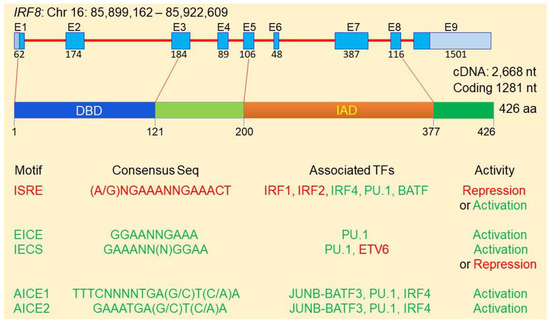
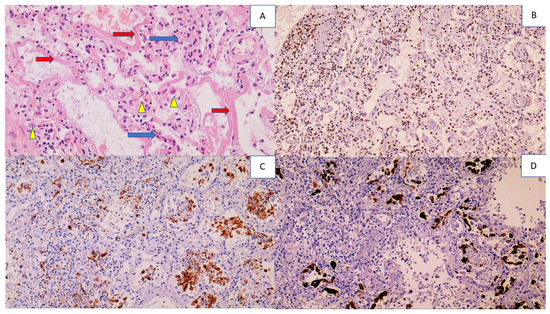
Immunity, Inflammation, Oxidative Stress and Cancer
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Cellular Immunology".
Editor
Interests: oxidative stress; growth regulation; cancer; lipid peroxidation; 4-hydroxynonenal (HNE)
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
The ongoing pandemics force science to reconsider many fundamental aspects of major diseases, which were mostly considered to be non-transmissible, chronic and associated with stress and aging until the onset of COVID-19. When going to the molecular biomedicine level of these diseases, many researchers found oxidative stress either as their crucial pathogenic factor or as an important epiphenomenon that might help define novel biomarkers and/or adjuvant therapies according to the modern concepts of integrative biomedicine. Nowadays, these diseases are often denoted as “comorbidities” in respect to COVID-19; however, cancer was and is still of particular interest not only because of its severity and socio-medical relevance but also because it is a constant enigma for the immune system, which we assume acts as our crucial defense mechanism, due to the simplified perception of COVID-19. Similarly, since the 1970s, scientists have also assumed immunology will eventually provide effective solutions to cure cancer, while the ongoing pandemics demand reconsidering crucial pathophysiological aspects of immunology, notably of immunity and of inflammation in general (cytokine storm, trained immunity, etc.) and in respect to cancer in particular (the most fearsome, immunosuppressive “comorbidity”).
Therefore, the aim of this Topical Collection (TC) is to collect comprehensive reviews and original research papers that provide new findings on the complex relationship between cancer and the immune system, focusing on inflammation, cancer development and therapies in particular. The onset of oxidative stress and endogenous and exogenous pro- and anti-oxidants will be of particular interest for the scope of this SI that aims to provide a better understanding of immunity, inflammation and cancer, thus offering new ideas and concepts for better diagnostics and treatment protocols.
Prof. Dr. Neven Zarkovic
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- cancer
- immune system
- oxidative stress
- inflammation
- immunity