scholar] [CrossRef]
- Park, S.S.; Seo, Y.K. Excess accumulation of lipid impairs insulin sensitivity in skeletal muscle. Int. J. Mol. Sci. 2020, 21, 1949. [Google Scholar] [CrossRef]
- Boucher, J.; Kleinridders, A.; Kahn, C.R. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb. Perspect. Biol. 2014, 6, a009191. [Google Scholar] [CrossRef]
- Huang, X.; Liu, G.; Guo, J.; Su, Z. The pi3k/akt pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 2018, 14, 1483–1496. [Google Scholar] [CrossRef]
- Ewa, Ś.; Justyna, S.; Adam, W.; Janusz, S.; Józef, D.; Agnieszka, Ś. Role of pi3k/akt pathway in insulin-mediated glucose uptake. In Blood Glucose Levels; Leszek, S., Ed.; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar]
- Beals, J.W.; Burd, N.A.; Moore, D.R.; van Vliet, S. Obesity alters the muscle protein synthetic response to nutrition and exercise. Front. Nutr. 2019, 6, 87. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, S.Y.; Choi, C.S. Insulin resistance: From mechanisms to therapeutic strategies. Diabetes Metab. J. 2022, 46, 15–37. [Google Scholar] [CrossRef] [PubMed]
- White, J.P.; Gao, S.; Puppa, M.J.; Sato, S.; Welle, S.L.; Carson, J.A. Testosterone regulation of akt/mtorc1/foxo3a signaling in skeletal muscle. Mol. Cell. Endocrinol. 2013, 365, 174–186. [Google Scholar] [CrossRef]
- Glass, D.J. Pi3 kinase regulation of skeletal muscle hypertrophy and atrophy. Curr. Top. Microbiol. Immunol. 2010, 346, 267–278. [Google Scholar] [PubMed]
- Hitachi, K.; Nakatani, M.; Tsuchida, K. Myostatin signaling regulates akt activity via the regulation of mir-486 expression. Int. J. Biochem. Cell Biol. 2014, 47, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Lee, H.A.; Kim, M.; Lee, E.; Sohn, U.D.; Kim, I. Forkhead box o3 plays a role in skeletal muscle atrophy through expression of e3 ubiquitin ligases murf-1 and atrogin-1 in cushing’s syndrome. Am. J. Physiology. Endocrinol. Metab. 2017, 312, E495–E507. [Google Scholar] [CrossRef]
- Mancuso, P. The role of adipokines in chronic inflammation. ImmunoTargets Ther. 2016, 5, 47–56. [Google Scholar] [CrossRef]
- Bian, A.L.; Hu, H.Y.; Rong, Y.D.; Wang, J.; Wang, J.X.; Zhou, X.Z. A study on relationship between elderly sarcopenia and inflammatory factors il-6 and tnf-α. Eur. J. Med. Res. 2017, 22, 25. [Google Scholar] [CrossRef]
- Sahab, N.R.M.; Subroto, E.; Balia, R.L.; Utama, G.L. Γ-aminobutyric acid found in fermented foods and beverages: Current trends. Heliyon 2020, 6, e05526. [Google Scholar] [CrossRef]
- Dhakal, R.; Bajpai, V.K.; Baek, K.H. Production of gaba (γ—Aminobutyric acid) by microorganisms: A review. Braz. J. Microbiol. Publ. Braz. Soc. Microbiol. 2012, 43, 1230–1241. [Google Scholar] [CrossRef]
- Ngo, D.H.; Vo, T.S. An updated review on pharmaceutical properties of gamma-aminobutyric acid. Molecules 2019, 24, 2678. [Google Scholar] [CrossRef]
- Rezazadeh, H.; Sharifi, M.R.; Soltani, N. Insulin resistance and the role of gamma-aminobutyric acid. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2021, 26, 39. [Google Scholar]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, G.; Mitrou, P.; Lambadiari, V.; Maratou, E.; Raptis, S.A. Insulin effects in muscle and adipose tissue. Diabetes Res. Clin. Pract. 2011, 93 (Suppl. 1), S52–S59. [Google Scholar] [CrossRef] [PubMed]
- Stitt, T.N.; Drujan, D.; Clarke, B.A.; Panaro, F.; Timofeyva, Y.; Kline, W.O.; Gonzalez, M.; Yancopoulos, G.D.; Glass, D.J. The igf-1/pi3k/akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting foxo transcription factors. Mol. Cell 2004, 14, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Bentzinger, C.F.; Wang, Y.X.; Rudnicki, M.A. Building muscle: Molecular regulation of myogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008342. [Google Scholar] [CrossRef] [PubMed]
- Frasca, D.; Blomberg, B.B.; Paganelli, R. Aging, obesity, and inflammatory age-related diseases. Front. Immunol. 2017, 8, 1745. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Nguyen, T.T.; Zhang, Y.; Ryu, D.; Gariani, K. Sarcopenic obesity: Epidemiology, pathophysiology, cardiovascular disease, mortality, and management. Front. Endocrinol. 2023, 14, 1185221. [Google Scholar] [CrossRef]
- Liu, C.; Cheng KY, K.; Tong, X.; Cheung, W.H.; Chow SK, H.; Law, S.W.; Wong RM, Y. The role of obesity in sarcopenia and the optimal body composition to prevent against sarcopenia and obesity. Front. Endocrinol. 2023, 14, 1077255. [Google Scholar] [CrossRef]
- Huffman, D.M.; Barzilai, N. Role of visceral adipose tissue in aging. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2009, 1790, 1117–1123. [Google Scholar] [CrossRef]
- Hernandes Júnior, P.R.; Sardeli, A.V. The effect of aging on body temperature: A systematic review and meta-analysis. Curr. Aging Sci. 2021, 14, 191–200. [Google Scholar] [CrossRef]
- Park, M.J.; Choi, K.M. Interplay of skeletal muscle and adipose tissue: Sarcopenic obesity. Metab. Clin. Exp. 2023, 144, 155577. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Oh, H.-J.; Nah, S.-Y.; Lee, B.-Y. Gintonin-enriched fraction protects against sarcopenic obesity by promoting energy expenditure and attenuating skeletal muscle atrophy in high-fat diet-fed mice. J. Ginseng Res. 2022, 46, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Oh, H.-J.; Kim, J.; Lee, K.-P.; Han, X.; Lee, O.-H.; Lee, B.-Y. Effects of ecklonia stolonifera extract on the obesity and skeletal muscle regeneration in high-fat diet-fed mice. J. Funct. Foods 2021, 82, 104511. [Google Scholar] [CrossRef]
- Dantas, W.S.; Zunica, E.R.M.; Heintz, E.C.; Vandanmagsar, B.; Floyd, Z.E.; Yu, Y.; Fujioka, H.; Hoppel, C.L.; Belmont, K.P.; Axelrod, C.L.; et al. Mitochondrial uncoupling attenuates sarcopenic obesity by enhancing skeletal muscle mitophagy and quality control. J. Cachexia Sarcopenia Muscle 2022, 13, 1821–1836. [Google Scholar] [CrossRef]
- Ferrannini, E.; Iozzo, P.; Virtanen, K.A.; Honka, M.-J.; Bucci, M.; Nuutila, P. Adipose tissue and skeletal muscle insulin-mediated glucose uptake in insulin resistance: Role of blood flow and diabetes. Am. J. Clin. Nutr. 2018, 108, 749–758. [Google Scholar] [CrossRef]
- Schultze, S.M.; Hemmings, B.A.; Niessen, M.; Tschopp, O. Pi3k/akt, mapk and ampk signalling: Protein kinases in glucose homeostasis. Expert Rev. Mol. Med. 2012, 14, e1. [Google Scholar] [CrossRef]
- Bodine, S.C.; Baehr, L.M. Skeletal muscle atrophy and the e3 ubiquitin ligases murf1 and mafbx/atrogin-1. Am. J. Physiol. -Endocrinol. Metab. 2014, 307, E469–E484. [Google Scholar] [CrossRef]
- Fry, C.S.; Rasmussen, B.B. Skeletal muscle protein balance and metabolism in the elderly. Curr. Aging Sci. 2011, 4, 260–268. [Google Scholar] [CrossRef]
- Khanna, D.; Khanna, S.; Khanna, P.; Kahar, P.; Patel, B.M. Obesity: A chronic low-grade inflammation and its markers. Cureus 2022, 14, e22711. [Google Scholar] [CrossRef]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, H.; Oh, H.-J.; Lee, B.-Y. GABA Prevents Age-Related Sarcopenic Obesity in Mice with High-Fat-Diet-Induced Obesity. Cells 2023, 12, 2146. https://doi.org/10.3390/cells12172146
Jin H, Oh H-J, Lee B-Y. GABA Prevents Age-Related Sarcopenic Obesity in Mice with High-Fat-Diet-Induced Obesity. Cells. 2023; 12(17):2146. https://doi.org/10.3390/cells12172146
Chicago/Turabian StyleJin, Heegu, Hyun-Ji Oh, and Boo-Yong Lee. 2023. "GABA Prevents Age-Related Sarcopenic Obesity in Mice with High-Fat-Diet-Induced Obesity" Cells 12, no. 17: 2146. https://doi.org/10.3390/cells12172146