Promising Redox Flow Batteries
A special issue of Batteries (ISSN 2313-0105). This special issue belongs to the section "Battery Materials and Interfaces: Anode, Cathode, Separators and Electrolytes or Others".
Deadline for manuscript submissions: closed (31 March 2023) | Viewed by 9960
Special Issue Editors
Interests: polymer electrolyte membrane; anion exchange membrane; high temperature proton exchange membrane fuel cell; flow battery; water electrolyser
Interests: mathematical modeling for flow batteries; optimization of stack/module design and operational management; electrode design and modification for flow batteries
Special Issue Information
Dear Colleagues,
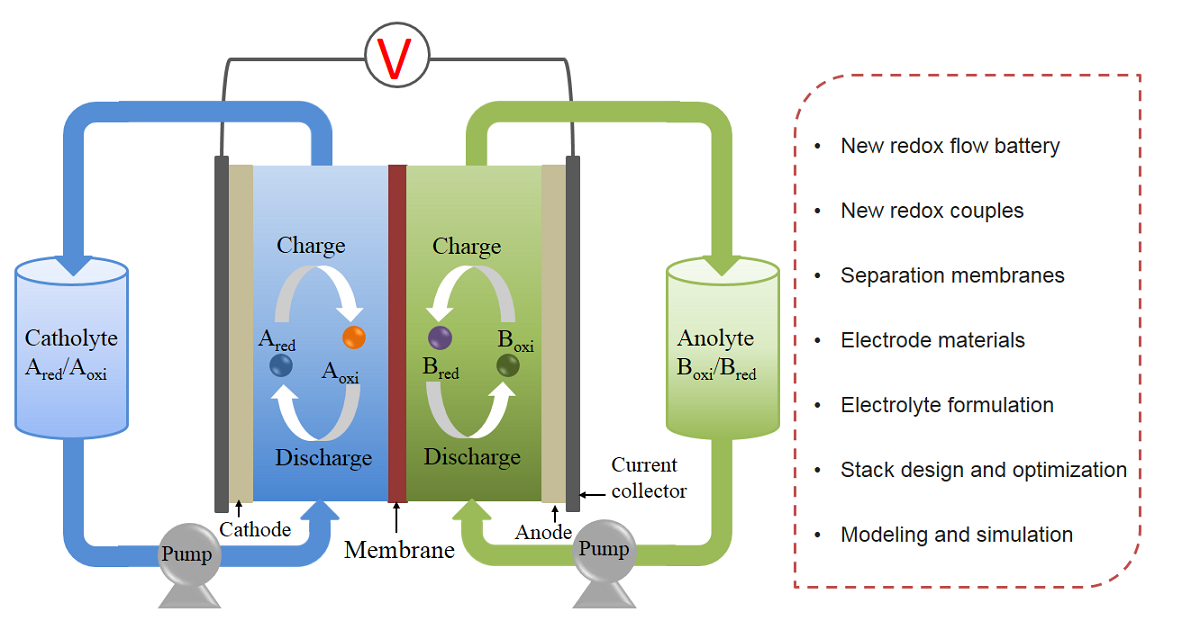
With the development of societies and economies, the energy crisis and the impact of massive carbon dioxide emissions on the environment are becoming more and more serious. The exploration and utilization of large-scale energy storage devices have attracted widespread attention, when unstable and intermittent renewable energies are being used efficiently worldwide. Among many energy storage devices, the redox flow battery (RFB) receives the attention of researchers, which brings advantages such as a quick response, flexible design, high safety, green environmental protection, and a long cycle life. Over the past few decades, RFBs have witnessed significant development: not only has the performance of conventional RFBs improved considerably, but a wide range of new battery chemistries/concepts has also been proposed. This Special Issue will cover various promising RFBs and related materials.
Topics of interest include, but are not limited to, the following:
- New redox flow battery and redox couples.
- Separation membrane materials.
- Electrode materials.
- Electrolyte formulation.
- Stack structural design and optimization.
- Modeling and simulation.
Dr. Jingshuai Yang
Dr. Hui Chen
Guest Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the special issue website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Batteries is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- redox flow battery
- energy storage
- membrane and electrode
- stack cell
- battery modeling






