Enhancement of Clover (Trifolium alexandrinum L.) Shade Tolerance and Nitrogen Fixation under Dense Stands-Based Cropping Systems
Abstract
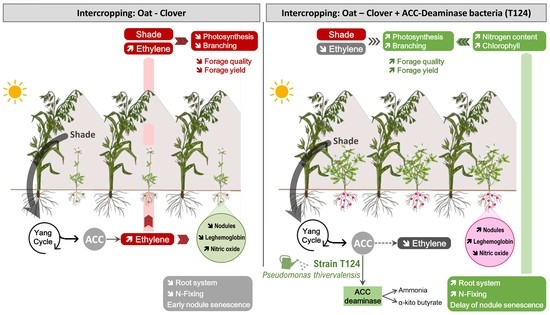
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Controlled Conditions Experiments
2.2.1. Experimental Design
2.2.2. Measurements
2.3. Field Experiments
2.3.1. Site Description
2.3.2. Experimental Design and Management
2.3.3. Measurements
2.4. Statistical Analysis
3. Results
3.1. Effect of Exogenous ACC and P. thivervalensis Strain T124 on Clover Growth under Normal and Reduced Light Conditions
3.2. Potentialities of P. thivervalensis Inoculation for the Improvement of Clover Nodulation Status and N-Fixing
3.3. Assessment of T124 and T618 Strains Inoculation Effects on Clover–Oat Intercrops under Field Conditions
3.3.1. Photosynthetic Active Radiation (PAR) and Photosynthesis Assimilation
3.3.2. Crops Status at Forage Cut (150 DAS)
3.3.3. Forage Yield
3.3.4. Nitrogen and Phosphorus Concentrations (% in Dry Matter)
4. Discussion
4.1. Potential Improvement of Clover Growth under Reduced Light by P. thivervalensis Strain T124 Inoculation
4.2. Supply of R. leguminosarum Strain T618 and P. thivervalensis Strain T124 Co-Inoculation under Oat-Clover Field Intercrops Condition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sadeghpour, A.; Jahanzad, E.; Esmaeili, A.; Hosseini, M.B.; Hashemi, M. Forage Yield, Quality and Economic Benefit of Intercropped Barley and Annual Medic in Semi-Arid Conditions: Additive Series. Field Crops Res. 2013, 148, 43–48. [Google Scholar] [CrossRef]
- Dordas, C.A.; Lithourgidis, A.S. Growth, Yield and Nitrogen Performance of Faba Bean Intercrops with Oat and Triticale at Varying Seeding Ratios. Grass Forage Sci. 2011, 66, 569–577. [Google Scholar] [CrossRef]
- Ross, S.M.; King, J.R.; O’Donovan, J.T.; Spaner, D. Intercropping Berseem Clover with Barley and Oat Cultivars for Forage. Agron. J. 2004, 96, 1719–1729. [Google Scholar] [CrossRef]
- Salama, H.S.A. Mixture Cropping of Berseem Clover with Cereals to Improve Forage Yield and Quality under Irrigated Conditions of the Mediterranean Basin. Ann. Agric. Sci. 2020, 65, 159–167. [Google Scholar] [CrossRef]
- Gecaitė, V.; Arlauskienė, A.; Cesevičienė, J. Competition Effects and Productivity in Oat–Forage Legume Relay Intercropping Systems under Organic Farming Conditions. Agriculture 2021, 11, 99. [Google Scholar] [CrossRef]
- Rodriguez, C.; Carlsson, G.; Englund, J.E.; Flöhr, A.; Pelzer, E.; Jeuffroy, M.H.; Makowski, D.; Jensen, E.S. Grain Legume-Cereal Intercropping Enhances the Use of Soil-Derived and Biologically Fixed Nitrogen in Temperate Agroecosystems. A Meta-Analysis. Eur. J. Agron. 2020, 118, 126077. [Google Scholar] [CrossRef]
- Li, C.; Dong, Y.; Li, H.; Shen, J.; Zhang, F. Shift from Complementarity to Facilitation on P Uptake by Intercropped Wheat Neighboring with Faba Bean When Available Soil P Is Depleted. Sci. Rep. 2016, 6, 18663. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, L.; Yan, M.; Zhang, Y.; Chen, Y.; Palta, J.A.; Zhang, S. Effect of Sowing Proportion on Above- and below-Ground Competition in Maize–Soybean Intercrops. Sci. Rep. 2021, 11, 15760. [Google Scholar] [CrossRef]
- Guenni, O.; Romero, E.; Guédez, Y.; Bravo de Guenni, L.; Pittermann, J. Influence of Low Light Intensity on Growth and Biomass Allocation, Leaf Photosynthesis and Canopy Radiation Interception and Use in Two Forage Species of Centrosema (DC.) Benth. Grass Forage Sci. 2018, 73, 967–978. [Google Scholar] [CrossRef]
- Ballaré, C.L.; Pierik, R. The Shade-Avoidance Syndrome: Multiple Signals and Ecological Consequences. Plant. Cell Environ. 2017, 40, 2530–2543. [Google Scholar] [CrossRef]
- Casal, J.J. Shade Avoidance. Arab. Book 2012, 10, e0157. [Google Scholar] [CrossRef] [PubMed]
- Wollenberg, A.C.; Strasser, B.; Cerdán, P.D.; Amasino, R.M. Acceleration of Flowering during Shade Avoidance in Arabidopsis Alters the Balance between FLOWERING LOCUS C-Mediated Repression and Photoperiodic Induction of Flowering. Plant Physiol. 2008, 148, 1681–1694. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.M.; King, J.R.; O’Donovan, J.T.; Spaner, D. The Productivity of Oats and Berseem Clover Intercrops. I. Primary Growth Characteristics and Forage Quality at Four Densities of Oats. Grass Forage Sci. 2005, 60, 74–86. [Google Scholar] [CrossRef]
- Mauro, R.P.; Occhipinti, A.; Longo, A.M.G.; Mauromicale, G. Effects of Shading on Chlorophyll Content, Chlorophyll Fluorescence and Photosynthesis of Subterranean Clover. J. Agron. Crop Sci. 2011, 197, 57–66. [Google Scholar] [CrossRef]
- Gommers, C.M.M.; Visser, E.J.W.; Onge, K.R.S.; Voesenek, L.A.C.J.; Pierik, R. Shade Tolerance: When Growing Tall Is Not an Option. Trends Plant Sci. 2013, 18, 65–71. [Google Scholar] [CrossRef]
- Dubois, M.; Van den Broeck, L.; Inzé, D. The Pivotal Role of Ethylene in Plant Growth. Trends Plant Sci. 2018, 23, 311–323. [Google Scholar] [CrossRef]
- Wang, X.; Gao, X.; Liu, Y.; Fan, S.; Ma, Q. Progress of Research on the Regulatory Pathway of the Plant Shade-Avoidance Syndrome. Front. Plant Sci. 2020, 11, 439. [Google Scholar] [CrossRef]
- Chen, Y.; Bonkowski, M.; Shen, Y.; Griffiths, B.S.; Jiang, Y.; Wang, X.; Sun, B. Root Ethylene Mediates Rhizosphere Microbial Community Reconstruction When Chemically Detecting Cyanide Produced by Neighbouring Plants. Microbiome 2020, 8, 4. [Google Scholar] [CrossRef]
- Kegge, W.; Pierik, R. Biogenic Volatile Organic Compounds and Plant Competition. Trends Plant Sci. 2010, 15, 126–132. [Google Scholar] [CrossRef]
- Pierik, R.; Djakovic-Petrovic, T.; Keuskamp, D.H.; de Wit, M.; Voesenek, L.A.C.J. Auxin and Ethylene Regulate Elongation Responses to Neighbor Proximity Signals Independent of Gibberellin and DELLA Proteins in Arabidopsis. Plant Physiol. 2009, 149, 1701–1712. [Google Scholar] [CrossRef] [Green Version]
- Pierik, R.; Whitelam, G.C.; Voesenek, L.A.C.J.; De Kroon, H.; Visser, E.J.W. Canopy Studies on Ethylene-Insensitive Tobacco Identify Ethylene as a Novel Element in Blue Light and Plant–Plant Signalling. Plant J. 2004, 38, 310–319. [Google Scholar] [CrossRef]
- Zhu, F.; Deng, J.; Chen, H.; Liu, P.; Zheng, L.; Ye, Q.; Li, R.; Brault, M.; Wen, J.; Frugier, F.; et al. A CEP Peptide Receptor-Like Kinase Regulates Auxin Biosynthesis and Ethylene Signaling to Coordinate Root Growth and Symbiotic Nodulation in Medicago Truncatula. Plant Cell 2020, 32, 2855–2877. [Google Scholar] [CrossRef] [PubMed]
- Lithourgidis, A.S.; Vlachostergios, D.N.; Dordas, C.A.; Damalas, C.A. Dry Matter Yield, Nitrogen Content, and Competition in Pea–Cereal Intercropping Systems. Eur. J. Agron. 2011, 34, 287–294. [Google Scholar] [CrossRef]
- Toukabri, W.; Ferchichi, N.; Hlel, D.; Jadlaoui, M.; Kheriji, O.; Zribi, F.; Taamalli, W.; Mhamdi, R.; Trabelsi, D. Improvements of Durum Wheat Main Crop in Weed Control, Productivity and Grain Quality through the Inclusion of FenuGreek and Clover as Companion Plants: Effect of N FertilizaTion Regime. Agronomy 2020, 11, 78. [Google Scholar] [CrossRef]
- Raza, M.A.; Feng, L.Y.; van der Werf, W.; Iqbal, N.; Khalid, M.H.B.; Chen, Y.K.; Wasaya, A.; Ahmed, S.; Ud Din, A.M.; Khan, A.; et al. Maize Leaf-Removal: A New Agronomic Approach to Increase Dry Matter, Flower Number and Seed-Yield of Soybean in Maize Soybean Relay Intercropping System. Sci. Rep. 2019, 9, 13453. [Google Scholar] [CrossRef]
- Pantazopoulou, C.K.; Bongers, F.J.; Pierik, R. Reducing Shade Avoidance Can Improve Arabidopsis Canopy Performance against Competitors. Plant. Cell Environ. 2021, 44, 1130–1141. [Google Scholar] [CrossRef]
- Carriedo, L.G.; Maloof, J.N.; Brady, S.M. Molecular Control of Crop Shade Avoidance. Curr. Opin. Plant Biol. 2016, 30, 151–158. [Google Scholar] [CrossRef]
- Adams, D.O.; Yang, S.F. Ethylene Biosynthesis: Identification of 1-Aminocyclopropane-1-Carboxylic Acid as an Intermediate in the Conversion of Methionine to Ethylene. Proc. Natl. Acad. Sci. USA 1979, 76, 170–174. [Google Scholar] [CrossRef]
- Honma, M.; Shimomura, T. Metabolism of 1-Aminocyclopropane-1-Carboxylic Acid. Agric. Biol. Chem. 1978, 42, 1825–1831. [Google Scholar] [CrossRef]
- Glick, B.R.; Cheng, Z.; Czarny, J.; Duan, J. Promotion of Plant Growth by ACC Deaminase-Producing Soil Bacteria. In New Perspectives and Approaches in Plant Growth-Promoting Rhizobacteria Research; Springer: Dordrecht, The Netherlands, 2007; pp. 329–339. [Google Scholar] [CrossRef]
- Zafar-Ul-Hye, M.; Danish, S.; Abbas, M.; Ahmad, M.; Munir, T.M. ACC Deaminase Producing PGPR Bacillus Amyloliquefaciens and Agrobacterium Fabrum along with Biochar Improve Wheat Productivity under Drought Stress. Agronomy 2019, 9, 343. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.d.C.; Duan, J.; DiBernardo, M.; Zetter, E.; Campos-García, J.; Glick, B.R.; Santoyo, G. The Production of ACC Deaminase and Trehalose by the Plant Growth Promoting Bacterium Pseudomonas Sp. UW4 Synergistically Protect Tomato Plants Against Salt Stress. Front. Microbiol. 2019, 10, 1392. [Google Scholar] [CrossRef] [PubMed]
- Bal, H.B.; Adhya, T.K. Alleviation of Submergence Stress in Rice Seedlings by Plant Growth-Promoting Rhizobacteria With ACC Deaminase Activity. Front. Sustain. Food Syst. 2021, 5, 606158. [Google Scholar] [CrossRef]
- Duan, J.; Müller, K.M.; Charles, T.C.; Vesely, S.; Glick, B.R. 1-Aminocyclopropane-1-Carboxylate (ACC) Deaminase Genes in Rhizobia from Southern Saskatchewan. Microb. Ecol. 2008, 57, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Mhamdi, R.; Laguerre, G.; Aouani, M.E.; Mars, M.; Amarger, N. Different Species and Symbiotic Genotypes of Field Rhizobia Can Nodulate Phaseolus Vulgaris in Tunisian Soils. FEMS Microbiol. Ecol. 2002, 41, 77–84. [Google Scholar] [CrossRef]
- Ferchichi, N.; Toukabri, W.; Boularess, M.; Smaoui, A.; Mhamdi, R.; Trabelsi, D. Isolation, Identification and Plant Growth Promotion Ability of Endophytic Bacteria Associated with Lupine Root Nodule Grown in Tunisian Soil. Arch. Microbiol. 2019, 201, 1333–1349. [Google Scholar] [CrossRef]
- Ben Zineb, A.; Trabelsi, D.; Ayachi, I.; Barhoumi, F.; Aroca, R.; Mhamdi, R. Inoculation with Elite Strains of Phosphate-Solubilizing Bacteria Enhances the Effectiveness of Fertilization with Rock Phosphates. Geomicrobiol. J. 2019, 37, 22–30. [Google Scholar] [CrossRef]
- Johnson, J.F.; Allan, D.L.; Vance, C.P. Phosphorus Stress-Induced Proteoid Roots Show Altered Metabolism in Lupinus Albus. Plant Physiol. 1994, 104, 657–665. [Google Scholar] [CrossRef]
- Vincent, J. A Manual for the Practical Study of Root-Nodule Bacteria; Published for the International Biological Programme; Blackwell Scientific: Oxford, UK, 1970; ISBN 9780632064106. [Google Scholar]
- Lichtenthaler, H.K. [34] Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1987; Volume 148, pp. 350–382. ISBN 0076-6879. [Google Scholar]
- Liu, Y.; Wu, R.; Wan, Q.; Xie, G.; Bi, Y. Glucose-6-Phosphate Dehydrogenase Plays a Pivotal Role in Nitric Oxide-Involved Defense Against Oxidative Stress Under Salt Stress in Red Kidney Bean Roots. Plant Cell Physiol. 2007, 48, 511–522. [Google Scholar] [CrossRef]
- Sergiev, I.; Alexieva, V.; Karanov, E.; Karanov, E.; Sergiev, L.; Karanova, E.; Alexieva, V. Effect of Spermine, Atrazine and Combination between Them on Some Endogenous Protective Systems and Stress Markers in Plants. Compt. Rend Acad. Bulg. Sci. 1997, 51, 121–124. [Google Scholar]
- Zhou, B.; Guo, Z.; Xing, J.; Huang, B. Nitric Oxide Is Involved in Abscisic Acid-Induced Antioxidant Activities in Stylosanthes Guianensis. J. Exp. Bot. 2005, 56, 3223–3228. [Google Scholar] [CrossRef] [Green Version]
- LaRue, T.A.; Child, J.J. Sensitive Fluorometric Assay for Leghemoglobin. Anal. Biochem. 1979, 92, 11–15. [Google Scholar] [CrossRef]
- Drabkin, D.L.; Austin, J.H. Spectrophotometric studies: I. spectrophotometric constants for common hemoglobin derivatives in human, dog, and rabbit blood. J. Biol. Chem. 1932, 98, 719–733. [Google Scholar] [CrossRef]
- Becana, M.; Gogorcena, Y.; Aparicio-Tejo, P.M.; Sánchez-Díaz, M. Nitrogen Fixation and Leghemoglobin Content during Vegetative Growth of Alfalfa. J. Plant Physiol. 1986, 123, 117–125. [Google Scholar] [CrossRef]
- Kjeldahl, C. A New Method for the Determination of Nitrogen in Organic Matter. Z. Anal. Chem. 1883, 22, 366. [Google Scholar] [CrossRef]
- Mendes Filho, P.F.; Vasconcellos, R.L.F.; De Paula, A.M.; Cardoso, E. Evaluating the Potential of Forest Species under “Microbial Management” for the Restoration of Degraded Mining Areas. Water. Air. Soil Pollut. 2010, 208, 79–89. [Google Scholar] [CrossRef]
- Borah, M.; Das, S.; Bora, S.S.; Boro, R.C.; Barooah, M. Comparative Assessment of Multi-Trait Plant Growth-Promoting Endophytes Associated with Cultivated and Wild Oryza Germplasm of Assam, India. Arch. Microbiol. 2021, 203, 2007–2028. [Google Scholar] [CrossRef]
- Thorsted, M.D.; Weiner, J.; Olesen, J.E. Above- and below-Ground Competition between Intercropped Winter Wheat Triticum Aestivum and White Clover Trifolium Repens. J. Appl. Ecol. 2006, 43, 237–245. [Google Scholar] [CrossRef]
- Vaidya, P.; Stinchcombe, J.R. The Potential for Genotype-by-Environment Interactions to Maintain Genetic Variation in a Model Legume-Rhizobia Mutualism. Plant Commun. 2020, 1, 100114. [Google Scholar] [CrossRef]
- Shigeyama, T.; Tominaga, A.; Arima, S.; Sakai, T.; Inada, S.; Jikumaru, Y.; Kamiya, Y.; Uchiumi, T.; Abe, M.; Hashiguchi, M.; et al. Additional Cause for Reduced JA-Ile in the Root of a Lotus Japonicus PhyB Mutant. Plant Signal. Behav. 2012, 7, 746–748. [Google Scholar] [CrossRef]
- Suzuki, A.; Suriyagoda, L.; Shigeyama, T.; Tominaga, A.; Sasaki, M.; Hiratsuka, Y.; Yoshinaga, A.; Arima, S.; Agarie, S.; Sakai, T.; et al. Lotus Japonicus Nodulation Is Photomorphogenetically Controlled by Sensing the Red/Far Red (R/FR) Ratio through Jasmonic Acid (JA) Signaling. Proc. Natl. Acad. Sci. USA 2011, 108, 16837. [Google Scholar] [CrossRef] [Green Version]
- Zdarska, M.; Dobisová, T.; Gelová, Z.; Pernisová, M.; Dabravolski, S.; Hejátko, J. Illuminating Light, Cytokinin, and Ethylene Signalling Crosstalk in Plant Development. J. Exp. Bot. 2015, 66, 4913–4931. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Y.; Cai, X.; Wan, Y.T.; Fu, Y.F.; Yang, X.Y.; Zhang, Z.W.; Yuan, S. Relatively Low Light Intensity Promotes Phosphorus Absorption and Enhances the Ethylene Signaling Component EIN3 in Maize, Wheat, and Oilseed Rape. Agronomy 2022, 12, 427. [Google Scholar] [CrossRef]
- Shaharoona, B.; Arshad, M.; Zahir, Z.A. Effect of Plant Growth Promoting Rhizobacteria Containing ACC-Deaminase on Maize (Zea mays L.) Growth under Axenic Conditions and on Nodulation in Mung Bean (Vigna radiata L.). Lett. Appl. Microbiol. 2006, 42, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Tittabutr, P.; Awaya, J.D.; Li, Q.X.; Borthakur, D. The Cloned 1-Aminocyclopropane-1-Carboxylate (ACC) Deaminase Gene from Sinorhizobium Sp. Strain BL3 in Rhizobium Sp. Strain TAL1145 Promotes Nodulation and Growth of Leucaena Leucocephala. Syst. Appl. Microbiol. 2008, 31, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Santoyo, G.; Moreno-Hagelsieb, G.; del Carmen Orozco-Mosqueda, M.; Glick, B.R. Plant Growth-Promoting Bacterial Endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef]
- Lemaire, B.; Van Cauwenberghe, J.; Chimphango, S.; Stirton, C.; Honnay, O.; Smets, E.; Muthama Muasya, A. Recombination and Horizontal Transfer of Nodulation and ACC Deaminase (AcdS) Genes within Alpha- and Betaproteobacteria Nodulating Legumes of the Cape Fynbos Biome. FEMS Microbiol. Ecol. 2015, 91, 118. [Google Scholar] [CrossRef]
- González, E.M.; Gálvez, L.; Arrese-Igor, C. Abscisic Acid Induces a Decline in Nitrogen Fixation That Involves Leghaemoglobin, but Is Independent of Sucrose Synthase Activity. J. Exp. Bot. 2001, 52, 285–293. [Google Scholar] [CrossRef]
- Cam, Y.; Pierre, O.; Boncompagni, E.; Hérouart, D.; Meilhoc, E.; Bruand, C. Nitric Oxide (NO): A Key Player in the Senescence of Medicago Truncatula Root Nodules. New Phytol. 2012, 196, 548–560. [Google Scholar] [CrossRef]
- Kato, K.; Kanahama, K.; Kanayama, Y. Involvement of Nitric Oxide in the Inhibition of Nitrogenase Activity by Nitrate in Lotus Root Nodules. J. Plant Physiol. 2010, 167, 238–241. [Google Scholar] [CrossRef]
- Wang, X.; Guo, X.; Yu, Y.; Cui, H.; Wang, R.; Guo, W. Increased Nitrogen Supply Promoted the Growth of Non-N-Fixing Woody Legume Species but Not the Growth of N-Fixing Robinia Pseudoacacia. Sci. Rep. 2018, 8, 17896. [Google Scholar] [CrossRef] [Green Version]
- Kaschuk, G.; Yin, X.; Hungria, M.; Leffelaar, P.A.; Giller, K.E.; Kuyper, T.W. Photosynthetic Adaptation of Soybean Due to Varying Effectiveness of N2 Fixation by Two Distinct Bradyrhizobium Japonicum Strains. Environ. Exp. Bot. 2012, 76, 1–6. [Google Scholar] [CrossRef]
- Pearcy, R.W.; Seemann, J.R. Photosynthetic Induction State of Leaves in a Soybean Canopy in Relation to Light Regulation of Ribulose-1-5-Bisphosphate Carboxylase and Stomatal Conductance. Plant Physiol. 1990, 94, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Wang, H.; Guo, H.; Zhou, A.; Huang, Y.; Sun, Y.; Li, M. Effects of Shade Treatments on Photosynthetic Characteristics, Chloroplast Ultrastructure, and Physiology of Anoectochilus Roxburghii. PLoS ONE 2014, 9, e85996. [Google Scholar] [CrossRef]
- Yang, F.; Liao, D.; Wu, X.; Gao, R.; Fan, Y.; Raza, M.A.; Wang, X.; Yong, T.; Liu, W.; Liu, J.; et al. Effect of Aboveground and Belowground Interactions on the Intercrop Yields in Maize-Soybean Relay Intercropping Systems. Filed Crops Res. 2017, 203, 16–23. [Google Scholar] [CrossRef]
- Kaschuk, G.; Kuyper, T.W.; Leffelaar, P.A.; Hungria, M.; Giller, K.E. Are the Rates of Photosynthesis Stimulated by the Carbon Sink Strength of Rhizobial and Arbuscular Mycorrhizal Symbioses? Soil Biol. Biochem. 2009, 41, 1233–1244. [Google Scholar] [CrossRef]
- Wu, H.; Lin, M.; Rensing, C.; Qin, X.; Zhang, S.; Chen, J.; Wu, L.; Zhao, Y.; Lin, S.; Lin, W. Plant-Mediated Rhizospheric Interactions in Intraspecific Intercropping Alleviate the Replanting Disease of Radix Pseudostellariae. Plant Soil 2020, 454, 411–430. [Google Scholar] [CrossRef]
- Fustec, J.; Lesuffleur, F.; Mahieu, S.; Cliquet, J.B. Nitrogen Rhizodeposition of Legumes. A Review. Agron. Sustain. Dev. 2010, 30, 57–66. [Google Scholar] [CrossRef]
- Alemneh, A.A.; Zhou, Y.; Ryder, M.H.; Denton, M.D. Mechanisms in Plant Growth-Promoting Rhizobacteria That Enhance Legume–Rhizobial Symbioses. J. Appl. Microbiol. 2020, 129, 1133–1156. [Google Scholar] [CrossRef]
- Ríos-Ruiz, W.F.; Torres-Chávez, E.E.; Torres-Delgado, J.; Rojas-García, J.C.; Bedmar, E.J.; Valdez-Nuñez, R.A. Inoculation of Bacterial Consortium Increases Rice Yield (Oryza sativa L.) Reducing Applications of Nitrogen Fertilizer in San Martin Region, Peru. Rhizosphere 2020, 14, 100200. [Google Scholar] [CrossRef]





| Strain T618 | Strain T124 | |
|---|---|---|
| 16S identification | ||
| Closest relative species | Rhizobium leguminosarum bv. trifolii strain ECRI 10A | Pseudomonas thivervalensis isolate 2.C.10 |
| Accession number | MW375693 | MW375690 |
| Similarity (%) | 100% | 97.35% |
| ACC deaminase | ||
| Presence of acdS gene | + | + |
| ACC deaminase activity (μM keto mg protein−1 h−1) | 0.7 | 19.42 |
| Other PGPR trails | ||
| Auxin production (μg mL−1) | 83.79 | 42.88 |
| Phosphate solubilization (μg mL−1) | 214.91 | − |
| Siderophore production (psu) | − | − |
| Alternaria alternata inhibition | − | + |
| Macrophomina phaseolina inhibition | − | − |
| N-Fixing ability | ||
| Presence of nifH gene | + | − |
| Presence of nodC gene | + | − |
| Nodulation | + | nd |
| Efficiency | 318% | nd |
| BEG | JBS | |||||||
|---|---|---|---|---|---|---|---|---|
| 2017 | 2018 | 2017 | 2018 | |||||
| Clover | Oat | Clover | Oat | Clover | Oat | Clover | Oat | |
| Plant height (cm) | ||||||||
| C | 79 B | 106 A | 65 B | 101 A | 69 B | 97 A | 78 B | 111 A |
| P | 77 B | 109 A | 69 B | 103 A | 67 B | 99 A | 70 B | 97 A |
| R | 77 B | 116 A | 70 B | 114 A | 70 B | 99 A | 76 B | 105 A |
| R + P | 75 B | 115 A | 71 B | 103 A | 73 B | 103 A | 79 B | 105 A |
| SPAD values | ||||||||
| C | 30.3 c | 43.7 b | 32.3 c | 44.7 a | 36.3 c | 43.9 b | 34.3 c | 44.5 ab |
| P | 33.3 ab | 44.6 ab | 35.5 ab | 42.8 a | 39.6 ab | 43.8 b | 37 ab | 43.6 b |
| R | 32 bc | 45.4 a | 33.6 bc | 44.5 a | 38.8 b | 45.4 a | 35.5 bc | 46 a |
| R + P | 34.9 a | 44.2 ab | 36.2 a | 43.4 a | 40.9 a | 44.3 ab | 38.6 a | 45 ab |
| Clover shoots branching | ||||||||
| C | 1.8 (73) | 2.1 (63) | 2.2 (62) | 1.8 (65) | ||||
| P | 2.7 (49) | 2.7 (37) | 2.7 (43) | 2.8 (31) | ||||
| R | 2.2 (62) | 1.8 (56) | 2.2 (58) | 2.3 (47) | ||||
| R + P | 2.9 (40) | 2.9 (40) | 3.3 (30) | 2.8 (36) | ||||
| Clover nodules number | ||||||||
| C | 26 b (36) | 33 a (47) | 22 c (65) | 21 c (48) | ||||
| P | 34 ab (27) | 43 a (31) | 24 bc (49) | 25 bc (36) | ||||
| R | 30 ab (36) | 37 a (35) | 37 ab (29) | 33 ab (34) | ||||
| R + P | 38 a (24) | 47 a (27) | 39 a (34) | 39 a (26) | ||||
| BEG | JBS | |||||||
|---|---|---|---|---|---|---|---|---|
| 2017 | 2018 | 2017 | 2018 | |||||
| Clover | Oat | Clover | Oat | Clover | Oat | Clover | Oat | |
| N concentration (%) | ||||||||
| C | 2.12 c | 1.87 b | 1.93 b | 2.31 b | 2.13 c | 1.61 b | 2.47 c | 1.83 b |
| P | 2.65 a | 1.91 bc | 2.51 a | 2.49 ab | 2.73 ab | 1.83 ab | 2.93 b | 1.86 b |
| R | 2.34 b | 2.32 a | 2.09 b | 2.84 a | 2.43 bc | 2.04 a | 2.56 c | 2.18 a |
| R + P | 2.73 a | 2.2 ab | 2.63 a | 2.53 ab | 3.04 ab | 1.92 ab | 3.24 a | 2.25 a |
| P concentration (%) | ||||||||
| C | 0.64 b | 0.35 c | 0.73 b | 0.38 b | 0.5 b | 0.31 b | 0.56 b | 0.35 a |
| P | 0.67 b | 0.38 bc | 0.73 b | 0.39 b | 0.52 b | 0.33 ab | 0.59 b | 0.37 a |
| R | 0.78 a | 0.47 a | 0.83 a | 0.48 a | 0.63 a | 0.36 a | 0.63 ab | 0.36 a |
| R + P | 0.75 a | 0.44 ab | 0.8 a | 0.45 a | 0.6 a | 0.35 a | 0.66 a | 0.35 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toukabri, W.; Ferchichi, N.; Barbouchi, M.; Hlel, D.; Jadlaoui, M.; Bahri, H.; Mhamdi, R.; Cheikh M’hamed, H.; Annabi, M.; Trabelsi, D. Enhancement of Clover (Trifolium alexandrinum L.) Shade Tolerance and Nitrogen Fixation under Dense Stands-Based Cropping Systems. Agronomy 2022, 12, 2332. https://doi.org/10.3390/agronomy12102332
Toukabri W, Ferchichi N, Barbouchi M, Hlel D, Jadlaoui M, Bahri H, Mhamdi R, Cheikh M’hamed H, Annabi M, Trabelsi D. Enhancement of Clover (Trifolium alexandrinum L.) Shade Tolerance and Nitrogen Fixation under Dense Stands-Based Cropping Systems. Agronomy. 2022; 12(10):2332. https://doi.org/10.3390/agronomy12102332
Chicago/Turabian StyleToukabri, Wael, Nouha Ferchichi, Meriem Barbouchi, Dorsaf Hlel, Mohamed Jadlaoui, Haithem Bahri, Ridha Mhamdi, Hatem Cheikh M’hamed, Mohamed Annabi, and Darine Trabelsi. 2022. "Enhancement of Clover (Trifolium alexandrinum L.) Shade Tolerance and Nitrogen Fixation under Dense Stands-Based Cropping Systems" Agronomy 12, no. 10: 2332. https://doi.org/10.3390/agronomy12102332