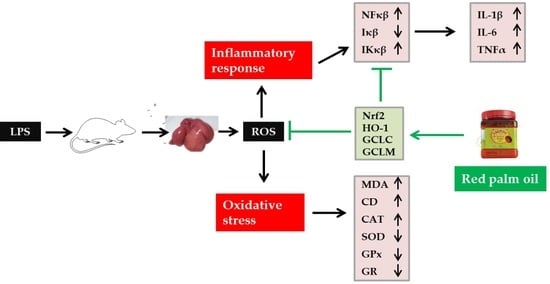
Red Palm Oil Ameliorates Oxidative Challenge and Inflammatory Responses Associated with Lipopolysaccharide-Induced Hepatic Injury by Modulating NF-κβ and Nrf2/GCL/HO-1 Signaling Pathways in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Animals
2.1.2. Ethical Approval
2.2. Methods
2.2.1. Induction of Hepatic Injury
2.2.2. Study Design
2.2.3. Histopathological Analysis
2.2.4. Preparation of Liver Homogenate
2.3. Biochemical Analysis
2.3.1. Vitamin E and Carotenoids Content of RPO
2.3.2. Hepatic Antioxidant Capacity
2.3.3. Markers of Hepatocyte Damage
2.3.4. Estimation of Hepatic Lipid Peroxidation
2.3.5. Determination of Hepatic Glutathione
2.3.6. Determination of Hepatic Antioxidant Enzymes Activity
2.3.7. Analysis of Inflammatory Biomarkers
2.3.8. Gene Expression Analysis by Real Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.4. Statistical Analyses
3. Results
3.1. Vitamin E and Cartenoid Content of Red Palm Oil
3.2. Weight Parameters, Vitamin E and Carotene Intake and Liver Antioxidant Capacity
3.3. RPO Protects against LPS-Induced Hepatic Injury
3.4. RPO Attenuates Oxidative Stress, Enhances Antioxidant Enzymes and Restores GSH Redox Status in the Liver of LPS-Induced Rats
3.5. RPO Inhibits LPS-Induced NF-κβ Activation and Reduces Production of Inflammatory Cytokines
3.6. RPO Activates Nrf2 and Upregulates mRNA Expression of Downstream Targets HO-1 and GCL
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mayeux, P.R. Pathobiology of lipopolysaccharide. J. Toxicol. Environ. Health 1997, 51, 415–435. [Google Scholar] [CrossRef] [PubMed]
- Berg, B.M.; Godbout, J.P.; Kelley, K.W.; Johnson, R.W. α-tocopherol attenuates lipopolysaccharide-induced sickness behavior in mice. Brain Behav. Immun. 2004, 18, 149–157. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, X.; Wang, F.; Wang, Y.; Wang, Y.; Li, H.; Lv, X.; Lu, D.; Wang, H. Yohimbine promotes cardiac NE release and prevents LPS-induced cardiac dysfunction via blockade of presynaptic α2A-adrenergic receptor. PLoS ONE 2013, 8, e63622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tucsek, Z. Effect of a Red Wine Compound on LPS-Induced Inflammatory Processes In Vivo and In Vitro. Ph.D. Thesis, University of Pécs, Pécs, Hungary, 2011. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Haegens, A.; Heeringa, P.; van Suylen, R.J.; Steele, C.; Aratani, Y.; O’Donoghue, R.J.; Mutsaers, S.E.; Mossman, B.T.; Wouters, E.F.; Vernooy, J.H. Myeloperoxidase deficiency attenuates lipopolysaccharide-induced acute lung inflammation and subsequent cytokine and chemokine production. J. Immunol. 2009, 182, 7990–7996. [Google Scholar] [CrossRef]
- Mohamadin, A.M.; Elberry, A.A.; Elkablawy, M.A.; Gawad, H.S.A.; Al-Abbasi, F.A. Montelukast, a leukotriene receptor antagonist abrogates lipopolysaccharide-induced toxicity and oxidative stress in rat liver. Pathophysiology 2011, 18, 235–242. [Google Scholar] [CrossRef]
- Ahmed, N.; El-Agamy, D.S.; Mohammed, G.A.; Abo-Haded, H.; Elkablawy, M.; Ibrahim, S.R.M. Suppression of LPS-induced hepato-and cardiotoxic effects by Pulicaria petiolaris via NF-κB dependent mechanism. Cardiovasc. Toxicol. 2020, 20, 121–129. [Google Scholar] [CrossRef]
- Depboylu, B.; Olgaç, V.; Doğru-Abbasoğlu, S.; Uysal, M. Response of liver to lipopolysaccharide treatment in male and female rats. Exp. Toxicol. Pathol. 2013, 65, 645–650. [Google Scholar] [CrossRef]
- Ghanim, B.Y.; Qinna, N.A. Nrf2/ARE axis signalling in hepatocyte cellular death. Mol. Biol. Rep. 2022, 49, 4039–4053. [Google Scholar] [CrossRef]
- Torrente, L.; DeNicola, G.M. Targeting NRF2 and its downstream processes: Opportunities and challenges. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 279–300. [Google Scholar] [CrossRef]
- Yerra, V.G.; Negi, G.; Sharma, S.S.; Kumar, A. Potential therapeutic effects of the simultaneous targeting of the Nrf2 and NF-κB pathways in diabetic neuropathy. Redox Biol. 2013, 1, 394–397. [Google Scholar] [CrossRef] [Green Version]
- Park, C.; Cha, H.J.; Lee, H.; Kim, G.Y.; Choi, Y.H. The regulation of the TLR4/NF-κB and Nrf2/HO-1 signaling pathways is involved in the inhibition of lipopolysaccharide-induced inflammation and oxidative reactions by morroniside in RAW 264.7 macrophages. Arch. Biochem. Biophys. 2021, 706, 108926. [Google Scholar] [CrossRef]
- Ajuwon, O.R.; Katengua-Thamahane, E.; Van Rooyen, J.; Oguntibeju, O.O.; Marnewick, J.L. Protective effects of rooibos (Aspalathus linearis) and/or red palm oil (Elaeis guineensis) supplementation on tert-butyl hydroperoxide-induced oxidative hepatotoxicity in Wistar rats. Evid.-Based Complement. Altern. Med. 2013, 19, 984273. [Google Scholar]
- Krajka-Kuźniak, V.; Baer-Dubowska, W. Modulation of Nrf2 and NF-κB signaling pathways by naturally occurring compounds in relation to cancer prevention and therapy. Are combinations better than single compounds? Int. J. Mol. Sci. 2021, 22, 8223. [Google Scholar] [CrossRef]
- Lawal, A.O.; Oluyede, D.M.; Adebimpe, M.O.; Olumegbon, L.T.; Awolaja, O.O.; Elekofehinti, O.O.; Crown, O.O. The cardiovascular protective effects of rooibos (Aspalathus linearis) extract on diesel exhaust particles induced inflammation and oxidative stress involve NF-κB-and Nrf2-dependent pathways modulation. Heliyon 2019, 5, e01426. [Google Scholar] [CrossRef] [Green Version]
- Mishra, P.; Paital, B.; Jena, S.; Swain, S.S.; Kumar, S.; Yadav, M.K.; Chainy, G.B.; Samanta, L. Possible activation of NRF2 by Vitamin E/Curcumin against altered thyroid hormone induced oxidative stress via NFĸB/AKT/mTOR/KEAP1 signalling in rat heart. Sci. Rep. 2019, 9, 1–16. [Google Scholar]
- Oluyede, D.M.; Lawal, A.O.; Adebimpe, M.O.; Olumegbon, L.T.; Elekofehinti, O.O. Biochemical and molecular effects of naringenin on the cardiovascular oxidative and pro-inflammatory effects of oral exposure to diesel exhaust particles in rats. Air Qual. Atmos. Health 2021, 14, 935–953. [Google Scholar] [CrossRef]
- Engelbrecht, A.M.; Odendaal, L.; Du Toit, E.F.; Kupai, K.; Csont, T.; Ferdinandy, P.; Van Rooyen, J. The effect of dietary red palm oil on the functional recovery of the ischaemic/reperfused isolated rat heart: The involvement of the PI3-Kinase signaling pathway. Lipids Health Dis. 2009, 8, 18. [Google Scholar] [CrossRef] [Green Version]
- Sambanthamurthi, R.; Sundram, K.; Tan, Y.A. Chemistry and biochemistry of palm oil. Prog. Lipid Res. 2000, 39, 507–558. [Google Scholar] [CrossRef]
- Budin, S.B.; Muhd Hanis, M.; Hamid, Z.A.; Mohamed, J. Tocotrienol-rich fraction of palm oil reduced pancreatic damage and oxidative stress in streptozotocin-induced diabetic rats. Aust. J. Basic Appl. Sci. 2011, 5, 2367–2374. [Google Scholar]
- Oluba, O.M.; Adeyemi, O.; Ojieh, G.C.; Aboluwoye, C.O.; Eidangbe, G.O. Comparative effect of soybean oil and palm oil on serum lipids and some serum enzymes in cholesterol-fed rats. Eur. J. Sci. Res. 2008, 23, 559–566. [Google Scholar]
- Yam, M.L.; Abdul Hafid, S.R.; Cheng, H.M.; Nesaretnam, K. Tocotrienols suppress pro-inflammatory markers and cyclooxygenase-2 expression in RAW264. 7 macrophages. Lipids 2009, 44, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Ohsaki, Y.; Shirakawa, H.; Hiwatashi, K.; Furukawa, Y.; Mizutani, T.; Komai, M. Vitamin K suppresses lipopolysaccharide-induced inflammation in the rat. Biosci. Biotechnol. Biochem. 2006, 70, 926–932. [Google Scholar] [CrossRef] [Green Version]
- Katengua-Thamahane, E.; Marnewick, J.L.; Ajuwon, O.R.; Chegou, N.N.; Szűcs, G.; Ferdinandy, P.; Csont, T.; Csonka, C.; Van Rooyen, J. The combination of red palm oil and rooibos show anti-inflammatory effects in rats. J. Inflamm. 2014, 11, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szucs, G.; Bester, D.J.; Kupai, K.; Csont, T.; Csonka, C.; Esterhuyse, A.J.; Ferdinandy, P.; Van Rooyen, J. Dietary red palm oil supplementation decreases infarct size in cholesterol fed rats. Lipids Health Dis. 2011, 10, 103. [Google Scholar] [CrossRef] [Green Version]
- Aboua, Y.G.; Brooks, N.; Mahfouz, R.Z.; Agarwal, A.; Du Plessis, S.S. A red palm oil diet can reduce the effects of oxidative stress on rat spermatozoa. Andrologia 2012, 44, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Bhanger, M.I.; Anwar, F. Antioxidant properties and components of bran extracts from selected wheat varieties commercially available in Pakistan. LWT—Food Sci. Technol. 2007, 40, 361–367. [Google Scholar] [CrossRef]
- Rautenbach, F.; Faber, M.; Laurie, S.; Laurie, R. Antioxidant capacity and antioxidant content in roots of 4 sweet potato varieties. J. Food Sci. 2010, 75, C400–C405. [Google Scholar] [CrossRef]
- Robles-Sánchez, M.; Astiazarán-García, H.; Martín-Belloso, O.; Gorinstein, S.; Alvarez-Parrilla, E.; Laura, A.; Yepiz-Plascencia, G.; González-Aguilar, G.A. Influence of whole and fresh-cut mango intake on plasma lipids and antioxidant capacity of healthy adults. Food Res. Int. 2011, 44, 1386–1391. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Khoschsorur, G.A.; Winklhofer-Roob, B.M.; Rabl, H.; Auer, T.; Peng, Z.; Schaur, R.J. Evaluation of a sensitive HPLC method for the determination of malondialdehyde, and application of the method to different biological materials. Chromatographia 2000, 52, 181–184. [Google Scholar] [CrossRef]
- Recknagel, R.O.; Glende, E.A., Jr. Spectrophotometric detection of lipid conjugated dienes. Methods Enzymol. 1984, 105, 331–337. [Google Scholar]
- Tietze, F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal. Biochem. 1969, 27, 502–522. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Crosti, N.; Servidei, T.; Bajer, J.; Serra, A. Modification of the 6-hydroxydopamine technique for the correct determination of superoxide dismutase. J. Clin. Chem. Clin. Biochem. 1987, 25, 265–266. [Google Scholar]
- Ellerby, L.M.; Bredesen, D.E. Measurement of cellular oxidation, reactive oxygen species, and antioxidant enzymes during apoptosis. Methods Enzymol. 2000, 322, 413–421. [Google Scholar]
- Staal, G.E.; Visser, J.; Veeger, C. Purification and properties of glutathione reductase of human erythrocytes. Biochim. Biophys. Acta (BBA)—Enzymol. 1969, 185, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Zhang, W.; Dong, S.; Song, L.; Zhao, S.; Wu, C.; Wang, X.; Liu, F.; Xie, J.; Wang, J.; et al. Protective effects of sea buckthorn polysaccharide extracts against LPS/d-GalN-induced acute liver failure in mice via suppressing TLR4-NF-κB signaling. J. Ethnopharmacol. 2015, 176, 69–78. [Google Scholar] [CrossRef]
- El Kamouni, S.; El Kebbaj, R.; Andreoletti, P.; El Ktaibi, A.; Rharrassi, I.; Essamadi, A.; Mandard, S.; Latruffe, N.; Vamecq, J.; Nasser, B.; et al. Protective effect of argan and olive oils against LPS-induced oxidative stress and inflammation in mice livers. Int. J. Mol. Sci. 2017, 18, 2181. [Google Scholar] [CrossRef] [Green Version]
- Al-Dossari, M.H.; Fadda, L.M.; Attia, H.A.; Hasan, I.H.; Mahmoud, A.M. Curcumin and selenium prevent lipopolysaccharide/diclofenac-induced liver injury by suppressing inflammation and oxidative stress. Biol. Trace Elem. Res. 2020, 196, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Hamesch, K.; Borkham-Kamphorst, E.; Strnad, P.; Weiskirchen, R. Lipopolysaccharide-induced inflammatory liver injury in mice. Lab. Anim. 2015, 49, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Sha, J.; Zhang, H.; Zhao, Y.; Feng, X.; Hu, X.; Wang, C.; Song, M.; Fan, H. Dexmedetomidine attenuates lipopolysaccharide-induced liver oxidative stress and cell apoptosis in rats by increasing GSK-3β/MKP-1/Nrf2 pathway activity via the α2 adrenergic receptor. Toxicol. Appl. Pharmacol. 2019, 364, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Li, W.; Cheng, L.M.; Wang, G.G. Lycopene attenuates LPS-induced liver injury by inactivation of NF-κB/COX-2 signaling. Int. J. Clin. Exp. Pathol. 2019, 12, 817–825. [Google Scholar]
- McGill, M.R. The past and present of serum aminotransferases and the future of liver injury biomarkers. EXCLI J. 2016, 15, 817–828. [Google Scholar]
- Fu, Y.; Zheng, S.; Lin, J.; Ryerse, J.; Chen, A. Curcumin protects the rat liver from CCl4-caused injury and fibrogenesis by attenuating oxidative stress and suppressing inflammation. Mol. Pharmacol. 2008, 73, 399–409. [Google Scholar] [CrossRef] [Green Version]
- El-Feki, M.A.; Amin, H.M.; Abdalla, A.A.; Fesal, M. Immunomodulatory and anti-oxidant effects of alpha-lipoic acid and vitamin E on lipopolysaccharide-induced liver Injury in rats. Middle East J. Appl. Sci. 2016, 6, 460–467. [Google Scholar]
- Indu, R.; Azhar, T.S.; Nair, A.; Nair, C.K.K. Amelioration of doxorubicin induced cardio-and hepato-toxicity by carotenoids. J. Cancer Res. Ther. 2014, 10, 62–67. [Google Scholar]
- Pandir, D.; Per, S.; Doganyigit, Z.; Bekdemir, F.O.; Gok, G.; Demirbag, A. All aspects of the toxic effects of lipopolysaccharide on rat liver and the protective effect of vitamin E and sodium selenite. Turk. J. Zool. 2019, 43, 566–579. [Google Scholar] [CrossRef]
- Ajuwon, O.R.; Oguntibeju, O.O.; Marnewick, J.L. Amelioration of lipopolysaccharide-induced liver injury by aqueous rooibos (Aspalathus linearis) extract via inhibition of pro-inflammatory cytokines and oxidative stress. BMC Complement. Altern. Med. 2014, 14, 392. [Google Scholar] [CrossRef] [Green Version]
- Woolbright, B.L.; Jaeschke, H. Mechanisms of inflammatory liver injury and drug-induced hepatotoxicity. Curr. Pharmacol. Rep. 2018, 4, 346–357. [Google Scholar] [CrossRef]
- Zhong, W.; Qian, K.; Xiong, J.; Ma, K.; Wang, A.; Zou, Y. Curcumin alleviates lipopolysaccharide induced sepsis and liver failure by suppression of oxidative stress-related inflammation via PI3K/AKT and NF-κB related signaling. Biomed. Pharmacother. 2016, 83, 302–313. [Google Scholar] [CrossRef]
- Kawata, A.; Murakami, Y.; Suzuki, S.; Fujisawa, S. Anti-inflammatory activity of β-carotene, lycopene and tri-n-butylborane, a scavenger of reactive oxygen species. In Vivo 2018, 32, 255–264. [Google Scholar]
- Shibata, A.; Nakagawa, K.; Kawakami, Y.; Tsuzuki, T.; Miyazawa, T. Suppression of γ-tocotrienol on UVB induced inflammation in HaCaT keratinocytes and HR-1 hairless mice via inflammatory mediators multiple signaling. J. Agric. Food Chem. 2010, 58, 7013–7020. [Google Scholar] [CrossRef]
- Tan, S.W.; bin Israf Ali, D.A.; Khaza’ai, H.; Wong, J.W.; Vidyadaran, S. Cellular uptake and anti-inflammatory effects of palm oil-derived delta (δ)-tocotrienol in microglia. Cell. Immunol. 2020, 357, 104200. [Google Scholar] [CrossRef]
- Wu, S.J.; Liu, P.L.; Ng, L.T. Tocotrienol-rich fraction of palm oil exhibits anti-inflammatory property by suppressing the expression of inflammatory mediators in human monocytic cells. Mol. Nutr. Food Res. 2008, 52, 921–929. [Google Scholar] [CrossRef]
- Zainal, Z.; Abdul Rahim, A.; Khaza’ai, H.; Chang, S.K. Effects of palm oil tocotrienol-rich fraction (TRF) and carotenes in ovalbumin (ova)-challenged asthmatic Brown Norway rats. Int. J. Mol. Sci. 2019, 20, 1764. [Google Scholar] [CrossRef] [Green Version]
- Zainal, Z.; Rahim, A.A.; Radhakrishnan, A.K.; Chang, S.K.; Khaza’ai, H. Investigation of the curative effects of palm vitamin E tocotrienols on autoimmune arthritis disease in vivo. Sci. Rep. 2019, 9, 16793. [Google Scholar] [CrossRef]
- De Paula Ramos, M.F.; Oliveira, O.B.; Razvickas, C.V.; de Andrade Pessoa, E.; da Silva, R.F.; Pereira, A.M.S.; Convento, M.B.; Borges, F.T.; Schor, N. Comparison of olive leaf, olive oil, palm oil, and omega-3 oil in acute kidney injury induced by sepsis in rats. PeerJ 2019, 7, e7219. [Google Scholar] [CrossRef]
- Jaffer, U.; Wade, R.G.; Gourlay, T. Cytokines in the systemic inflammatory response syndrome: A review. HSR Proc. Intensive Care Cardiovasc. Anesth. 2010, 2, 161. [Google Scholar]
- Chaudhry, H.; Zhou, J.; Zhong, Y.I.N.; Ali, M.M.; McGuire, F.; Nagarkatti, P.S.; Nagarkatti, M. Role of cytokines as a double-edged sword in sepsis. In Vivo 2013, 27, 669–684. [Google Scholar] [PubMed]
- Abdel-Salam, O.M.; Youness, E.R.; Mohammed, N.A.; Abd-Elmoniem, M.; Omara, E.; Sleem, A.A. Neuroprotective and hepatoprotective effects of micronized purified flavonoid fraction (Daflon®) in lipopolysaccharide-treated rats. Drug Discov. Ther. 2012, 6, 306–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.; Yang, L.; Deng, S.; Liang, M. Daidzein ameliorates LPS-induced hepatocyte injury by inhibiting inflammation and oxidative stress. Eur. J. Pharmacol. 2020, 885, 173399. [Google Scholar] [CrossRef] [PubMed]
- Kono, Y.; Fridovich, I. Superoxide radical inhibits catalase. J. Biol. Chem. 1982, 257, 5751–5754. [Google Scholar] [CrossRef]
- Catanzaro, R.; Zerbinati, N.; Solimene, U.; Marcellino, M.; Mohania, D.; Italia, A.; Ayala, A.; Marotta, F. Beneficial effect of refined red palm oil on lipid peroxidation and monocyte tissue factor in HCV-related liver disease: A randomized controlled study. Hepatobiliary Pancreat. Dis. Int. 2016, 15, 165–172. [Google Scholar] [CrossRef]
- Li, W.; Khor, T.O.; Xu, C.; Shen, G.; Jeong, W.S.; Yu, S.; Kong, A.N. Activation of Nrf2-antioxidant signaling attenuates NFκB-inflammatory response and elicits apoptosis. Biochem. Pharmacol. 2008, 76, 1485–1489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, C.Y.; Xu, L.Q.; Zhang, Z.B.; Chen, C.H.; Huang, Y.Z.; Su, Z.Q.; Guo, H.Z.; Chen, X.Y.; Zhang, X.; Liu, Y.H.; et al. Protective effects of pogostone against LPS-induced acute lung injury in mice via regulation of Keap1–Nrf2/NF-κB signaling pathways. Int. Immunopharmacol. 2016, 32, 55–61. [Google Scholar] [CrossRef]
- Sun, S.C. Non-canonical NF-kappaB signaling pathway. Cell Res. 2011, 21, 71–85. [Google Scholar] [CrossRef] [Green Version]
- Vallabhapurapu, S.; Karin, M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu. Rev. Immunol. 2009, 27, 693–733. [Google Scholar] [CrossRef]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Ye, J.; Guan, M.; Lu, Y.; Zhang, D.; Li, C.; Zhou, C. Arbutin attenuates LPS-induced lung injury via Sirt1/Nrf2/NF-κBp65 pathway. Pulm. Pharmacol. Ther. 2019, 54, 53–59. [Google Scholar] [CrossRef]
- Johnson, D.A.; Amirahmadi, S.; Ward, C.; Fabry, Z.; Johnson, J.A. The absence of the pro-antioxidant transcription factor Nrf2 exacerbates experimental autoimmune encephalomyelitis. Toxicol. Sci. 2010, 114, 237–246. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.; Thimmulappa, R.; Kombairaju, P.; Biswal, S. NADPH oxidase-dependent reactive oxygen species mediate amplified TLR4 signaling and sepsis-induced mortality in Nrf2-deficient mice. J. Immunol. 2010, 185, 569–577. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Lu, H.; Bai, Y. Nrf2 in cancers: A double-edged sword. Cancer Med. 2019, 8, 2252–2267. [Google Scholar] [CrossRef]
- Kim, J.E.; You, D.J.; Lee, C.; Ahn, C.; Seong, J.Y.; Hwang, J.I. Suppression of NF-kappaB signaling by KEAP1 regulation of IKKbeta activity through autophagic degradation and inhibition of phosphorylation. Cell Signal. 2010, 22, 1645–1654. [Google Scholar] [CrossRef]
- Ranneh, Y.; Akim, A.M.; Hamid, H.A.; Khazaai, H.; Fadel, A.; Mahmoud, A.M. Stingless bee honey protects against lipopolysaccharide induced-chronic subclinical systemic inflammation and oxidative stress by modulating Nrf2, NF-κB and p38 MAPK. Nutr. Metab. 2019, 16, 15. [Google Scholar] [CrossRef]
- Aladaileh, S.H.; Abukhalil, M.H.; Saghir, S.A.; Hanieh, H.; Alfwuaires, M.A.; Almaiman, A.A.; Bin-Jumah, M.; Mahmoud, A.M. Galangin activates Nrf2 signaling and attenuates oxidative damage, inflammation, and apoptosis in a rat model of cyclophosphamide-induced hepatotoxicity. Biomolecules 2019, 9, 346. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, A.M.; Germoush, M.O.; Alotaibi, M.F.; Hussein, O.E. Possible involvement of Nrf2 and PPARγ up-regulation in the protective effect of umbelliferone against cyclophosphamide-induced hepatotoxicity. Biomed. Pharmacother. 2017, 86, 297–306. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Mohammed, H.M.; Khadrawy, S.M.; Galaly, S.R. Hesperidin protects against chemically induced hepatocarcinogenesis via modulation of Nrf2/ARE/HO-1, PPARγ and TGF-β1/Smad3 signaling, and amelioration of oxidative stress and inflammation. Chem. Biol. Interact. 2017, 277, 146–158. [Google Scholar] [CrossRef]
- Mittal, R.; Kumar, A.; Singh, D.P.; Bishnoi, M.; Nag, T.C. Ameliorative potential of rutin in combination with nimesulide in STZ model of diabetic neuropathy: Targeting Nrf2/HO-1/NF-kB and COX signalling pathway. Inflammopharmacology 2018, 26, 755–768. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An overview of Nrf2 signaling pathway and its role in inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]
- Son, Y.; Lee, J.H.; Chung, H.T.; Pae, H.O. Therapeutic roles of heme oxygenase-1 in metabolic diseases: Curcumin and resveratrol analogues as possible inducers of heme oxygenase-1. Oxid. Med. Cell. Longev. 2013, 2013, 639541. [Google Scholar] [CrossRef] [Green Version]
- Wunder, C.; Potter, R.F. The heme oxygenase system: Its role in liver inflammation. Cardiovasc. Hematol. Disord. Drug Tagets 2003, 3, 199–208. [Google Scholar] [CrossRef]
- Choo, Y.Y.; Lee, S.; Nguyen, P.H.; Lee, W.; Woo, M.H.; Min, B.S.; Lee, J.H. Caffeoylglycolic acid methyl ester, a major constituent of sorghum, exhibits anti-inflammatory activity via the Nrf2/heme oxygenase-1 pathway. RSV Adv. 2015, 5, 17786–17796. [Google Scholar] [CrossRef]
- Consoli, V.; Sorrenti, V.; Grosso, S.; Vanella, L. Heme oxygenase-1 signaling and redox homeostasis in physiopathological conditions. Biomolecules 2021, 11, 589. [Google Scholar] [CrossRef]
- Paine, A.; Eiz-Vesper, B.; Blasczyk, R.; Immenschuh, S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem. Pharmacol. 2010, 80, 1895–1903. [Google Scholar] [CrossRef] [Green Version]
- Peh, H.Y.; Ho, W.E.; Cheng, C.; Chan, T.K.; Seow, A.C.G.; Lim, A.Y.; Fong, C.W.; Seng, K.Y.; Ong, C.N.; Wong, W.F. Vitamin E isoform γ-tocotrienol downregulates house dust mite–induced asthma. J. Immunol. 2015, 195, 437–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Gene | Sequence (5′->3′) |
|---|---|
| Nrf2 (NM_031789.3) | Forward: TGGATCTGTCAGCTACTCCCA Reverse: ATCCAGGGCAAGCGACTCAT |
| HO-1 (NM_012580.2) | Forward: CAGAAGGGTCAGGTGTCCAG Reverse: GAAGGCCATGTCCTGCTCTA |
| GCLC (NM_012815.2) | Forward: TCTGGATGATGCCAACGAGT Reverse: CCTCCATTGGTCGGAACTCT |
| GCLM (NM_017305.2) | Forward: CGCCTGCGGAAAAAGTGTC Reverse: TTCCACTGCATGGGACATGG |
| NFκβ (NM_199267.2) | Forward: CTGGCCATGGACGATCTGTT Reverse: GCACTTGTAACGGAAACGCA |
| Iκβ (NM_030867.2) | Forward: CCACTCCATGTAGCTGTCATC Reverse: ACACGTAGGCTCCGGTTTATT |
| IκKβ (NM_053355.3) | Forward: AGCTCTGGAACCTCCTGAAGA Reverse: CACTGGAAGGCTGGGACATT |
| β-actin (NM_031144.3) | Forward: CTCCCTGGAGAAGAGCTATGA Reverse: CAGGAAGGAAGGCTGGAAGA |
| Constituents | Concentration |
|---|---|
| α-tocopherol (µg/g) | 75.37 ± 10.50 |
| β/γ-tocopherol(µg/g) | 8.33 ± 1.05 |
| δ-tocopherol (µg/g) | 18.79 ± 4.80 |
| α-tocotrienol (µg/g) | 65.26 ± 6.43 |
| β/γ-tocotrienol (µg/g) | 242.21 ± 9.76 |
| δ-tocotrienol (µg/g) | 91.45 ± 5.94 |
| α-carotene (µg/g) | 20.44 ± 1.16 |
| β-carotene (µg/g) | 29.78 ± 2.92 |
| SFA (%) | 51 |
| MUFA (%) | 38 |
| PUFA (%) | 11 |
| Parameter | NC | LPS | RPO | RPO + LPS |
|---|---|---|---|---|
| Weight gain (g) | 89.21 ± 17.31 a | 91.19 ± 11.57 a | 89.56 ± 5.09 a | 89.94 ± 7.93 a |
| Liver weight (g) | 11.83 ± 0.82 a | 12.36 ± 1.25 a | 11.83 ± 0.89 a | 12.06 ± 1.44 a |
| Relative liver weight (%) | 3.15 ± 0.22 a | 3.36 ± 0.43 a | 3.11 ± 0.28 a | 3.23 ± 0.27 a |
| Intake of tocopherol/day (μg/100 g body weight) | Not determined | Not determined | 4.52 ± 0.14 a | 4.63 ± 0.24 a |
| Intake of tocotrienol/day (μg/100 g body weight) | Not determined | Not determined | 18.02 ± 0.67 a | 18.20 ± 0.94 a |
| Intake of α-carotene/day (μg/100 g body weight) | Not determined | Not determined | 1.09 ± 0.04 a | 1.11 ± 0.06 a |
| Intake of β-carotene/day (μg/100 g body weight) | Not determined | Not determined | 1.35 ± 0.04 a | 1.38 ± 0.07 a |
| Parameters | NC | LPS | RPO | RPO + LPS |
|---|---|---|---|---|
| FRAP (μmol AAE/g tissue) | 2.87 ± 0.25 a | 2.63 ± 0.13 b | 2.90 ± 0.17 a | 2.91 ± 0.18 a |
| ORAC (μmol TE/g tissue) | 22.14 ± 10.56 a | 17.40 ± 4.14 a | 19.61 ± 5.13 a | 17.72 ± 4.48 a |
| TEAC (μmol TE/g tissue) | 57.29 ± 3.48 a | 57.42 ± 4.37 a | 57.97 ± 9.02 a | 58.20 ± 4.45 a |
| CD (nmol/g tissue) | 10.38 ± 1.12 b | 12.78 ± 0.79 a | 11.15 ± 0.74 b | 11.12 ± 0.34 b |
| MDA (µmol/g tissue) | 64.83 ± 5.46 c | 92.65 ± 7.57 a | 70.62 ± 5.77 bc | 79.03 ± 13.91 b |
| CAT (µmol H2O2 consumed/min/µg protein) | 0.11 ± 0.01 c | 0.23 ± 0.03 a | 0.12 ± 0.03 c | 0.18 ± 0.02 b |
| SOD (U/mg protein) | 55.01 ± 5.27 a | 42.06 ± 6.26 b | 59.12 ± 13.82 a | 46.62 ± 7.09 b |
| GR (µmol NADPH oxidized/min/µg protein) | 3.99 ± 1.12 a | 2.69 ± 0.62 b | 4.10 ± 1.06 a | 3.71 ± 0.86 a |
| GPx (nmol NADPH oxidized/min/µg protein) | 0.16 ± 0.02 a | 0.12 ± 0.02 b | 0.17 ± 0.02 a | 0.15 ± 0.03 a |
| GSH | 6.38 ± 0.58 a | 6.37 ± 0.83 a | 6.50 ± 1.36 a | 6.20 ± 0.97 a |
| GSSG | 0.32 ± 0.10 b | 0.46 ± 0.09 a | 0.29 ± 0.04 b | 0.34 ± 0.05 b |
| GSH:GSSG | 21.42 ± 6.32 a | 13.64 ± 2.39 b | 23.09 ± 6.23 a | 18.72 ± 3.82 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajuwon, O.R.; Marnewick, J.L.; Oguntibeju, O.O.; Davids, L.M. Red Palm Oil Ameliorates Oxidative Challenge and Inflammatory Responses Associated with Lipopolysaccharide-Induced Hepatic Injury by Modulating NF-κβ and Nrf2/GCL/HO-1 Signaling Pathways in Rats. Antioxidants 2022, 11, 1629. https://doi.org/10.3390/antiox11081629
Ajuwon OR, Marnewick JL, Oguntibeju OO, Davids LM. Red Palm Oil Ameliorates Oxidative Challenge and Inflammatory Responses Associated with Lipopolysaccharide-Induced Hepatic Injury by Modulating NF-κβ and Nrf2/GCL/HO-1 Signaling Pathways in Rats. Antioxidants. 2022; 11(8):1629. https://doi.org/10.3390/antiox11081629
Chicago/Turabian StyleAjuwon, Olawale R., Jeanine L. Marnewick, Oluwafemi O. Oguntibeju, and Lester M. Davids. 2022. "Red Palm Oil Ameliorates Oxidative Challenge and Inflammatory Responses Associated with Lipopolysaccharide-Induced Hepatic Injury by Modulating NF-κβ and Nrf2/GCL/HO-1 Signaling Pathways in Rats" Antioxidants 11, no. 8: 1629. https://doi.org/10.3390/antiox11081629