Glycosylation and Cell Biology
A topical collection in Cells (ISSN 2073-4409).
Viewed by 56523Editor
Interests: mucin-type O-glycosylation; O-glycoproteins; mucins; MUC1; cancer; innate immunity; galactosemia; glycomics; (glyco)proteomics; mass spectrometry
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
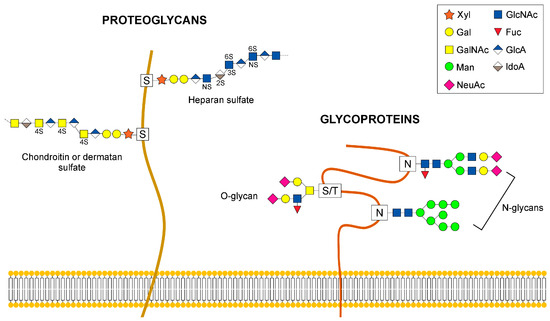
The complex interplay of sugar metabolism and protein glycosylation (evident in a series of congenital disorders) and the impact of protein dys-glycosylation on protein sorting for targeted transport and targeted protein transport to plasma membranes are currently attracting researchers’ attention. These challenging topics are related to cellular functions at higher organizational levels, such as receptor-mediated cell signaling (for example, glycosylation can be a main regulator of growth and death factor receptors’ signaling) and associated molecular pathomechanisms. A series of questions could be addressed; for instance: How is cell signaling modulated by receptor glycosylation? How is it regulated, by direct effects on protein structure (see VEGFR in endothelial cells) or by indirect effects, like altered raft sorting? What is the molecular basis of glycosylation-dependent effects on protein sorting for targeted transport to their subcellular destinations?
Another topic of high research actuality refers to rare O-glycosylation types and their regulatory influence on cellular pathways (O-GlcNAc), on cell–cell interactions (O-mannosylation of cadherins) and cell–matrix interactions, mediated by O-mannosylated lecticans in the perineural net. What is the functional implication of glycans exhibiting the protein-specific, peripheral sugar modulation LacdiNAc (e.g., in self-renewal of embryonic stem cells by regulating LIF/STAT3 signaling)?
There are many more open questions related to complex glycosylation of proteins and its functional impact at the molecular and cellular levels which could be addressed in this Topical Collection, which is designed as a platform for featuring the most recent original work on these topics together with high-standard surveys.
Prof. Franz-Georg Hanisch
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- cell signaling
- trafficking (targeted transport)
- lipid rafts
- extracellular matrix
- glycoproteome
- glycomics
- N/O-glycosylation
- rare O-glycosylation