Bees and Their Symbionts
Share This Topical Collection
Editors
 Dr. Kirk E. Anderson
Dr. Kirk E. Anderson
 Dr. Kirk E. Anderson
Dr. Kirk E. Anderson
E-Mail
Website
Guest Editor
Carl Hayden Bee Research Center, USDA-ARS, Tucson, AZ 85719, USA
Interests: honey bee microbiota
 Dr. Quinn S. McFrederick
Dr. Quinn S. McFrederick
 Dr. Quinn S. McFrederick
Dr. Quinn S. McFrederick
E-Mail
Website
Guest Editor
Department of Entomology, University of California, Riverside, CA 92521, USA
Interests: wild bee symbiosis
Topical Collection Information
Dear Colleagues,
Bee pollination is critical for agricultural production and ecosystem health. While honey bees are an emerging model for gut bacterial symbiosis, microbes associated with wild and solitary bees are understudied. In contrast to honey bees and their relatives, many bee species may reflect a deeper reliance on flower microbes and microbes adapted to nutrient-rich niches. In this special edition, we invite a broad perspective of bee-microbe symbiosis, including the niche-specific or context dependent factors that shape microbial community diversity and composition, stability and succession. How might variations in the nesting, social and pollination environment effect microbial exposure and establishment at multiple scales including floral-insect transmission, seasonal cycles, agroecosystems and pollinator guilds? We solicit ecological questions, including host behavioral roles, dysbiotic states, susceptibility to disease, immune training, host gland and plant secretions, nutrition and aging, detoxification, fungal associations and pathogen defense. We encourage synthesis and invite research on all bee species.
Dr. Kirk E. Anderson
Dr. Quinn S. McFrederick
Guest Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Insects is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2600 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- Microbiome
- transmission
- function
- behavior
- host-microbe interactions
- niche
- gut bacteria
- pollinator
- flower
- agriculture
- fungi
- virus
- phage
- protozoa
Published Papers (13 papers)
Open AccessArticle
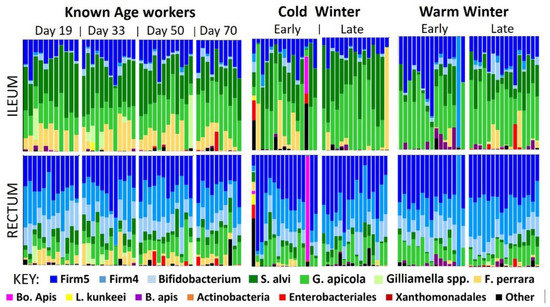
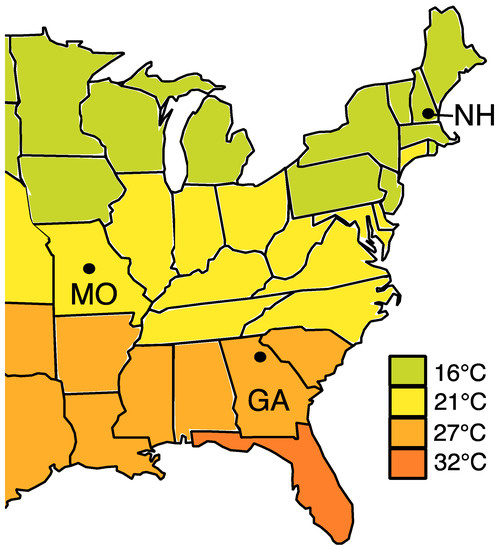
Overwintering Honey Bee Colonies: Effect of Worker Age and Climate on the Hindgut Microbiota
by
Patrick W. Maes, Amy S. Floyd, Brendon M. Mott and Kirk E. Anderson
Cited by 14 | Viewed by 3371
Abstract
Honey bee overwintering health is essential to meet the demands of spring pollination. Managed honey bee colonies are overwintered in a variety of climates, and increasing rates of winter colony loss have prompted investigations into overwintering management, including indoor climate controlled overwintering. Central
[...] Read more.
Honey bee overwintering health is essential to meet the demands of spring pollination. Managed honey bee colonies are overwintered in a variety of climates, and increasing rates of winter colony loss have prompted investigations into overwintering management, including indoor climate controlled overwintering. Central to colony health, the worker hindgut gut microbiota has been largely ignored in this context. We sequenced the hindgut microbiota of overwintering workers from both a warm southern climate and controlled indoor cold climate. Congruently, we sampled a cohort of known chronological age to estimate worker longevity in southern climates, and assess age-associated changes in the core hindgut microbiota. We found that worker longevity over winter in southern climates was much lower than that recorded for northern climates. Workers showed decreased bacterial and fungal load with age, but the relative structure of the core hindgut microbiome remained stable. Compared to cold indoor wintering, collective microbiota changes in the southern outdoor climate suggest compromised host physiology. Fungal abundance increased by two orders of magnitude in southern climate hindguts and was positively correlated with non-core, likely opportunistic bacteria. Our results contribute to understanding overwintering honey bee biology and microbial ecology and provide insight into overwintering strategies.
Full article
►▼
Show Figures
Open AccessArticle
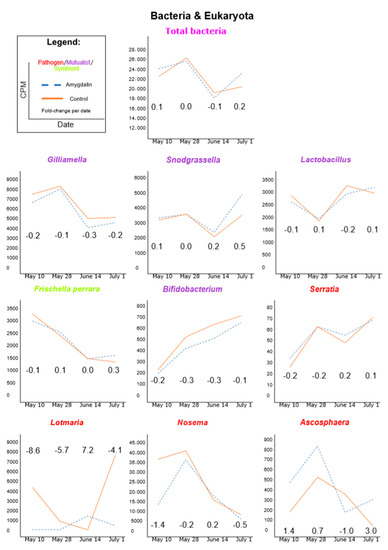
Colony-Level Effects of Amygdalin on Honeybees and Their Microbes
by
James P. Tauber, Cansu Ö. Tozkar, Ryan S. Schwarz, Dawn Lopez, Rebecca E. Irwin, Lynn S. Adler and Jay D. Evans
Cited by 7 | Viewed by 2587
Abstract
Amygdalin, a cyanogenic glycoside, is found in the nectar and pollen of almond trees, as well as in a variety of other crops, such as cherries, nectarines, apples and others. It is inevitable that western honeybees (
Apis mellifera) consistently consume amygdalin
[...] Read more.
Amygdalin, a cyanogenic glycoside, is found in the nectar and pollen of almond trees, as well as in a variety of other crops, such as cherries, nectarines, apples and others. It is inevitable that western honeybees (
Apis mellifera) consistently consume amygdalin during almond pollination season because almond crops are almost exclusively pollinated by honeybees. This study tests the effects of a field-relevant concentration of amygdalin on honeybee microbes and the activities of key honeybee genes. We executed a two-month field trial providing sucrose solutions with or without amygdalin
ad libitum to free-flying honeybee colonies. We collected adult worker bees at four time points and used RNA sequencing technology and our HoloBee database to assess global changes in microbes and honeybee transcripts. Our hypothesis was that amygdalin will negatively affect bee microbes and possibly immune gene regulation. Using a log
2 fold-change cutoff at two and intraday comparisons, we show no large change of bacterial counts, fungal counts or key bee immune gene transcripts, due to amygdalin treatment in relation to the control. However, relatively large titer decreases in the amygdalin treatment relative to the control were found for several viruses. Chronic bee paralysis virus levels had a sharp decrease (−14.4) with titers then remaining less than the control, Black queen cell virus titers were lower at three time points (<−2) and Deformed wing virus titers were lower at two time points (<−6) in amygdalin-fed compared to sucrose-fed colonies. Titers of
Lotmaria passim were lower in the treatment group at three of the four dates (<−4). In contrast, Sacbrood virus had two dates with relative increases in its titers (>2). Overall, viral titers appeared to fluctuate more so than bacteria, as observed by highly inconstant patterns between treatment and control and throughout the season. Our results suggest that amygdalin consumption may reduce several honeybee viruses without affecting other microbes or colony-level expression of immune genes.
Full article
►▼
Show Figures
Open AccessArticle
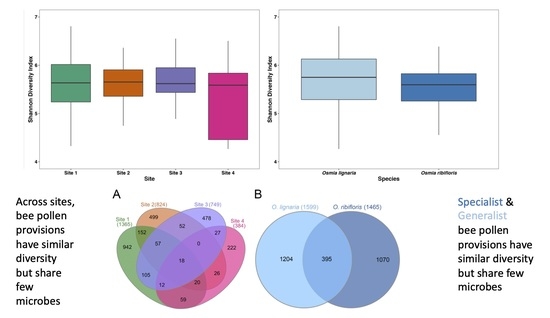
Diet Breadth Affects Bacterial Identity but Not Diversity in the Pollen Provisions of Closely Related Polylectic and Oligolectic Bees
by
Jason A. Rothman, Diana L. Cox-Foster, Corey Andrikopoulos and Quinn S. McFrederick
Cited by 10 | Viewed by 3224
Abstract
Mounting evidence suggests that microbes found in the pollen provisions of wild and solitary bees are important drivers of larval development. As these microbes are also known to be transmitted via the environment, most likely from flowers, the diet breadth of a bee
[...] Read more.
Mounting evidence suggests that microbes found in the pollen provisions of wild and solitary bees are important drivers of larval development. As these microbes are also known to be transmitted via the environment, most likely from flowers, the diet breadth of a bee may affect the diversity and identity of the microbes that occur in its pollen provisions. Here, we tested the hypothesis that, due to the importance of floral transmission of microbes, diet breadth affects pollen provision microbial community composition. We collected pollen provisions at four sites from the polylectic bee
Osmia lignaria and the oligolectic bee
Osmia ribifloris. We used high-throughput sequencing of the bacterial 16S rRNA gene to characterize the bacteria found in these provisions. We found minimal overlap in the specific bacterial variants in pollen provisions across the host species, even when the bees were constrained to foraging from the same flowers in cages at one site. Similarly, there was minimal overlap in the specific bacterial variants across sites, even within the same host species. Together, these findings highlight the importance of environmental transmission and host specific sorting influenced by diet breadth for microbes found in pollen provisions. Future studies addressing the functional consequences of this filtering, along with tests for differences between more species of oligoletic and polylectic bees will provide rich insights into the microbial ecology of solitary bees.
Full article
►▼
Show Figures
Open AccessArticle
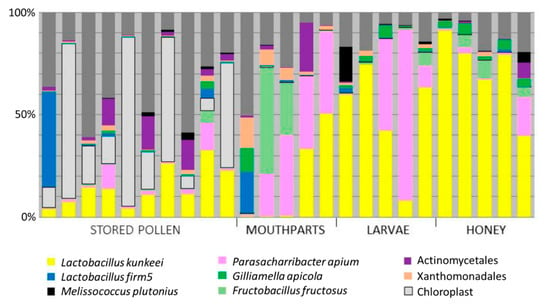
Microbial Ecology of European Foul Brood Disease in the Honey Bee (Apis mellifera): Towards a Microbiome Understanding of Disease Susceptibility
by
Amy S. Floyd, Brendon M. Mott, Patrick Maes, Duan C. Copeland, Quinn S. McFrederick and Kirk E. Anderson
Cited by 27 | Viewed by 4690
Abstract
European honey bees (
Apis mellifera Linnaeus) are beneficial insects that provide essential pollination services for agriculture and ecosystems worldwide. Modern commercial beekeeping is plagued by a variety of pathogenic and environmental stressors often confounding attempts to understand colony loss. European foulbrood (EFB)
[...] Read more.
European honey bees (
Apis mellifera Linnaeus) are beneficial insects that provide essential pollination services for agriculture and ecosystems worldwide. Modern commercial beekeeping is plagued by a variety of pathogenic and environmental stressors often confounding attempts to understand colony loss. European foulbrood (EFB) is considered a larval-specific disease whose causative agent,
Melissococcus plutonius, has received limited attention due to methodological challenges in the field and laboratory. Here, we improve the experimental and informational context of larval disease with the end goal of developing an EFB management strategy. We sequenced the bacterial microbiota associated with larval disease transmission, isolated a variety of
M.plutonius strains, determined their virulence against larvae in vitro, and explored the potential for probiotic treatment of EFB disease. The larval microbiota was a low diversity environment similar to honey, while worker mouthparts and stored pollen contained significantly greater bacterial diversity. Virulence of
M. plutonius against larvae varied markedly by strain and inoculant concentration. Our chosen probiotic,
Parasaccharibacter apium strain C6, did not improve larval survival when introduced alone, or in combination with a virulent EFB strain. We discuss the importance of positive and negative controls for in vitro studies of the larval microbiome and disease.
Full article
►▼
Show Figures
Open AccessBrief Report
The Gut–Brain–Microbiome Axis in Bumble Bees
by
Laura Leger and Quinn S. McFrederick
Cited by 10 | Viewed by 4457
Abstract
The brain-gut–microbiome axis is an emerging area of study, particularly in vertebrate systems. Existing evidence suggests that gut microbes can influence basic physiological functions and that perturbations to the gut microbiome can have deleterious effects on cognition and lead to neurodevelopmental disorders. While
[...] Read more.
The brain-gut–microbiome axis is an emerging area of study, particularly in vertebrate systems. Existing evidence suggests that gut microbes can influence basic physiological functions and that perturbations to the gut microbiome can have deleterious effects on cognition and lead to neurodevelopmental disorders. While this relationship has been extensively studied in vertebrate systems, little is known about this relationship in insects. We hypothesized that because of its importance in bee health, the gut microbiota influences learning and memory in adult bumble bees. As an initial test of whether there is a brain-gut–microbiome axis in bumble bees, we reared microbe-inoculated and microbe-depleted bees from commercial
Bombus impatiens colonies. We then conditioned experimental bees to associate a sucrose reward with a color and tested their ability to learn and remember the rewarding color. We found no difference between microbe-inoculated and microbe-depleted bumble bees in performance during the behavioral assay. While these results suggest that the brain-gut–microbiome axis is not evident in
Bombus impatiens, future studies with different invertebrate systems are needed to further investigate this phenomenon.
Full article
►▼
Show Figures
Open AccessArticle
Diverse Diets with Consistent Core Microbiome in Wild Bee Pollen Provisions
by
Rebecca M. Dew, Quinn S. McFrederick and Sandra M. Rehan
Cited by 15 | Viewed by 3941
Abstract
Bees collect pollen from flowers for their offspring, and by doing so contribute critical pollination services for our crops and ecosystems. Unlike many managed bee species, wild bees are thought to obtain much of their microbiome from the environment. However, we know surprisingly
[...] Read more.
Bees collect pollen from flowers for their offspring, and by doing so contribute critical pollination services for our crops and ecosystems. Unlike many managed bee species, wild bees are thought to obtain much of their microbiome from the environment. However, we know surprisingly little about what plant species bees visit and the microbes associated with the collected pollen. Here, we addressed the hypothesis that the pollen and microbial components of bee diets would change across the range of the bee, by amplicon sequencing pollen provisions of a widespread small carpenter bee,
Ceratina calcarata, across three populations.
Ceratina calcarata was found to use a diversity of floral resources across its range, but the bacterial genera associated with pollen provisions were very consistent.
Acinetobacter,
Erwinia,
Lactobacillus,
Sodalis,
Sphingomonas and
Wolbachia were among the top ten bacterial genera across all sites.
Ceratina calcarata uses both raspberry (
Rubus) and sumac (
Rhus) stems as nesting substrates, however nests within these plants showed no preference for host plant pollen. Significant correlations in plant and bacterial co-occurrence differed between sites, indicating that many of the most common bacterial genera have either regional or transitory floral associations. This range-wide study suggests microbes present in brood provisions are conserved within a bee species, rather than mediated by climate or pollen composition. Moving forward, this has important implications for how these core bacteria affect larval health and whether these functions vary across space and diet. These data increase our understanding of how pollinators interact with and adjust to their changing environment.
Full article
►▼
Show Figures
Open AccessArticle
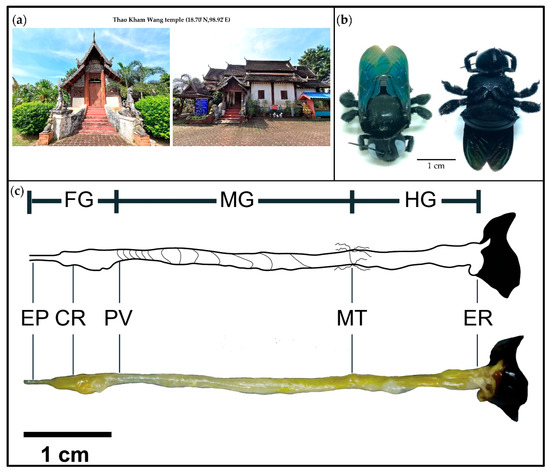
Bacterial Communities in Three Parts of Intestinal Tracts of Carpenter Bees (Xylocopa tenuiscapa)
by
Phakamas Subta, Phongsathon Yodsuwan, Rujipas Yongsawas, Ammarin In-on, Natapot Warrit, Somsak Panha, Kitiphong Khongphinitbunjong, Panuwan Chantawannakul, Korrawat Attasopa and Terd Disayathanoowat
Cited by 8 | Viewed by 3577
Abstract
This study investigated different bacterial communities in three intestinal parts (foregut, midgut and hindgut) of
Xylocopatenuiscapa to understand the roles of gut bacteria. Our phylogenetic analysis revealed that
X. tenuiscapa is closely related to
Xylocopa latipes. The 16S rRNA gene in
[...] Read more.
This study investigated different bacterial communities in three intestinal parts (foregut, midgut and hindgut) of
Xylocopatenuiscapa to understand the roles of gut bacteria. Our phylogenetic analysis revealed that
X. tenuiscapa is closely related to
Xylocopa latipes. The 16S rRNA gene in the genomic DNA samples from the gut was examined by illumina (Solexa) and a total of 998 operational taxonomic unit (OTUs) clusters were found. Taxonomic classification identified 16 bacterial phyla and unclassified bacteria. The dominant bacteria taxa in the three parts of
X. tenuiscapa gut were Proteobacteria, Firmicutes, Bacteroidetes and Actinobacteria. In the foregut, Lactobacillales and Enterobacteriaceae were predominantly found. The population in the midgut was similar to that in the foregut, with the addition of
Gilliamella, which was also abundant. The most dominant bacteria identified in the hindgut were similar to those in the midgut and Lactobacillales, Enterobacteriaceae,
Gilliamella, Bifidobacteriaceae and Flavobacteriaceae appeared in abundance. Moreover, our results suggest that a community structure of bacteria in different parts of
X. tenuiscapa’s gut may be an important indicator of carpenter bees’ health. This functional study of bacterial communities revealed significant differences among the three intestinal parts and is the first report of the gut bacteria structure in solitary bees.
Full article
►▼
Show Figures
Open AccessArticle
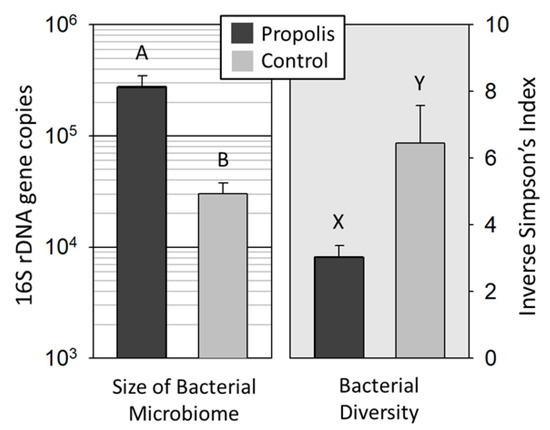
Propolis Envelope Promotes Beneficial Bacteria in the Honey Bee (Apis mellifera) Mouthpart Microbiome
by
Hollie Dalenberg, Patrick Maes, Brendon Mott, Kirk E. Anderson and Marla Spivak
Cited by 22 | Viewed by 5847
Abstract
Honey bees collect and apply plant resins to the interior of their nest cavity, in order to form a layer around the nest cavity called a propolis envelope. Propolis displays antimicrobial activity against honey bee pathogens, but the effect of propolis on the
[...] Read more.
Honey bees collect and apply plant resins to the interior of their nest cavity, in order to form a layer around the nest cavity called a propolis envelope. Propolis displays antimicrobial activity against honey bee pathogens, but the effect of propolis on the honey bee microbiome is unknown. Honey bees do not intentionally consume propolis, but they do manipulate propolis with their mouthparts. Because honey bee mouthparts are used for collecting and storing nectar and pollen, grooming and trophallaxis between adults, feeding larvae, and cleaning the colony, they are an important interface between the bees’ external and internal environments and serve as a transmission route for core gut bacteria and pathogens alike. We hypothesized that the antimicrobial activity of an experimentally applied propolis envelope would influence the bacterial diversity and abundance of the worker mouthpart microbiome. The results revealed that the mouthparts of worker bees in colonies with a propolis envelope exhibited a significantly lower bacterial diversity and significantly higher bacterial abundance compared to the mouthparts of bees in colonies without a propolis envelope. Based on the taxonomic results, the propolis envelope appeared to reduce pathogenic or opportunistic microbes and promote the proliferation of putatively beneficial microbes on the honey bee mouthparts, thus reinforcing the core microbiome of the mouthpart niche.
Full article
►▼
Show Figures
Open AccessArticle
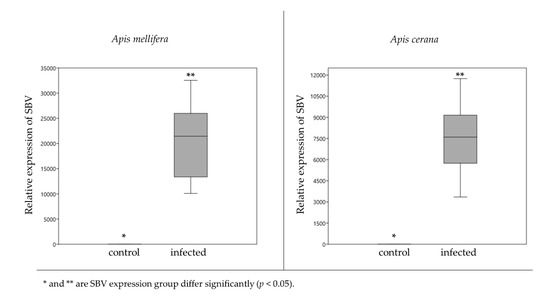
Impact of Sacbrood Virus on Larval Microbiome of Apis mellifera and Apis cerana
by
Rujipas Yongsawas, Veeranan Chaimanee, Jeffery S. Pettis, Humberto Freire Boncristiani Junior, Dawn Lopez, Ammarin In-on, Panuwan Chantawannakul and Terd Disayathanoowat
Cited by 6 | Viewed by 3645
Abstract
In this study, we examined the impact of Sacbrood virus (SBV), the cause of larval honeybee (
Apis mellifera) death, producing a liquefied a larva sac, on the gut bacterial communities on two larval honeybee species,
Apis mellifera and
Apis cerana.
[...] Read more.
In this study, we examined the impact of Sacbrood virus (SBV), the cause of larval honeybee (
Apis mellifera) death, producing a liquefied a larva sac, on the gut bacterial communities on two larval honeybee species,
Apis mellifera and
Apis cerana. SBV was added into a worker jelly food mixture and bee larvae were grafted into each of the treatment groups for 24 h before DNA/RNA extraction. Confirmation of SBV infection was achieved using quantitative reverse transcription polymerase chain reaction (RT-qPCR) and visual symptomology. The 16S rDNA was sequenced by Illumina sequencing. The results showed the larvae were infected with SBV. The gut communities of infected
A. cerana larvae exhibited a dramatic change compared with
A. mellifera. In
A. mellifera larvae, the Illumina sequencing revealed the proportion of
Gilliamella,
Snodgrassella and
Fructobacillus was not significantly different, whereas in
A. cerana,
Gilliamella was significantly decreased (from 35.54% to 2.96%), however, with significant increase in
Snodgrassella and
Fructobacillus. The possibility of cross-infection should be further investigated.
Full article
►▼
Show Figures
Open AccessArticle
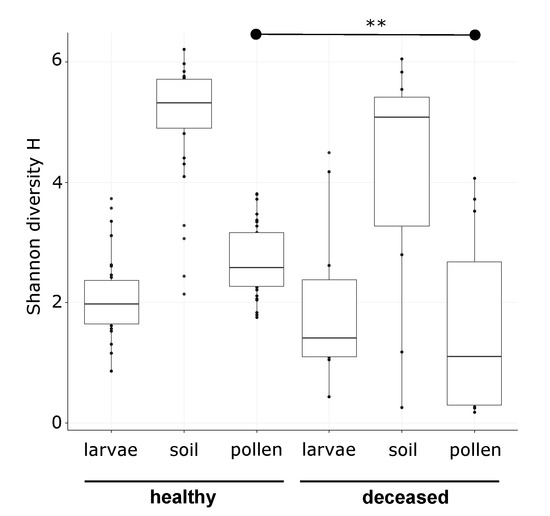
Susceptibility of Red Mason Bee Larvae to Bacterial Threats Due to Microbiome Exchange with Imported Pollen Provisions
by
Anna Voulgari-Kokota, Ingolf Steffan-Dewenter and Alexander Keller
Cited by 22 | Viewed by 4129
Abstract
Solitary bees are subject to a variety of pressures that cause severe population declines. Currently, habitat loss, temperature shifts, agrochemical exposure, and new parasites are identified as major threats. However, knowledge about detrimental bacteria is scarce, although they may disturb natural microbiomes, disturb
[...] Read more.
Solitary bees are subject to a variety of pressures that cause severe population declines. Currently, habitat loss, temperature shifts, agrochemical exposure, and new parasites are identified as major threats. However, knowledge about detrimental bacteria is scarce, although they may disturb natural microbiomes, disturb nest environments, or harm the larvae directly. To address this gap, we investigated 12
Osmia bicornis nests with deceased larvae and 31 nests with healthy larvae from the same localities in a 16S ribosomal RNA (rRNA) gene metabarcoding study. We sampled larvae, pollen provisions, and nest material and then contrasted bacterial community composition and diversity in healthy and deceased nests. Microbiomes of pollen provisions and larvae showed similarities for healthy larvae, whilst this was not the case for deceased individuals. We identified three bacterial taxa assigned to
Paenibacillus sp. (closely related to
P. pabuli/amylolyticus/xylanexedens),
Sporosarcina sp., and
Bacillus sp. as indicative for bacterial communities of deceased larvae, as well as
Lactobacillus for corresponding pollen provisions. Furthermore, we performed a provisioning experiment, where we fed larvae with untreated and sterilized pollens, as well as sterilized pollens inoculated with a
Bacillus sp. isolate from a deceased larva. Untreated larval microbiomes were consistent with that of the pollen provided. Sterilized pollen alone did not lead to acute mortality, while no microbiome was recoverable from the larvae. In the inoculation treatment, we observed that larval microbiomes were dominated by the seeded bacterium, which resulted in enhanced mortality. These results support that larval microbiomes are strongly determined by the pollen provisions. Further, they underline the need for further investigation of the impact of detrimental bacterial acquired via pollens and potential buffering by a diverse pollen provision microbiome in solitary bees.
Full article
►▼
Show Figures
Open AccessArticle
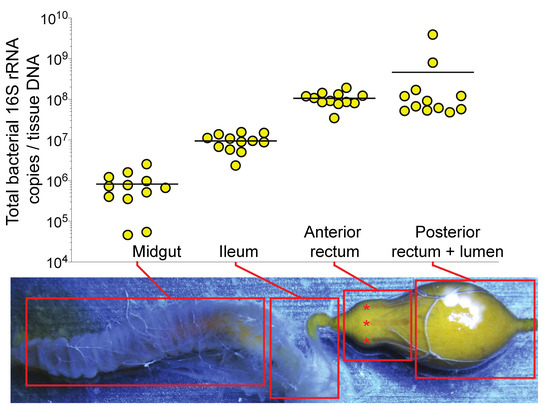
Probing the Honey Bee Diet-Microbiota-Host Axis Using Pollen Restriction and Organic Acid Feeding
by
Vincent A. Ricigliano and Kirk E. Anderson
Cited by 15 | Viewed by 4636
Abstract
Microbial metabolites are considered important drivers of diet-based microbiota influence on the host, however, mechanistic models are confounded by interactions between diet, microbiota function, and host physiology. The honey bee harbors a simple microbiota that produces organic acids as fermentation products of dietary
[...] Read more.
Microbial metabolites are considered important drivers of diet-based microbiota influence on the host, however, mechanistic models are confounded by interactions between diet, microbiota function, and host physiology. The honey bee harbors a simple microbiota that produces organic acids as fermentation products of dietary nectar and pollen, making it a model for gut microbiota research. Herein, we demonstrate that bacterial abundance in the honey bee gut is partially associated with the anterior rectum epithelium. We used dietary pollen restriction and organic acid feeding treatments to obtain information about the role of undigested pollen as a microbiota growth substrate and the impact of bacterial fermentation products on honey bee enteroendocrine signaling. Pollen restriction markedly reduced total and specific bacterial 16S rRNA abundance in the anterior rectum but not in the ileum. Anterior rectum expression levels of bacterial fermentative enzyme gene transcripts (acetate kinase, lactate dehydrogenase, and hydroxybutyryl-CoA dehydrogenase) were reduced in association with diet-induced microbiota shifts. To evaluate the effects of fermentative metabolites on host enteroendocrine function, pollen-restricted bees were fed an equimolar mixture of organic acid sodium salts (acetate, lactate, butyrate, formate, and succinate). Organic acid feeding significantly impacted hindgut enteroendocrine signaling gene expression, rescuing some effects of pollen restriction. This was specifically manifested by tissue-dependent expression patterns of neuropeptide F and allatostatin pathways, which are implicated in energy metabolism and feeding behaviors. Our findings provide new insights into the diet-microbiota-host axis in honey bees and may inform future efforts to improve bee health through diet-based microbiota manipulations.
Full article
►▼
Show Figures
Open AccessArticle
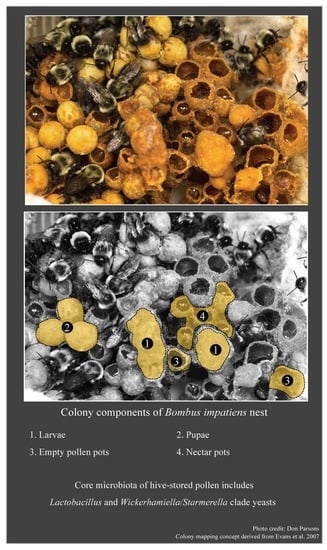
Microbial Diversity Associated with the Pollen Stores of Captive-Bred Bumble Bee Colonies
by
Prarthana S. Dharampal, Luis Diaz-Garcia, Max A. B. Haase, Juan Zalapa, Cameron R. Currie, Chris Todd Hittinger and Shawn A. Steffan
Cited by 23 | Viewed by 5997
Abstract
The pollen stores of bumble bees host diverse microbiota that influence overall colony fitness. Yet, the taxonomic identity of these symbiotic microbes is relatively unknown. In this descriptive study, we characterized the microbial community of pollen provisions within captive-bred bumble bee hives obtained
[...] Read more.
The pollen stores of bumble bees host diverse microbiota that influence overall colony fitness. Yet, the taxonomic identity of these symbiotic microbes is relatively unknown. In this descriptive study, we characterized the microbial community of pollen provisions within captive-bred bumble bee hives obtained from two commercial suppliers located in North America. Findings from 16S rRNA and ITS gene-based analyses revealed that pollen provisions from the captive-bred hives shared several microbial taxa that have been previously detected among wild populations. While diverse microbes across phyla Firmicutes, Proteobacteria, Bacteroidetes, Actinobacteria, and Ascomycota were detected in all commercial hives, significant differences were detected at finer-scale taxonomic resolution based on the supplier source. The causative agent of chalkbrood disease in honey bees,
Ascosphaera apis, was detected in all hives obtained from one supplier source, although none of the hives showed symptoms of infection. The shared core microbiota across both commercial supplier sources consisted of two ubiquitous bee-associated groups,
Lactobacillus and
Wickerhamiella/Starmerella clade yeasts that potentially contribute to the beneficial function of the microbiome of bumble bee pollen provisions.
Full article
►▼
Show Figures
Open AccessReview
Diversity and Global Distribution of Viruses of the Western Honey Bee, Apis mellifera
by
Alexis Beaurepaire, Niels Piot, Vincent Doublet, Karina Antunez, Ewan Campbell, Panuwan Chantawannakul, Nor Chejanovsky, Anna Gajda, Matthew Heerman, Delphine Panziera, Guy Smagghe, Orlando Yañez, Joachim R. de Miranda and Anne Dalmon
Cited by 126 | Viewed by 16351
Abstract
In the past centuries, viruses have benefited from globalization to spread across the globe, infecting new host species and populations. A growing number of viruses have been documented in the western honey bee,
Apis mellifera. Several of these contribute significantly to honey
[...] Read more.
In the past centuries, viruses have benefited from globalization to spread across the globe, infecting new host species and populations. A growing number of viruses have been documented in the western honey bee,
Apis mellifera. Several of these contribute significantly to honey bee colony losses. This review synthetizes the knowledge of the diversity and distribution of honey-bee-infecting viruses, including recent data from high-throughput sequencing (HTS). After presenting the diversity of viruses and their corresponding symptoms, we surveyed the scientific literature for the prevalence of these pathogens across the globe. The geographical distribution shows that the most prevalent viruses (deformed wing virus, sacbrood virus, black queen cell virus and acute paralysis complex) are also the most widely distributed. We discuss the ecological drivers that influence the distribution of these pathogens in worldwide honey bee populations. Besides the natural transmission routes and the resulting temporal dynamics, global trade contributes to their dissemination. As recent evidence shows that these viruses are often multihost pathogens, their spread is a risk for both the beekeeping industry and the pollination services provided by managed and wild pollinators.
Full article
►▼
Show Figures