Mechanism of Immunotherapy in Cancers
A topical collection in Cancers (ISSN 2072-6694). This collection belongs to the section "Cancer Immunology and Immunotherapy".
Viewed by 168332Editors
Interests: acute leukemias; immunotherapy; tolerance; NK cells; clinical trials
Interests: de novo acute myeloid leukemia; secondary acute myeloid leukemia; primary mielofibrosis; bone marrow microenvinronment; clonal evolution; stem cell transplantation; chemotherapy; targeted therapy; immunotherapy
Interests: lung cancer; thoracic oncology; immunotherapy; target therapy
Topical Collection Information
Dear Colleagues,
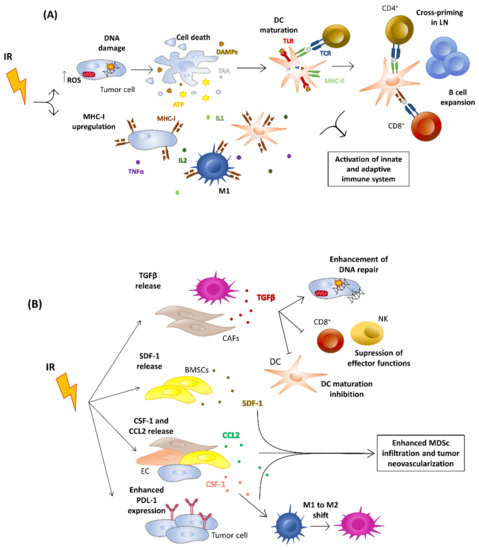
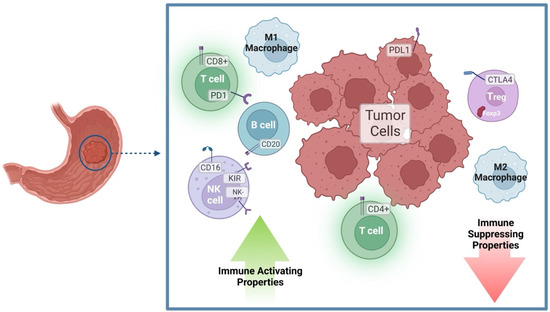
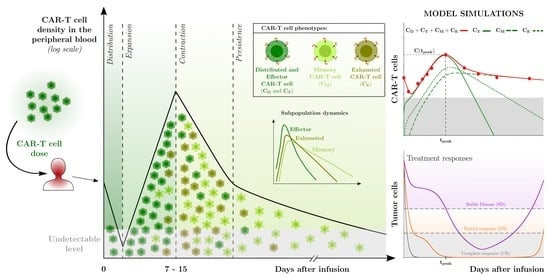
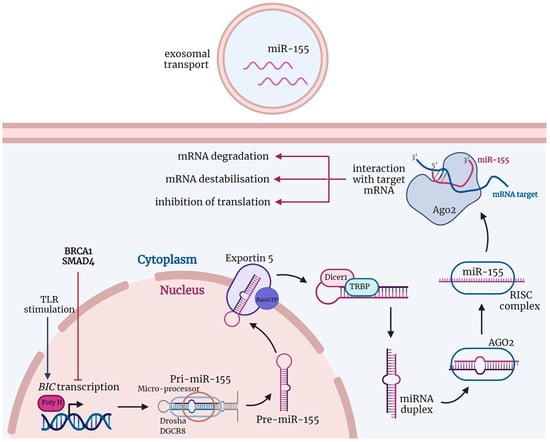
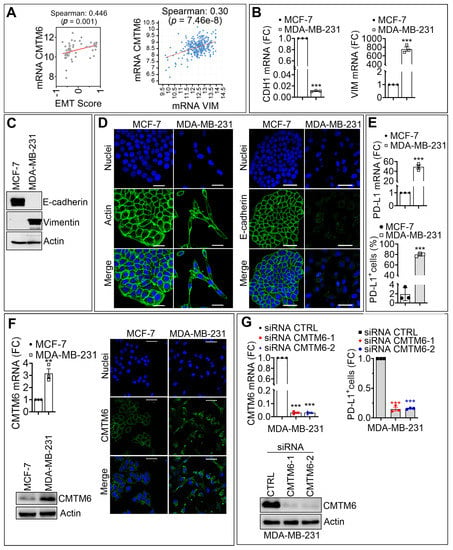
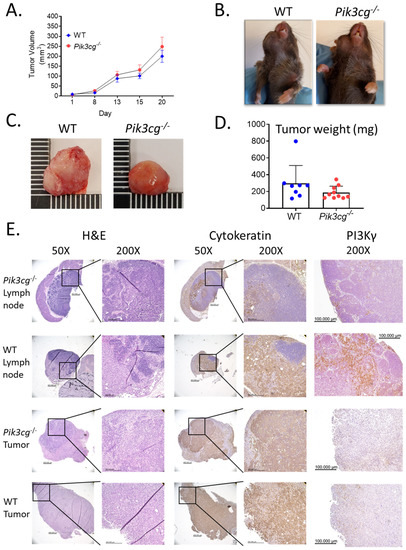
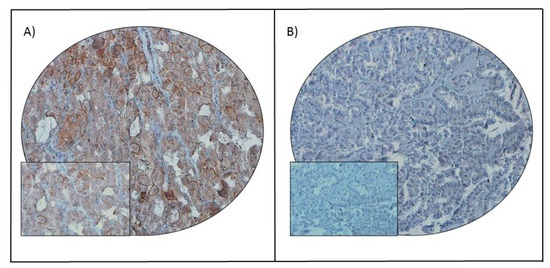
In cancers, a better knowledge of the mechanisms leading to immunological activation/tolerance, as well as the identification of critical regulators, such as immune checkpoints, are paving the way for a fast-track development of a huge number of novel drugs and therapeutic strategies. Although major strides have been made in the deep understanding of the mechanisms underlying immunotherapy in cancers, a long list of unanswered questions is still awaiting response for a full exploitation of immunotherapy into the clinical ground. In the immunotherapy era, it is time to refine stratification and prognostication of cancer patients under a new immunological-driven biological approach. Moreover, few, if any, immune-based biomarkers, predictive of response to immune therapy, are under clinical use, and the impact of genetic alterations of cancer cells, which have a role in shaping immunological microenvironment, has not been fully elucidated.
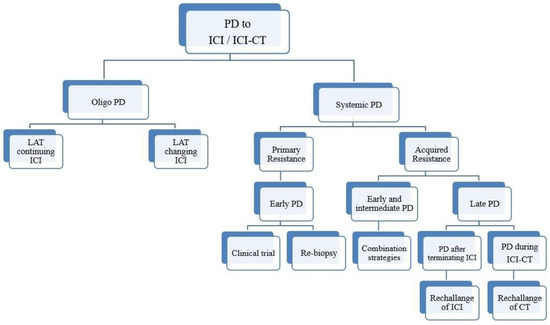
In a series of manuscripts authored by experts in the field, we plan to focus on a mechanism-based overview of novel immunotherapies in cancers. We will discuss open questions, regarding efficacy and safety signals, ideal therapeutic settings, mechanisms of resistance, the importance and incorporation of predictive biomarkers in clinical trials, and the overall potential for future development of immunotherapy in the cancer landscape.
Dr. Antonio Curti
Dr. Alessandro Isidori
Dr. Giuseppe Lo Russo
Dr. Marina Chiara Garassino
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cancers is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2900 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- cancers
- solid tumors
- hematological neoplasms
- immunotherapy
- immune response
- tolerance
- resistance
- clinical trials