Electrochemical, Thermal, and Safety Properties of Lithium and Post-Li Materials and Cells II
A special issue of Batteries (ISSN 2313-0105). This special issue belongs to the section "Battery Performance, Ageing, Reliability and Safety".
Deadline for manuscript submissions: closed (31 December 2022) | Viewed by 42208
Special Issue Editor
Interests: lithium and post-lithium-ion batteries; battery calorimetry; thermal characterization of materials/cells/batteries; safety testing; thermal management; multiscale electric, electrochemical, and thermal modeling of cells and batteries
Special Issues, Collections and Topics in MDPI journals
Special Issue Information
Dear Colleagues,
New cheaper, safer, and more sustainable battery materials and technology concepts are urgently required for the decarbonization of the energy system and an extensive market penetration of electric vehicles and stationary storage systems. So-called post-lithium batteries based on, e.g., Na or Mg ions, which no longer rely on Li are promising alternatives that offer a huge potential. Therefore, characterization of electrochemical, thermal, and safety properties of the cells and their individual active and passive materials is required to obtain quantitative and reliable data, which are necessary to improve the current understanding in order to design and develop better materials and cells. This Special Issue addresses all techniques, which are necessary for a holistic assessment from materials to cell level. I warmly invite you to publish your original research paper or a review paper in this Special Issue.
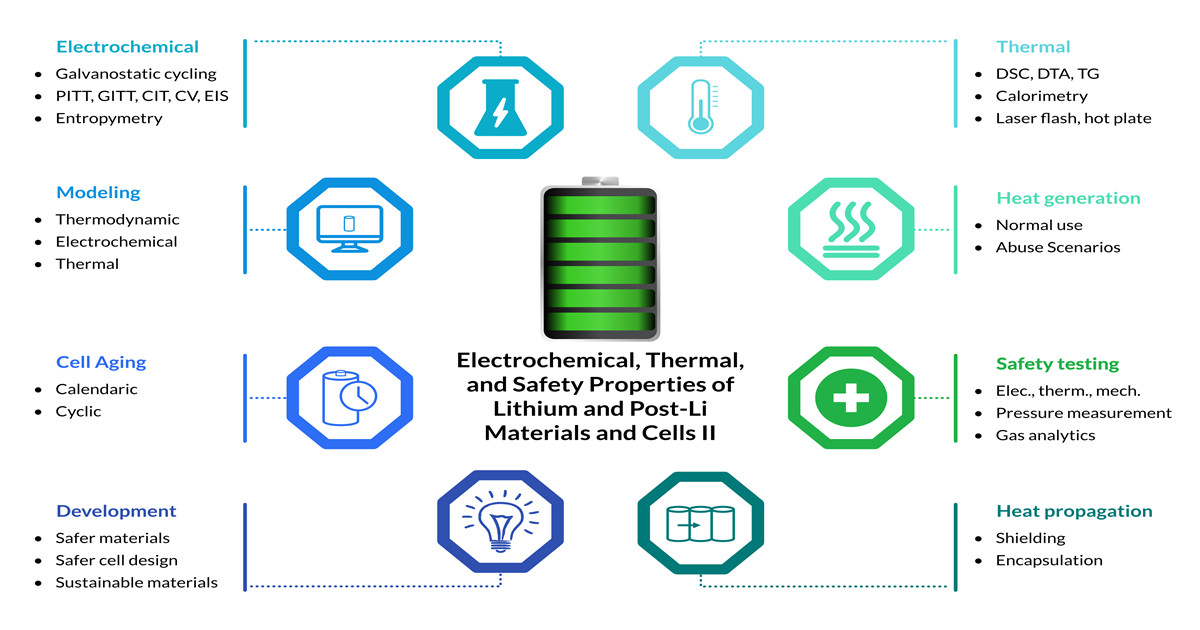
Potential topics include but are not limited to:
- Electrochemical characterization techniques (galvanostatic cycling, PITT, GITT, CIT, CV, EIS, entropymetry);
- Thermal characterization techniques (DSC, DTA, TG, drop solution calorimetry, battery calorimetry, laser flash, hot-plate, thermography, etc.) for materials and cells;
- Determination of heat generation of cells under normal use;
- Safety testing (mechanical, electrical, thermal abuse) combined with pressure measurement and gas analytics;
- Cell aging studies;
- Thermal propagation mitigation;
- Development of safer materials and cell designs;
- Development of more sustainable materials;
- Thermodynamic modeling of battery materials (CALPHAD, kinetic modeling) and database generation;
- Electrical, thermal, and electrochemical modeling.
Share your results to get a deeper understanding of the electrochemical and thermal processes under both normal use and abuse conditions. This will be an important milestone to increase their safety and to exploit their full potential.
Dr. Carlos Ziebert
Guest Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the special issue website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Batteries is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- post-lithium batteries
- electrochemical and thermal characterization
- battery calorimetry, safety testing, and gas analytics
- cell aging studies
- thermal propagation mitigation
- development of safer and more sustainable materials
- thermodynamic modelling
- electrical, thermal, and electrochemical modelling





