A Holistic Approach to Hard-to-Treat Cancers: The Future of Immunotherapy for Glioblastoma, Triple Negative Breast Cancer, and Advanced Prostate Cancer
Abstract
:1. Introduction
2. Glioblastoma (GBM)
3. Triple Negative Breast Cancer (TNBC)
4. Advanced Prostate Cancer (PCa)
5. Chronic Stress and Cancer
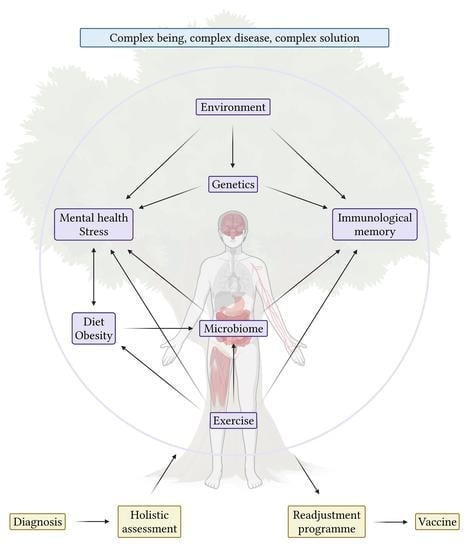
6. Conclusions and Future Directions for Immunotherapy
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Twomey, J.D.; Zhang, B. Cancer Immunotherapy Update: FDA-Approved Checkpoint Inhibitors and Companion Diagnostics. APPS J. 2021, 23, 39. [Google Scholar] [CrossRef]
- Conry, R.M.; Westbrook, B.; McKee, S.; Norwood, T.G. Talimogene Laherparepvec: First in Class Oncolytic Virotherapy. Hum. Vaccin. Immunother. 2018, 14, 839–846. [Google Scholar] [CrossRef] [Green Version]
- Ostrom, Q.T.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014–2018. Neuro-Oncology 2021, 23, iii1–iii105. [Google Scholar] [CrossRef]
- Ohgaki, H. Epidemiology of Brain Tumors. In Cancer Epidemiology: Modifiable Factors; Verma, M., Ed.; Humana Press: Totowa, NJ, USA, 2009; pp. 323–342. ISBN 978-1-60327-492-0. [Google Scholar]
- Grossman, S.A.; Batara, J.F. Current Management of Glioblastoma Multiforme. Semin. Oncol. 2004, 31, 635–644. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, X.; Zhou, B.; Zhang, L. The Prognostic Value of MGMT Promoter Methylation in Glioblastoma Multiforme: A Meta-Analysis. Fam. Cancer 2013, 12, 4492013458. [Google Scholar] [CrossRef]
- Malkki, H. Glioblastoma Vaccine Therapy Disappointment in Phase III Trial. Nat. Rev. Neurol. 2016, 12, 190. [Google Scholar] [CrossRef]
- Ravi, V.M.; Neidert, N.; Will, P.; Joseph, K.; Maier, J.P.; Kückelhaus, J.; Vollmer, L.; Goeldner, J.M.; Behringer, S.P.; Scherer, F.; et al. T-Cell Dysfunction in the Glioblastoma Microenvironment Is Mediated by Myeloid Cells Releasing Interleukin-10. Nat. Commun. 2022, 13, 925. [Google Scholar] [CrossRef] [PubMed]
- Woroniecka, K.I.; Rhodin, K.E.; Chongsathidkiet, P.; Keith, K.A.; Fecci, P.E. T-Cell Dysfunction in Glioblastoma: Applying a New Framework. Clin. Cancer Res. 2018, 24, 3792–3802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirzaei, R.; Sarkar, S.; Yong, V.W. T Cell Exhaustion in Glioblastoma: Intricacies of Immune Checkpoints. Trends Immunol. 2017, 38, 104–115. [Google Scholar] [CrossRef]
- Rudnick, J.D.; Fink, K.L.; Landolfi, J.C.; Markert, J.; Piccioni, D.E.; Glantz, M.J.; Swanson, S.J.; Gringeri, A.; Yu, J. Immunological Targeting of CD133 in Recurrent Glioblastoma: A Multi-Center Phase I Translational and Clinical Study of Autologous CD133 Dendritic Cell Immunotherapy. JCO 2017, 35, 2059. [Google Scholar] [CrossRef]
- Fadul, C.E.; Fisher, J.L.; Hampton, T.H.; Lallana, E.C.; Li, Z.; Gui, J.; Szczepiorkowski, Z.M.; Tosteson, T.D.; Rhodes, C.H.; Wishart, H.A.; et al. Immune Response in Patients with Newly Diagnosed Glioblastoma Multiforme Treated with Intranodal Autologous Tumor Lysate-Dendritic Cell Vaccination After Radiation Chemotherapy. J. Immunother. 2011, 34, 382. [Google Scholar] [CrossRef] [Green Version]
- Woroniecka, K.; Fecci, P.E. Immuno-Synergy? Neoantigen Vaccines and Checkpoint Blockade in Glioblastoma. Neuro-Oncology 2020, 22, 1233–1234. [Google Scholar] [CrossRef] [PubMed]
- Dohnal, A.M.; Witt, V.; Hügel, H.; Holter, W.; Gadner, H.; Felzmann, T. Phase I Study of Tumor Ag-Loaded IL-12 Secreting Semi-Mature DC for the Treatment of Pediatric Cancer. Cytotherapy 2007, 9, 755–770. [Google Scholar] [CrossRef]
- Chang, C.-N.; Huang, Y.-C.; Yang, D.-M.; Kikuta, K.; Wei, K.-J.; Kubota, T.; Yang, W.-K. A Phase I/II Clinical Trial Investigating the Adverse and Therapeutic Effects of a Postoperative Autologous Dendritic Cell Tumor Vaccine in Patients with Malignant Glioma. J. Clin. Neurosci. 2011, 18, 1048–1054. [Google Scholar] [CrossRef]
- Wen, P.Y.; Reardon, D.A.; Armstrong, T.S.; Phuphanich, S.; Aiken, R.D.; Landolfi, J.C.; Curry, W.T.; Zhu, J.-J.; Glantz, M.; Peereboom, D.M.; et al. A Randomized Double-Blind Placebo-Controlled Phase II Trial of Dendritic Cell Vaccine ICT-107 in Newly Diagnosed Patients with Glioblastoma. Clin. Cancer Res. 2019, 25, 5799–5807. [Google Scholar] [CrossRef] [PubMed]
- Inogés, S.; Tejada, S.; de Cerio, A.L.-D.; Gállego Pérez-Larraya, J.; Espinós, J.; Idoate, M.A.; Domínguez, P.D.; de Eulate, R.G.; Aristu, J.; Bendandi, M.; et al. A Phase II Trial of Autologous Dendritic Cell Vaccination and Radiochemotherapy Following Fluorescence-Guided Surgery in Newly Diagnosed Glioblastoma Patients. J. Transl. Med. 2017, 15, 104. [Google Scholar] [CrossRef] [Green Version]
- Wen, P.Y.; Reardon, D.A.; Forst, D.A.; Lee, E.Q.; Haas, B.; Daoud, T.; Berthoud, T.; Diaz-Mitoma, F.; Anderson, D.E.; Lassman, A.B.; et al. Evaluation of Tumor Responses and Overall Survival in Patients with Recurrent Glioblastoma (GBM) from a Phase IIa Trial of a CMV Vaccine Immunotherapeutic Candidate (VBI-1901). JCO 2022, 40, 2014. [Google Scholar] [CrossRef]
- Wang, Q.-T.; Nie, Y.; Sun, S.-N.; Lin, T.; Han, R.-J.; Jiang, J.; Li, Z.; Li, J.-Q.; Xiao, Y.-P.; Fan, Y.-Y.; et al. Tumor-Associated Antigen-Based Personalized Dendritic Cell Vaccine in Solid Tumor Patients. Cancer Immunol. Immunother. 2020, 69, 1375–1387. [Google Scholar] [CrossRef]
- Berneman, Z.N.; Anguille, S.; Willemen, Y.; de Velde, A.V.; Germonpre, P.; Huizing, M.; Van Tendeloo, V.; Saevels, K.; Rutsaert, L.; Vermeulen, K.; et al. Vaccination of Cancer Patients with Dendritic Cells Electroporated with MRNA Encoding the Wilms’ Tumor 1 Protein (WT1): Correlation of Clinical Effect and Overall Survival with T-Cell Response. Cytotherapy 2019, 21, S10. [Google Scholar] [CrossRef]
- Peereboom, D.M.; Nabors, L.B.; Kumthekar, P.; Badruddoja, M.A.; Fink, K.L.; Lieberman, F.S.; Phuphanich, S.; Dunbar, E.M.; Walbert, T.; Schiff, D.; et al. Phase 2 Trial of SL-701 in Relapsed/Refractory (r/r) Glioblastoma (GBM): Correlation of Immune Response with Longer-Term Survival. JCO 2018, 36, 2058. [Google Scholar] [CrossRef]
- Rahman, M.; Ghiaseddin, A.; Deleyrolle, P.; Peters, K.B.; Archer, G.; Sampson, J.; Mitchell, D. Phase II Randomized, Blinded, Placebo-Controlled Trial Testing Pp65 CMV MRNA Dendritic Cell Vaccine and Tetanus-Diphtheria Toxoid for Newly Diagnosed GBM (ATTAC II, NCT02465268). Neuro-Oncologyogy 2022, 24, vii60–vii61. [Google Scholar] [CrossRef]
- Dutoit, V.; Marinari, E.; Dietrich, P.-Y.; Migliorini, D. Combination of the IMA950/Poly-ICLC Multipeptide Vaccine with Pembrolizumab in Relapsing Glioblastoma Patients. Neuro Oncol. 2020, 22, ii34. [Google Scholar] [CrossRef]
- Sloan, A.E.; Dansey, R.; Zamorano, L.; Barger, G.; Hamm, C.; Diaz, F.; Baynes, R.; Wood, G. Adoptive Immunotherapy in Patients with Recurrent Malignant Glioma: Preliminary Results of Using Autologous Whole-Tumor Vaccine plus Granulocyte-Macrophage Colony-Stimulating Factor and Adoptive Transfer of Anti-CD3-Activated Lymphocytes. Neurosurg. Focus. 2000, 9, e9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batich, K.A.; Mitchell, D.A.; Healy, P.; Herndon, J.E.; Sampson, J.H. Once, Twice, Three Times a Finding: Reproducibility of Dendritic Cell Vaccine Trials Targeting Cytomegalovirus in Glioblastoma. Clin. Cancer Res. 2020, 26, 5297–5303. [Google Scholar] [CrossRef]
- Reardon, D.A.; Idbaih, A.; Vieito, M.; Tabatabai, G.; Stradella, A.; Ghiringhelli, F.; Burger, M.C.; Mildenberger, I.; González, M.; Hervieu, A.; et al. EO2401 Therapeutic Vaccine for Patients with Recurrent Glioblastoma: Phase 1/2 ROSALIE Study (NCT04116658). Neuro-Oncology 2022, 24, vii63. [Google Scholar] [CrossRef]
- Tarakanovskaya, M.G.; Chinburen, J.; Batchuluun, P.; Munkhzaya, C.; Purevsuren, G.; Dandii, D.; Hulan, T.; Oyungerel, D.; Kutsyna, G.A.; Reid, A.A.; et al. Open-Label Phase II Clinical Trial in 75 Patients with Advanced Hepatocellular Carcinoma Receiving Daily Dose of Tableted Liver Cancer Vaccine, Hepcortespenlisimut-L. J. Hepatocell. Carcinoma 2017, 4, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Luo, F.; Tang, C.; Chen, D.; Qin, Z.; Hua, W.; Xu, M.; Zhong, P.; Yu, S.; Chen, D.; et al. Molecular Subgroups and B7-H4 Expression Levels Predict Responses to Dendritic Cell Vaccines in Glioblastoma: An Exploratory Randomized Phase II Clinical Trial. Cancer Immunol. Immunother. 2018, 67, 1777–1788. [Google Scholar] [CrossRef]
- Hu, J.L.; Omofoye, O.A.; Rudnick, J.D.; Kim, S.; Tighiouart, M.; Phuphanich, S.; Wang, H.; Mazer, M.; Ganaway, T.; Chu, R.M.; et al. A Phase I Study of Autologous Dendritic Cell Vaccine Pulsed with Allogeneic Stem-like Cell Line Lysate in Patients with Newly Diagnosed or Recurrent Glioblastoma. Clin. Cancer Res. 2022, 28, 689–696. [Google Scholar] [CrossRef]
- Parney, I.F.; Anderson, S.K.; Gustafson, M.P.; Steinmetz, S.; Peterson, T.E.; Kroneman, T.N.; Raghunathan, A.; O’Neill, B.P.; Buckner, J.C.; Solseth, M.; et al. Phase I Trial of Adjuvant Mature Autologous Dendritic Cell/Allogeneic Tumor Lysate Vaccines in Combination with Temozolomide in Newly Diagnosed Glioblastoma. Neuro-Oncology Adv. 2022, 4, vdac089. [Google Scholar] [CrossRef]
- Batich, K.A.; Reap, E.A.; Archer, G.E.; Sanchez-Perez, L.; Nair, S.K.; Schmittling, R.J.; Norberg, P.; Xie, W.; Herndon, J.E., II; Healy, P.; et al. Long-Term Survival in Glioblastoma with Cytomegalovirus Pp65-Targeted Vaccination. Clin. Cancer Res. 2017, 23, 1898–1909. [Google Scholar] [CrossRef] [Green Version]
- Ahluwalia, M.S.; Reardon, D.A.; Abad, A.P.; Curry, W.T.; Wong, E.T.; Figel, S.A.; Mechtler, L.L.; Peereboom, D.M.; Hutson, A.D.; Withers, H.G.; et al. Phase IIa Study of SurVaxM Plus Adjuvant Temozolomide for Newly Diagnosed Glioblastoma. J. Clin. Oncol. 2023, 41, 1453–1465. [Google Scholar] [CrossRef] [PubMed]
- Wick, W.; Dietrich, P.-Y.; Kuttruff, S.; Hilf, N.; Frenzel, K.; Admon, A.; van der Burg, S.H.; von Deimling, A.; Gouttefangeas, C.; Kroep, J.R.; et al. GAPVAC-101: First-in-Human Trial of a Highly Personalized Peptide Vaccination Approach for Patients with Newly Diagnosed Glioblastoma. JCO 2018, 36, 2000. [Google Scholar] [CrossRef]
- Kodysh, J.; Bozkus, C.C.; Saxena, M.; Meseck, M.; Rubinsteyn, A.; O’Donnell, T.; Thin, T.H.; Brody, R.; Mandeli, J.; Bhardwaj, N.; et al. Phase I Study of Safety and Activity of Personalized Neoantigen-Based Vaccines in Combination with Tumor Treating Fields for Newly Diagnosed Glioblastoma Patients. J. Immunother. Cancer 2021, 9, 334. [Google Scholar] [CrossRef]
- Wheeler, L.A.; Manzanera, A.G.; Bell, S.D.; Cavaliere, R.; McGregor, J.M.; Grecula, J.C.; Newton, H.B.; Lo, S.S.; Badie, B.; Portnow, J.; et al. Phase II Multicenter Study of Gene-Mediated Cytotoxic Immunotherapy as Adjuvant to Surgical Resection for Newly Diagnosed Malignant Glioma. Neuro-Oncology 2016, 18, 1137–1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jouanneau, E.; Black, K.L.; Veiga, L.; Cordner, R.; Goverdhana, S.; Zhai, Y.; Zhang, X.; Panwar, A.; Mardiros, A.; Wang, H.; et al. Intrinsically De-Sialylated CD103+ CD8 T Cells Mediate Beneficial Anti-Glioma Immune Responses. Cancer Immunol. Immunother. 2014, 63, 911–924. [Google Scholar] [CrossRef]
- Chiocca, E.A.; Aguilar, L.K.; Bell, S.D.; Kaur, B.; Hardcastle, J.; Cavaliere, R.; McGregor, J.; Lo, S.; Ray-Chaudhuri, A.; Chakravarti, A.; et al. Phase IB Study of Gene-Mediated Cytotoxic Immunotherapy Adjuvant to up-Front Surgery and Intensive Timing Radiation for Malignant Glioma. J. Clin. Oncol. 2011, 29, 3611–3619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moertel, C.; Pluhar, G.E.; Olin, M. Use of a Pan-Peptide Checkpoint Inhibitor in the Treatment of Central Nervous System Tumors. Neuro Oncol. 2023, 25, i81. [Google Scholar] [CrossRef]
- Zakharia, Y.; Johnson, T.S.; Colman, H.; Vahanian, N.N.; Link, C.J.; Kennedy, E.; Sadek, R.F.; Kong, F.M.; Vender, J.; Munn, D.; et al. A Phase I/II Study of the Combination of Indoximod and Temozolomide for Adult Patients with Temozolomide-Refractory Primary Malignant Brain Tumors. JCO 2014, 32, TPS2107. [Google Scholar] [CrossRef]
- Vik-Mo, E.O.; Nyakas, M.; Mikkelsen, B.V.; Moe, M.C.; Due-Tønnesen, P.; Suso, E.M.I.; Sæbøe-Larssen, S.; Sandberg, C.; Brinchmann, J.E.; Helseth, E.; et al. Therapeutic Vaccination against Autologous Cancer Stem Cells with MRNA-Transfected Dendritic Cells in Patients with Glioblastoma. Cancer Immunol. Immunother. 2013, 62, 1499–1509. [Google Scholar] [CrossRef] [Green Version]
- Bankiewicz, K.; Achrol, A.; Aghi, M.; Bexon, M.; Brenner, A.; Butowski, N.; Elder, B.; Floyd, J.; Lonser, R.; Merchant, F.; et al. MRI-Guided Convective Delivery of MDNA55, an Interleukin-4 Receptor Targeted Immunotherapy for the Treatment of Recurrent Glioblastoma. Neuro-Oncology 2017, 19, vi29. [Google Scholar] [CrossRef] [Green Version]
- Carpentier, A.F.; Verlut, C.; Ghiringhelli, F.; Bronnimann, C.; Ursu, R.; Fumet, J.D.; Gherga, E.; Lefort, F.; Belin, C.; Vernerey, D.; et al. Anti-Telomerase Vaccine in Patients with Newly Diagnosed, Unmethylated MGMT Glioblastoma: A Phase II Study. JCO 2023, 41, 2005. [Google Scholar] [CrossRef]
- Crittenden, M.; Bahjat, K.S.; Li, R.; Gore, P.; Fountain, C.; Hanson, B.; Skoble, J.; Lauer, P.; Murphy, A.L.; Dubensky, T.; et al. Phase I Study of Safety and Immunogenicity of ADU-623, a Live-Attenuated Listeria Monocytogenes Vaccine (ΔactA/ΔinlB) Expressing EGFRVIII and NY-ESO-1, in Patients with Who Grade III/IV Astrocytomas. J. Immunother. Cancer 2015, 3, P162. [Google Scholar] [CrossRef] [Green Version]
- Wick, W.; Wick, A.; Nowosielski, M.; Sahm, F.; Riehl, D.; Arzt, M.; von Deimling, A.; Bendszus, M.; Kickingereder, P.; Bonekamp, D.; et al. VXM01 Phase I Study in Patients with Resectable Progression of a Glioblastoma. JCO 2017, 35, 2061. [Google Scholar] [CrossRef]
- Wick, W.; Wick, A.; Chinot, O.L.; Van Den Bent, M.J.; De Vos, F.Y.F.L.; Mansour, M.; Podola, L.; Lubenau, H.; Platten, M. Oral DNA Vaccination Targeting VEGFR2 Combined with Anti-PDL1 Avelumab in Patients with Progressive Glioblastoma: Safety Run-in Results—NCT03750071. JCO 2020, 38, 3001. [Google Scholar] [CrossRef]
- Liau, L.M.; Ashkan, K.; Brem, S.; Campian, J.L.; Trusheim, J.E.; Iwamoto, F.M.; Tran, D.D.; Ansstas, G.; Cobbs, C.S.; Heth, J.A.; et al. Association of Autologous Tumor Lysate-Loaded Dendritic Cell Vaccination With Extension of Survival Among Patients With Newly Diagnosed and Recurrent Glioblastoma: A Phase 3 Prospective Externally Controlled Cohort Trial. JAMA Oncol. 2023, 9, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Reardon, D.A.; Schuster, J.; Tran, D.D.; Fink, K.L.; Nabors, L.B.; Li, G.; Bota, D.A.; Lukas, R.V.; Desjardins, A.; Ashby, L.S.; et al. ReACT: Overall Survival from a Randomized Phase II Study of Rindopepimut (CDX-110) plus Bevacizumab in Relapsed Glioblastoma. JCO 2015, 33, 2009. [Google Scholar] [CrossRef]
- De Groot, J.F.; Cloughesy, T.F.; Pitz, M.W.; Narita, Y.; Nonomura, T. A Randomized, Multicenter Phase 2 Study of DSP-7888 Dosing Emulsion in Combination with Bevacizumab (Bev) versus Bev Alone in Patients with Recurrent or Progressive Glioblastoma. JCO 2018, 36, TPS2071. [Google Scholar] [CrossRef]
- Bloch, O.; Raizer, J.J.; Lim, M.; Sughrue, M.; Komotar, R.; Abrahams, J.; O’Rourke, D.; D’Ambrosio, A.; Bruce, J.N.; Parsa, A. Newly Diagnosed Glioblastoma Patients Treated with an Autologous Heat Shock Protein Peptide Vaccine: PD-L1 Expression and Response to Therapy. JCO 2015, 33, 2011. [Google Scholar] [CrossRef]
- Plautz, G.E.; Barnett, G.H.; Miller, D.W.; Cohen, B.H.; Prayson, R.A.; Krauss, J.C.; Luciano, M.; Kangisser, D.B.; Shu, S. Systemic T Cell Adoptive Immunotherapy of Malignant Gliomas. J. Neurosurg. 1998, 89, 42–51. [Google Scholar] [CrossRef] [Green Version]
- Sampson, J.H.; Schmittling, R.J.; Archer, G.E.; Congdon, K.L.; Nair, S.K.; Reap, E.A.; Desjardins, A.; Friedman, A.H.; Friedman, H.S.; Ii, J.E.H.; et al. A Pilot Study of IL-2Rα Blockade during Lymphopenia Depletes Regulatory T-Cells and Correlates with Enhanced Immunity in Patients with Glioblastoma. PLoS ONE 2012, 7, e31046. [Google Scholar] [CrossRef]
- Vlahovic, G.; Archer, G.E.; Reap, E.; Desjardins, A.; Peters, K.B.; Randazzo, D.; Healy, P.; Herndon, J.E.; Friedman, A.H.; Friedman, H.S.; et al. Phase I Trial of Combination of Antitumor Immunotherapy Targeted against Cytomegalovirus (CMV) plus Regulatory T-Cell Inhibition in Patients with Newly-Diagnosed Glioblastoma Multiforme (GBM). JCO 2016, 34, e13518. [Google Scholar] [CrossRef]
- Schuster, J.; Lai, R.K.; Recht, L.D.; Reardon, D.A.; Paleologos, N.A.; Groves, M.D.; Mrugala, M.M.; Jensen, R.; Baehring, J.M.; Sloan, A.; et al. A Phase II, Multicenter Trial of Rindopepimut (CDX-110) in Newly Diagnosed Glioblastoma: The ACT III Study. Neuro-Oncology 2015, 17, 854–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenstermaker, R.A.; Ciesielski, M.J.; Qiu, J.; Yang, N.; Frank, C.L.; Lee, K.P.; Mechtler, L.R.; Belal, A.; Ahluwalia, M.S.; Hutson, A.D. Clinical Study of a Survivin Long Peptide Vaccine (SurVaxM) in Patients with Recurrent Malignant Glioma. Cancer Immunol. Immunother. 2016, 65, 1339–1352. [Google Scholar] [CrossRef] [Green Version]
- Bota, D.A.; Taylor, T.H.; Piccioni, D.E.; Duma, C.M.; LaRocca, R.V.; Kesari, S.; Carrillo, J.A.; Abedi, M.; Aiken, R.D.; Hsu, F.P.K.; et al. Phase 2 Study of AV-GBM-1 (a Tumor-Initiating Cell Targeted Dendritic Cell Vaccine) in Newly Diagnosed Glioblastoma Patients: Safety and Efficacy Assessment. J. Exp. Clin. Cancer Res. 2022, 41, 344. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; Butowski, N.; Tran, D.D.; Recht, L.D.; Lim, M.; Hirte, H.; Ashby, L.; Mechtler, L.; Goldlust, S.A.; Iwamoto, F.; et al. Rindopepimut with Temozolomide for Patients with Newly Diagnosed, EGFRvIII-Expressing Glioblastoma (ACT IV): A Randomised, Double-Blind, International Phase 3 Trial. Lancet Oncol. 2017, 18, 1373–1385. [Google Scholar] [CrossRef] [Green Version]
- Desjardins, A.; Sampson, J.H.; Peters, K.B.; Vlahovic, G.; Randazzo, D.; Threatt, S.; Herndon, J.E.; Boulton, S.; Lally-Goss, D.; McSherry, F.; et al. Patient Survival on the Dose Escalation Phase of the Oncolytic Polio/Rhinovirus Recombinant (PVSRIPO) against WHO Grade IV Malignant Glioma (MG) Clinical Trial Compared to Historical Controls. JCO 2016, 34, 2061. [Google Scholar] [CrossRef]
- Spira, A.; Hansen, A.R.; Harb, W.A.; Curtis, K.K.; Koga-Yamakawa, E.; Origuchi, M.; Li, Z.; Ertik, B.; Shaib, W.L. Multicenter, Open-Label, Phase I Study of DSP-7888 Dosing Emulsion in Patients with Advanced Malignancies. Targ. Oncol. 2021, 16, 461–469. [Google Scholar] [CrossRef]
- Migliorini, D.; Dutoit, V.; Allard, M.; Grandjean Hallez, N.; Marinari, E.; Widmer, V.; Philippin, G.; Corlazzoli, F.; Gustave, R.; Kreutzfeldt, M.; et al. Phase I/II Trial Testing Safety and Immunogenicity of the Multipeptide IMA950/Poly-ICLC Vaccine in Newly Diagnosed Adult Malignant Astrocytoma Patients. Neuro-Oncology 2019, 21, 923–933. [Google Scholar] [CrossRef] [Green Version]
- Prins, R.M.; Wang, X.; Soto, H.; Young, E.; Lisiero, D.N.; Fong, B.; Everson, R.; Yong, W.H.; Lai, A.; Li, G.; et al. Comparison of Glioma-Associated Antigen Peptide-Loaded Versus Autologous Tumor Lysate-Loaded Dendritic Cell Vaccination in Malignant Glioma Patients. J. Immunother. 2013, 36, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liau, L.M.; Prins, R.M.; Kiertscher, S.M.; Odesa, S.K.; Kremen, T.J.; Giovannone, A.J.; Lin, J.-W.; Chute, D.J.; Mischel, P.S.; Cloughesy, T.F.; et al. Dendritic Cell Vaccination in Glioblastoma Patients Induces Systemic and Intracranial T-Cell Responses Modulated by the Local Central Nervous System Tumor Microenvironment. Clin. Cancer Res. 2005, 11, 5515–5525. [Google Scholar] [CrossRef] [Green Version]
- Bloch, O.; Crane, C.A.; Fuks, Y.; Kaur, R.; Aghi, M.K.; Berger, M.S.; Butowski, N.A.; Chang, S.M.; Clarke, J.L.; McDermott, M.W.; et al. Heat-Shock Protein Peptide Complex–96 Vaccination for Recurrent Glioblastoma: A Phase II, Single-Arm Trial. Neuro-Oncology 2014, 16, 274–279. [Google Scholar] [CrossRef] [Green Version]
- Thompson, E.M.; Landi, D.; Brown, M.C.; Friedman, H.S.; McLendon, R.; Herndon, J.E.; Buckley, E.; Bolognesi, D.P.; Lipp, E.; Schroeder, K.; et al. Recombinant Polio-Rhinovirus Immunotherapy for Recurrent Paediatric High-Grade Glioma: A Phase 1b Trial. Lancet Child. Adolesc. Health 2023, 7, 471–478. [Google Scholar] [CrossRef]
- Fu, S.; Piccioni, D.E.; Liu, H.; Lukas, R.V.; Aregawi, D.; Yamaguchi, K.; Whicher, K.; Chen, Y.-L.; Poola, N.; Eddy, J.; et al. Initial Phase 1 Study of WT2725 Dosing Emulsion in Patients with Advanced Malignancies. JCO 2017, 35, 2066. [Google Scholar] [CrossRef]
- Dees, K.J.; Koo, H.; Humphreys, J.F.; Hakim, J.A.; Crossman, D.K.; Crowley, M.R.; Nabors, L.B.; Benveniste, E.N.; Morrow, C.D.; McFarland, B.C. Human Gut Microbial Communities Dictate Efficacy of Anti-PD-1 Therapy in a Humanized Microbiome Mouse Model of Glioma. Neuro-Oncol. Adv. 2021, 3, vdab023. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Garay, C.; Djouder, N. Dietary Interventions and Precision Nutrition in Cancer Therapy. Trends Mol. Med. 2023, 29, 489–511. [Google Scholar] [CrossRef]
- Lussier, D.M.; Woolf, E.C.; Johnson, J.L.; Brooks, K.S.; Blattman, J.N.; Scheck, A.C. Enhanced Immunity in a Mouse Model of Malignant Glioma Is Mediated by a Therapeutic Ketogenic Diet. BMC Cancer 2016, 16, 310. [Google Scholar] [CrossRef] [Green Version]
- Hansen, A.; Søgaard, K.; Minet, L.R. Development of an Exercise Intervention as Part of Rehabilitation in a Glioblastoma Multiforme Survivor during Irradiation Treatment: A Case Report. Disabil. Rehabil. 2019, 41, 1608–1614. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.-W.; Chang, Y.-H.; Lee, H.-H.; Wu, J.-Y.; Huang, J.-X.; Chung, Y.-H.; Hsu, S.-T.; Chow, L.-P.; Wei, K.-C.; Huang, F.-T. Irisin, an Exercise Myokine, Potently Suppresses Tumor Proliferation, Invasion, and Growth in Glioma. FASEB J. 2020, 34, 9678–9693. [Google Scholar] [CrossRef]
- Conen, K.L.; Schüpbach, R.; Handschin, B.; Zwahlen, D.; Voss, M.; Eisele, G.; Rentsch, K.; Beyrau, R.; Vogt, D.R.; Katan, M.; et al. Prospective Evaluation of Stress in Patients with Newly Diagnosed Glioblastoma and in a Close Partner (TOGETHER-Study). JCO 2017, 35, e13524. [Google Scholar] [CrossRef]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.B.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of Radiotherapy with Concomitant and Adjuvant Temozolomide versus Radiotherapy Alone on Survival in Glioblastoma in a Randomised Phase III Study: 5-Year Analysis of the EORTC-NCIC Trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Keats, M.R.; Grandy, S.A.; Blanchard, C.; Fowles, J.R.; Neyedli, H.F.; Weeks, A.C.; MacNeil, M.V. The Impact of Resistance Exercise on Muscle Mass in Glioblastoma in Survivors (RESIST): Protocol for a Randomized Controlled Trial. JMIR Res. Protoc. 2022, 11, e37709. [Google Scholar] [CrossRef]
- Klein, P.; Tyrlikova, I.; Zuccoli, G.; Tyrlik, A.; Maroon, J.C. Treatment of Glioblastoma Multiforme with “Classic” 4:1 Ketogenic Diet Total Meal Replacement. Cancer Metab. 2020, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Yalamarty, S.S.K.; Filipczak, N.; Li, X.; Subhan, M.A.; Parveen, F.; Ataide, J.A.; Rajmalani, B.A.; Torchilin, V.P. Mechanisms of Resistance and Current Treatment Options for Glioblastoma Multiforme (GBM). Cancers 2023, 15, 2116. [Google Scholar] [CrossRef] [PubMed]
- Schreck, K.C.; Hsu, F.-C.; Berrington, A.; Henry-Barron, B.; Vizthum, D.; Blair, L.; Kossoff, E.H.; Easter, L.; Whitlow, C.T.; Barker, P.B.; et al. Feasibility and Biological Activity of a Ketogenic/Intermittent-Fasting Diet in Patients with Glioma. Neurology 2021, 97, e953–e963. [Google Scholar] [CrossRef] [PubMed]
- Martin-McGill, K.J.; Marson, A.G.; Tudur Smith, C.; Jenkinson, M.D. Ketogenic Diets as an Adjuvant Therapy in Glioblastoma (the KEATING Trial): Study Protocol for a Randomised Pilot Study. Pilot. Feasibility Stud. 2017, 3, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anjum, K.; Shagufta, B.I.; Abbas, S.Q.; Patel, S.; Khan, I.; Shah, S.A.A.; Akhter, N.; Hassan, S.S.U. Current Status and Future Therapeutic Perspectives of Glioblastoma Multiforme (GBM) Therapy: A Review. Biomed. Pharm. 2017, 92, 681–689. [Google Scholar] [CrossRef]
- Rieger, J.; Bähr, O.; Maurer, G.D.; Hattingen, E.; Franz, K.; Brucker, D.; Walenta, S.; Kämmerer, U.; Coy, J.F.; Weller, M.; et al. ERGO: A Pilot Study of Ketogenic Diet in Recurrent Glioblastoma. Int. J. Oncol. 2014, 44, 1843–1852. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, K.; Chang, H.T.; Nikolai, M.; Pernicone, J.; Rhee, S.; Olson, K.; Kurniali, P.C.; Hord, N.G.; Noel, M. Treatment of Glioma Patients with Ketogenic Diets: Report of Two Cases Treated with an IRB-Approved Energy-Restricted Ketogenic Diet Protocol and Review of the Literature. Cancer Metab. 2015, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Dardis, C.; Renda, L.; Honea, N.; Smith, K.; Nakaji, P.; Ashby, L.S.; Scheck, A.C. ACTR-15. Therapeutic Ketogenic Diet (KD) with Radiation and Chemotherapy for Newly Diagnosed Glioblastoma—Preliminary Results from NCT02046187. Neuro Oncol. 2017, 19, vi4. [Google Scholar] [CrossRef]
- Gresham, G.; Amaral, L.; Lockshon, L.; Levin, D.; Rudnick, J.; Provisor, A.; Shiao, S.; Bhowmick, N.; Irwin, S.; Freedland, S.; et al. ACTR-15. Phase 1 Trial of a Ketogenic Diet in Patients Receiving Standard-of-Care Treatment for Recently Diagnosed Glioblastoma. Neuro Oncol. 2019, 21, vi15. [Google Scholar] [CrossRef]
- Voss, M.; Wenger, K.J.; von Mettenheim, N.; Bojunga, J.; Vetter, M.; Diehl, B.; Franz, K.; Gerlach, R.; Ronellenfitsch, M.W.; Harter, P.N.; et al. Short-Term Fasting in Glioma Patients: Analysis of Diet Diaries and Metabolic Parameters of the ERGO2 Trial. Eur. J. Nutr. 2022, 61, 477–487. [Google Scholar] [CrossRef]
- Qayum, A.; Magotra, A.; Shah, S.M.; Nandi, U.; Sharma, P.R.; Shah, B.A.; Singh, S.K. Synergistic Combination of PMBA and 5-Fluorouracil (5-FU) in Targeting Mutant KRAS in 2D and 3D Colorectal Cancer Cells. Heliyon 2022, 8, e09103. [Google Scholar] [CrossRef]
- Brem, S.; Grossman, S.A.; Carson, K.A.; New, P.; Phuphanich, S.; Alavi, J.B.; Mikkelsen, T.; Fisher, J.D. New Approaches to Brain Tumor Therapy CNS Consortium Phase 2 Trial of Copper Depletion and Penicillamine as Antiangiogenesis Therapy of Glioblastoma. Neuro Oncol. 2005, 7, 246–253. [Google Scholar] [CrossRef]
- Cancer Research UK. Triple Negative Breast Cancer. Available online: https://www.cancerresearchuk.org/about-cancer/breast-cancer/types/triple-negative-breast-cancer (accessed on 22 January 2023).
- Breast Cancer Now. Triple Negative Breast Cancer. Available online: https://breastcancernow.org/information-support/facing-breast-cancer/diagnosed-breast-cancer/primary-breast-cancer/triple-negative-breast-cancer (accessed on 22 January 2023).
- Ghani, S.; Sochat, M.; Luo, J.; Tao, Y.; Ademuyiwa, F. Characteristics of Male Triple Negative Breast Cancer: A Population-Based Study. Breast J. 2020, 26, 1748–1755. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Triple-Negative Breast Cancer. Available online: https://www.cancer.org/cancer/types/breast-cancer/about/types-of-breast-cancer/triple-negative.html (accessed on 22 January 2023).
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-Negative Breast Cancer: Clinical Features and Patterns of Recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soares, R.F.; Garcia, A.R.; Monteiro, A.R.; Macedo, F.; Pereira, T.C.; Carvalho, J.C.; Pêgo, A.; Mariano, M.; Madeira, P.; Póvoa, S.; et al. Prognostic Factors for Early Relapse in Non-Metastatic Triple Negative Breast Cancer—Real World Data. Rep. Pract. Oncol. Radiother. 2021, 26, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Allison, K.H.; Hammond, M.E.H.; Dowsett, M.; McKernin, S.E.; Carey, L.A.; Fitzgibbons, P.L.; Hayes, D.F.; Lakhani, S.R.; Chavez-MacGregor, M.; Perlmutter, J.; et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: ASCO/CAP Guideline Update. J. Clin. Oncol. 2020, 38, 1346–1366. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, B. Targeted Therapeutic Options and Future Perspectives for HER2-Positive Breast Cancer. Sig. Transduct. Target. 2019, 4, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahba, H.A.; El-Hadaad, H.A. Current Approaches in Treatment of Triple-Negative Breast Cancer. Cancer Biol. Med. 2015, 12, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Biswas, T.; Efird, J.T.; Prasad, S.; Jindal, C.; Walker, P.R. The Survival Benefit of Neoadjuvant Chemotherapy and PCR among Patients with Advanced Stage Triple Negative Breast Cancer. Oncotarget 2017, 8, 112712–112719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianco, N.; Palazzo, A.; Pagan, E.; Bagnardi, V.; Milano, M.; De Maio, A.P.; Colleoni, M. Adjuvant Treatment for Triple Negative Breast Cancer with Residual Tumor after Neo-Adjuvant Chemotherapy. A Single Institutional Retrospective Analysis. Breast 2021, 59, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, H.; Merkher, Y.; Chen, L.; Liu, N.; Leonov, S.; Chen, Y. Recent Advances in Therapeutic Strategies for Triple-Negative Breast Cancer. J. Hematol. Oncol. 2022, 15, 121. [Google Scholar] [CrossRef]
- Tuohy, V.K.; Jaini, R.; Johnson, J.M.; Loya, M.G.; Wilk, D.; Downs-Kelly, E.; Mazumder, S. Targeted Vaccination against Human α-Lactalbumin for Immunotherapy and Primary Immunoprevention of Triple Negative Breast Cancer. Cancers 2016, 8, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landry, I.; Sumbly, V.; Vest, M. Advancements in the Treatment of Triple-Negative Breast Cancer: A Narrative Review of the Literature. Cureus 2022, 14, e21970. [Google Scholar] [CrossRef]
- Gandhi, S.; Forsyth, P.; Opyrchal, M.; Ahmed, K.; Khong, H.; Attwood, K.; Levine, E.; O’Connor, T.; Early, A.; Fenstermaker, R.; et al. Phase IIa Study of Alpha-DC1 Vaccine against HER2/HER3, Chemokine Modulation Regimen and Pembrolizumab in Patients with Asymptomatic Brain Metastasis from Triple Negative or HER2+ Breast Cancer. J. Immunother. Cancer 2020, 8, 320. [Google Scholar] [CrossRef]
- Makhoul, I.; Ibrahim, S.M.; Abu-Rmaileh, M.; Jousheghany, F.; Siegel, E.R.; Rogers, L.J.; Lee, J.J.; Pina-Oviedo, S.; Post, G.R.; Beck, J.T.; et al. P10s-PADRE Vaccine Combined with Neoadjuvant Chemotherapy in ER-Positive Breast Cancer Patients Induces Humoral and Cellular Immune Responses. Oncotarget 2021, 12, 2252–2265. [Google Scholar] [CrossRef] [PubMed]
- Isakoff, S.J.; Tung, N.M.; Yin, J.; Tayob, N.; Parker, J.; Rosenberg, J.; Bardia, A.; Spring, L.; Park, H.; Collins, M.; et al. A Phase 1b Study of PVX-410 Vaccine in Combination with Pembrolizumab in Metastatic Triple Negative Breast Cancer (MTNBC). Cancer Res. 2022, 82, P2-14-17. [Google Scholar] [CrossRef]
- Isakoff, S.J.; Tolaney, S.M.; Tung, N.M.; Adams, S.; Soliman, H.H.; Brachtel, E.F.; Habin, K.R.; Bauer, L.J.; Ellisen, L.W.; Severgnini, M.; et al. A Phase 1b Study of Safety and Immune Response to PVX-410 Vaccine Alone and in Combination with Durvalumab (MEDI4736) in HLA-A2+ Patients Following Adjuvant Therapy for Stage 2/3 Triple Negative Breast Cancer. JCO 2017, 35, TPS1126. [Google Scholar] [CrossRef]
- Disis, M.; Liu, Y.; Stanton, S.; Gwin, W.; Coveler, A.; Liao, J.; Childs, J.; Cecil, D. A Phase I Dose Escalation Study of STEMVAC, a Multi-Antigen, Multi-Epitope Th1 Selective Plasmid-Based Vaccine, Targeting Stem Cell Associated Proteins in Patients with Advanced Breast Cancer. J. Immunother. Cancer 2022, 10, 546. [Google Scholar] [CrossRef]
- Kalli, K.R.; Block, M.S.; Kasi, P.M.; Erskine, C.L.; Hobday, T.J.; Dietz, A.; Padley, D.; Gustafson, M.P.; Shreeder, B.; Puglisi-Knutson, D.; et al. Folate Receptor Alpha Peptide Vaccine Generates Immunity in Breast and Ovarian Cancer Patients. Clin. Cancer Res. 2018, 24, 3014–3025. [Google Scholar] [CrossRef] [Green Version]
- Gao, T.; Cen, Q.; Lei, H. A Review on Development of MUC1-Based Cancer Vaccine. Biomed. Pharmacother. 2020, 132, 110888. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, J.; Roberts, L.K.; Smith, J.L.; Levin, M.K.; Timis, R.; Finholt, J.P.; Burkeholder, S.B.; Tarnowski, J.; Muniz, L.S.; Melton, M.G.; et al. Safety and Initial Clinical Efficacy of a Dendritic Cell (DC) Vaccine in Locally Advanced, Triple-Negative Breast Cancer (TNBC) Patients (Pts). JCO 2016, 34, 1086. [Google Scholar] [CrossRef]
- Miao, L.; Zhang, Y.; Huang, L. MRNA Vaccine for Cancer Immunotherapy. Mol. Cancer 2021, 20, 41. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.S.; Chow, L.W.C.; Cortes, J.; Fasching, P.A.; Hsu, P.; Huang, C.-S.; Kim, S.-B.; Lu, Y.-S.; Melisko, M.E.; Nanda, R.; et al. Phase III, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of Adagloxad Simolenin (OBI-822) and OBI-821 Treatment in Patients with Early-Stage Triple-Negative Breast Cancer (TNBC) at High Risk for Recurrence. JCO 2020, 38, TPS599. [Google Scholar] [CrossRef]
- Jain, A.G.; Talati, C.; Pinilla-Ibarz, J. Galinpepimut-S (GPS): An Investigational Agent for the Treatment of Acute Myeloid Leukemia. Expert Opin. Investig. Drugs 2021, 30, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Chung, V.; Kos, F.J.; Hardwick, N.; Yuan, Y.; Chao, J.; Li, D.; Waisman, J.; Li, M.; Zurcher, K.; Frankel, P.; et al. Evaluation of Safety and Efficacy of P53MVA Vaccine Combined with Pembrolizumab in Patients with Advanced Solid Cancers. Clin. Transl. Oncol. 2019, 21, 363–372. [Google Scholar] [CrossRef]
- Modi-1—Scancell. Available online: https://www.scancell.co.uk/modi-1 (accessed on 24 June 2023).
- Gheybi, E.; Salmanian, A.H.; Fooladi, A.A.I.; Salimian, J.; Hosseini, H.M.; Halabian, R.; Amani, J. Immunogenicity of Chimeric MUC1-HER2 Vaccine against Breast Cancer in Mice. Iran. J. Basic. Med. Sci. 2018, 21, 26–32. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Das, B.; Nair, G.B. Homeostasis and Dysbiosis of the Gut Microbiome in Health and Disease. J. Biosci. 2019, 44, 117. [Google Scholar] [CrossRef]
- Helmink, B.A.; Khan, M.A.W.; Hermann, A.; Gopalakrishnan, V.; Wargo, J.A. The Microbiome, Cancer, and Cancer Therapy. Nat. Med. 2019, 25, 377–388. [Google Scholar] [CrossRef]
- Lynn, D.J.; Benson, S.C.; Lynn, M.A.; Pulendran, B. Modulation of Immune Responses to Vaccination by the Microbiota: Implications and Potential Mechanisms. Nat. Rev. Immunol. 2022, 22, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.F.; Reina-Pérez, I.; Astorga, J.M.; Rodríguez-Carrillo, A.; Plaza-Díaz, J.; Fontana, L. Breast Cancer and Its Relationship with the Microbiota. Int. J. Environ. Res. Public. Health 2018, 15, 1747. [Google Scholar] [CrossRef] [Green Version]
- Sadrekarimi, H.; Gardanova, Z.R.; Bakhshesh, M.; Ebrahimzadeh, F.; Yaseri, A.F.; Thangavelu, L.; Hasanpoor, Z.; Zadeh, F.A.; Kahrizi, M.S. Emerging Role of Human Microbiome in Cancer Development and Response to Therapy: Special Focus on Intestinal Microflora. J. Transl. Med. 2022, 20, 301. [Google Scholar] [CrossRef]
- Goedert, J.J.; Jones, G.; Hua, X.; Xu, X.; Yu, G.; Flores, R.; Falk, R.T.; Gail, M.H.; Shi, J.; Ravel, J.; et al. Investigation of the Association Between the Fecal Microbiota and Breast Cancer in Postmenopausal Women: A Population-Based Case-Control Pilot Study. J. Natl. Cancer Inst. 2015, 107, djv147. [Google Scholar] [CrossRef]
- Byrd, D.A.; Vogtmann, E.; Wu, Z.; Han, Y.; Wan, Y.; Clegg-Lamptey, J.-N.; Yarney, J.; Wiafe-Addai, B.; Wiafe, S.; Awuah, B.; et al. Associations of Fecal Microbial Profiles with Breast Cancer and Nonmalignant Breast Disease in the Ghana Breast Health Study. Int. J. Cancer 2021, 148, 2712–2723. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Qu, M.; Wang, X. Analysis of Gut Microbiota in Patients with Breast Cancer and Benign Breast Lesions. Pol. J. Microbiol. 2022, 71, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Travis, R.C.; Key, T.J. Oestrogen Exposure and Breast Cancer Risk. Breast Cancer Res. 2003, 5, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Parida, S.; Sharma, D. The Microbiome-Estrogen Connection and Breast Cancer Risk. Cells 2019, 8, 1642. [Google Scholar] [CrossRef] [Green Version]
- Devoy, C.; Flores Bueso, Y.; Tangney, M. Understanding and Harnessing Triple-Negative Breast Cancer-Related Microbiota in Oncology. Front. Oncol. 2022, 12, 1020121. [Google Scholar] [CrossRef]
- Treeck, O.; Schüler-Toprak, S.; Ortmann, O. Estrogen Actions in Triple-Negative Breast Cancer. Cells 2020, 9, 2358. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current Understanding of the Human Microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Urbaniak, C.; Cummins, J.; Brackstone, M.; Macklaim, J.M.; Gloor, G.B.; Baban, C.K.; Scott, L.; O’Hanlon, D.M.; Burton, J.P.; Francis, K.P.; et al. Microbiota of Human Breast Tissue. Appl. Environ. Microbiol. 2014, 80, 3007–3014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzeng, A.; Sangwan, N.; Jia, M.; Liu, C.-C.; Keslar, K.S.; Downs-Kelly, E.; Fairchild, R.L.; Al-Hilli, Z.; Grobmyer, S.R.; Eng, C. Human Breast Microbiome Correlates with Prognostic Features and Immunological Signatures in Breast Cancer. Genome Med. 2021, 13, 60. [Google Scholar] [CrossRef]
- Banerjee, S.; Wei, Z.; Tan, F.; Peck, K.N.; Shih, N.; Feldman, M.; Rebbeck, T.R.; Alwine, J.C.; Robertson, E.S. Distinct Microbiological Signatures Associated with Triple Negative Breast Cancer. Sci. Rep. 2015, 5, 15162. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Tian, T.; Wei, Z.; Shih, N.; Feldman, M.D.; Peck, K.N.; DeMichele, A.M.; Alwine, J.C.; Robertson, E.S. Distinct Microbial Signatures Associated with Different Breast Cancer Types. Front. Microbiol. 2018, 9, 951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, S.; Wei, Z.; Tian, T.; Bose, D.; Shih, N.N.C.; Feldman, M.D.; Khoury, T.; De Michele, A.; Robertson, E.S. Prognostic Correlations with the Microbiome of Breast Cancer Subtypes. Cell. Death Dis. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Warburton, D.E.R.; Nicol, C.W.; Bredin, S.S.D. Health Benefits of Physical Activity: The Evidence. CMAJ 2006, 174, 801–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieman, D.C.; Wentz, L.M. The Compelling Link between Physical Activity and the Body’s Defense System. J. Sport. Health Sci. 2019, 8, 201–217. [Google Scholar] [CrossRef]
- Hojman, P.; Stagaard, R.; Adachi-Fernandez, E.; Deshmukh, A.S.; Mund, A.; Olsen, C.H.; Keller, L.; Pedersen, B.K.; Gehl, J. Exercise Suppresses Tumor Growth Independent of High Fat Food Intake and Associated Immune Dysfunction. Sci. Rep. 2022, 12, 5476. [Google Scholar] [CrossRef]
- Wennerberg, E.; Lhuillier, C.; Rybstein, M.D.; Dannenberg, K.; Rudqvist, N.-P.; Koelwyn, G.J.; Jones, L.W.; Demaria, S. Exercise Reduces Immune Suppression and Breast Cancer Progression in a Preclinical Model. Oncotarget 2020, 11, 452–461. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, P.; Blank, A.; Cui, C.; Schoenfelt, K.Q.; Zhou, G.; Xu, Y.; Khramtsova, G.; Olopade, F.; Shah, A.M.; Khan, S.A.; et al. Metabolically Activated Adipose Tissue Macrophages Link Obesity to Triple-Negative Breast Cancer. J. Exp. Med. 2019, 216, 1345–1358. [Google Scholar] [CrossRef] [PubMed]
- Vulczak, A.; de Souza, A.O.; Ferrari, G.D.; Azzolini, A.E.C.S.; Pereira-da-Silva, G.; Alberici, L.C. Moderate Exercise Modulates Tumor Metabolism of Triple-Negative Breast Cancer. Cells 2020, 9, 628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Lu, W.; Zheng, W.; Gu, K.; Matthews, C.E.; Chen, Z.; Zheng, Y.; Shu, X.O. Exercise after Diagnosis of Breast Cancer in Association with Survival. Cancer Prev. Res. 2011, 4, 1409–1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janni, W.; Rack, B.; Friedl, T.; Müller, V.; Lorenz, R.; Rezai, M.; Tesch, H.; Heinrich, G.; Andergassen, U.; Harbeck, N.; et al. Lifestyle Intervention and Effect on Disease-Free Survival in Early Breast Cancer Pts: Interim Analysis from the Randomized SUCCESS C Study. Cancer Res. 2019, 79, GS5-03. [Google Scholar] [CrossRef]
- Lohmann, A.E.; Soldera, S.V.; Pimentel, I.; Ribnikar, D.; Ennis, M.; Amir, E.; Goodwin, P.J. Association of Obesity with Breast Cancer Outcome in Relation to Cancer Subtypes: A Meta-Analysis. J. Natl. Cancer Inst. 2021, 113, 1465–1475. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, P.; Schmidt, M.E.; Prentzell, M.T.; Berdel, B.; Wiskemann, J.; Kellner, K.H.; Debus, J.; Ulrich, C.; Opitz, C.A.; Steindorf, K. Resistance Exercise Reduces Kynurenine Pathway Metabolites in Breast Cancer Patients Undergoing Radiotherapy. Front. Oncol. 2019, 9, 962. [Google Scholar] [CrossRef]
- Heng, B.; Bilgin, A.A.; Lovejoy, D.B.; Tan, V.X.; Milioli, H.H.; Gluch, L.; Bustamante, S.; Sabaretnam, T.; Moscato, P.; Lim, C.K.; et al. Differential Kynurenine Pathway Metabolism in Highly Metastatic Aggressive Breast Cancer Subtypes: Beyond IDO1-Induced Immunosuppression. Breast Cancer Res. 2020, 22, 113. [Google Scholar] [CrossRef]
- Alizadeh, A.M.; Isanejad, A.; Sadighi, S.; Mardani, M.; Kalaghchi, B.; Hassan, Z.M. High-Intensity Interval Training Can Modulate the Systemic Inflammation and HSP70 in the Breast Cancer: A Randomized Control Trial. J. Cancer Res. Clin. Oncol. 2019, 145, 2583–2593. [Google Scholar] [CrossRef]
- Liubomirski, Y.; Lerrer, S.; Meshel, T.; Rubinstein-Achiasaf, L.; Morein, D.; Wiemann, S.; Körner, C.; Ben-Baruch, A. Tumor-Stroma-Inflammation Networks Promote Pro-Metastatic Chemokines and Aggressiveness Characteristics in Triple-Negative Breast Cancer. Front. Immunol. 2019, 10, 757. [Google Scholar] [CrossRef] [Green Version]
- Clinton, S.K.; Giovannucci, E.L.; Hursting, S.D. The World Cancer Research Fund/American Institute for Cancer Research Third Expert Report on Diet, Nutrition, Physical Activity, and Cancer: Impact and Future Directions. J. Nutr. 2020, 150, 663–671. [Google Scholar] [CrossRef]
- Aznab, M.; Shojae, S.; Sorkheh, A.G.; Pia, K.E.; Rezaei, M. The Survival of Patients with Triple Negative Breast Cancer Undergoing Chemotherapy Along with Lifestyle Change Interventions: Survival of TNBC Patients. Arch. Breast Cancer 2023, 10, 66–73. [Google Scholar] [CrossRef]
- Swisher, A.K.; Abraham, J.; Bonner, D.; Gilleland, D.; Hobbs, G.; Kurian, S.; Yanosik, M.A.; Vona-Davis, L. Exercise and Dietary Advice Intervention for Survivors of Triple-Negative Breast Cancer: Effects on Body Fat, Physical Function, Quality of Life, and Adipokine Profile. Support. Care Cancer 2015, 23, 2995–3003. [Google Scholar] [CrossRef] [Green Version]
- Liao, M.; Qin, R.; Huang, W.; Zhu, H.-P.; Peng, F.; Han, B.; Liu, B. Targeting Regulated Cell Death (RCD) with Small-Molecule Compounds in Triple-Negative Breast Cancer: A Revisited Perspective from Molecular Mechanisms to Targeted Therapies. J. Hematol. Oncol. 2022, 15, 44. [Google Scholar] [CrossRef] [PubMed]
- de Groot, S.; Lugtenberg, R.T.; Cohen, D.; Welters, M.J.P.; Ehsan, I.; Vreeswijk, M.P.G.; Smit, V.T.H.B.M.; de Graaf, H.; Heijns, J.B.; Portielje, J.E.A.; et al. Fasting Mimicking Diet as an Adjunct to Neoadjuvant Chemotherapy for Breast Cancer in the Multicentre Randomized Phase 2 DIRECT Trial. Nat. Commun. 2020, 11, 3083. [Google Scholar] [CrossRef] [PubMed]
- Fasching, P.A.; Hein, A.; Kolberg, H.-C.; Häberle, L.; Uhrig, S.; Rübner, M.; Belleville, E.; Hack, C.C.; Fehm, T.N.; Janni, W.; et al. Pembrolizumab in Combination with Nab-Paclitaxel for the Treatment of Patients with Early-Stage Triple-Negative Breast Cancer—A Single-Arm Phase II Trial (NeoImmunoboost, AGO-B-041). Eur. J. Cancer 2023, 184, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Harsini, S.; Wilson, D.; Saprunoff, H.; Allan, H.; Gleave, M.; Goldenberg, L.; Chi, K.N.; Kim-Sing, C.; Tyldesley, S.; Bénard, F. Outcome of Patients with Biochemical Recurrence of Prostate Cancer after PSMA PET/CT-Directed Radiotherapy or Surgery without Systemic Therapy. Cancer Imaging 2023, 23, 27. [Google Scholar] [CrossRef]
- Leslie, S.W.; Soon-Sutton, T.L.; Sajjad, H.; Siref, L.E. Prostate Cancer. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Stultz, J.; Fong, L. How to Turn up the Heat on the Cold Immune Microenvironment of Metastatic Prostate Cancer. Prostate Cancer Prostatic Dis. 2021, 24, 697–717. [Google Scholar] [CrossRef] [PubMed]
- Fay, E.K.; Graff, J.N. Immunotherapy in Prostate Cancer. Cancers 2020, 12, 1752. [Google Scholar] [CrossRef]
- Vitkin, N.; Nersesian, S.; Siemens, D.R.; Koti, M. The Tumor Immune Contexture of Prostate Cancer. Front. Immunol. 2019, 10, 603. [Google Scholar] [CrossRef] [Green Version]
- Fizazi, K.; Mella, P.G.; Castellano, D.; Minatta, J.N.; Kalebasty, A.R.; Shaffer, D.; Limón, J.C.V.; López, H.M.S.; Armstrong, A.J.; Horvath, L.; et al. Nivolumab plus Docetaxel in Patients with Chemotherapy-Naïve Metastatic Castration-Resistant Prostate Cancer: Results from the Phase II CheckMate 9KD Trial. Eur. J. Cancer 2022, 160, 61–71. [Google Scholar] [CrossRef]
- Fizazi, K.; Retz, M.; Petrylak, D.P.; Goh, J.C.; Perez-Gracia, J.; Lacombe, L.; Zschäbitz, S.; Burotto, M.; Mahammedi, H.; Gravis, G.; et al. Nivolumab plus Rucaparib for Metastatic Castration-Resistant Prostate Cancer: Results from the Phase 2 CheckMate 9KD Trial. J. Immunother. Cancer 2022, 10, e004761. [Google Scholar] [CrossRef]
- Linch, M.; Papai, Z.; Takacs, I.; Imedio, E.R.; Kühnle, M.-C.; Derhovanessian, E.; Vogler, I.; Renken, S.; Graham, P.; Sahin, U.; et al. A First-in-Human (FIH) Phase I/IIa Clinical Trial Assessing a Ribonucleic Acid Lipoplex (RNA-LPX) Encoding Shared Tumor Antigens for Immunotherapy of Prostate Cancer; Preliminary Analysis of PRO-MERIT. J. Immunother. Cancer 2021, 9, 421. [Google Scholar] [CrossRef]
- Lee, S.C.; Ma, J.S.Y.; Kim, M.S.; Laborda, E.; Choi, S.-H.; Hampton, E.N.; Yun, H.; Nunez, V.; Muldong, M.T.; Wu, C.N.; et al. A PSMA-Targeted Bispecific Antibody for Prostate Cancer Driven by a Small-Molecule Targeting Ligand. Sci. Adv. 2021, 7, eabi8193. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Trihy, L.; Romero, E.H.; Sam, S.L.; Rastogi, M.; Kouchkovsky, I.D.; Small, E.J.; Feng, F.; Kwon, D.; Friedlander, T.; et al. A Phase Ib Study of a Single Priming Dose of 177Lu-PSMA-617 Coupled with Pembrolizumab in Metastatic Castration Resistant Prostate Cancer (MCRPC). Ann. Oncol. 2022, 33, S1173. [Google Scholar] [CrossRef]
- Einstein, D.J.; Wei, X.X.; Werner, L.; Ye, H.; Calagua, C.; Bubley, G.; Balk, S.P. A Phase II Study of Nivolumab in Patients with High-Risk Biochemically Recurrent (BCR) Prostate Cancer (PCa). JCO 2019, 37, TPS341. [Google Scholar] [CrossRef]
- Terrisse, S.; Zitvogel, L.; Kroemer, G. Effects of the Intestinal Microbiota on Prostate Cancer Treatment by Androgen Deprivation Therapy. Microb. Cell 2022, 9, 202–206. [Google Scholar] [CrossRef]
- Fujita, K.; Matsushita, M.; De Velasco, M.A.; Hatano, K.; Minami, T.; Nonomura, N.; Uemura, H. The Gut-Prostate Axis: A New Perspective of Prostate Cancer Biology through the Gut Microbiome. Cancers 2023, 15, 1375. [Google Scholar] [CrossRef]
- Kure, A.; Tsukimi, T.; Ishii, C.; Aw, W.; Obana, N.; Nakato, G.; Hirayama, A.; Kawano, H.; China, T.; Shimizu, F.; et al. Gut Environment Changes Due to Androgen Deprivation Therapy in Patients with Prostate Cancer. Prostate Cancer Prostatic Dis. 2023, 26, 323–330. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, H. Compositional Differences of Gut Microbiome in Matched Hormone-Sensitive and Castration-Resistant Prostate Cancer. Transl. Urol. 2020, 9, 1937–1944. [Google Scholar] [CrossRef]
- Che, B.; Zhang, W.; Xu, S.; Yin, J.; He, J.; Huang, T.; Li, W.; Yu, Y.; Tang, K. Prostate Microbiota and Prostate Cancer: A New Trend in Treatment. Front. Oncol. 2021, 11, 805459. [Google Scholar] [CrossRef]
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [Green Version]
- Santa Mina, D.; Au, D.; Alibhai, S.M.H.; Jamnicky, L.; Faghani, N.; Hilton, W.J.; Stefanyk, L.E.; Ritvo, P.; Jones, J.; Elterman, D.; et al. A Pilot Randomized Trial of Conventional versus Advanced Pelvic Floor Exercises to Treat Urinary Incontinence after Radical Prostatectomy: A Study Protocol. BMC Urol. 2015, 15, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsang, D.S.; Jones, J.M.; Samadi, O.; Shah, S.; Mitsakakis, N.; Catton, C.N.; Jeon, W.; To, J.; Breunis, H.; Alibhai, S.M.H. Healthy Bones Study: Can a Prescription Coupled with Education Improve Bone Health for Patients Receiving Androgen Deprivation Therapy?—A before/after Study. Support. Care Cancer 2018, 26, 2861–2869. [Google Scholar] [CrossRef]
- Patel, D.I.; Gallegos, A.M.; Sheikh, B.; Vardeman, S.; Liss, M.A. A Randomized Controlled Trial of a Home-Based Exercise Program on Prognostic Biomarkers in Men with Prostate Cancer: A Study Protocol. Contemp. Clin. Trials Commun. 2020, 20, 100659. [Google Scholar] [CrossRef]
- Winters-Stone, K.M.; Dobek, J.C.; Bennett, J.A.; Dieckmann, N.F.; Maddalozzo, G.F.; Ryan, C.W.; Beer, T.M. Resistance Training Reduces Disability in Prostate Cancer Survivors on Androgen Deprivation Therapy: Evidence from a Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2015, 96, 7–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pernar, C.H.; Fall, K.; Rider, J.R.; Markt, S.C.; Adami, H.-O.; Andersson, S.-O.; Valdimarsdottir, U.; Andrén, O.; Mucci, L.A. A Walking Intervention among Men with Prostate Cancer: A Pilot Study. Clin. Genitourin. Cancer 2017, 15, e1021–e1028. [Google Scholar] [CrossRef] [PubMed]
- Thorsen, L.; Nilsen, T.S.; Raastad, T.; Courneya, K.S.; Skovlund, E.; Fosså, S.D. A Randomized Controlled Trial on the Effectiveness of Strength Training on Clinical and Muscle Cellular Outcomes in Patients with Prostate Cancer during Androgen Deprivation Therapy: Rationale and Design. BMC Cancer 2012, 12, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segal, R.J.; Reid, R.D.; Courneya, K.S.; Malone, S.C.; Parliament, M.B.; Scott, C.G.; Venner, P.M.; Quinney, H.A.; Jones, L.W.; Slovinec D’Angelo, M.E.; et al. Resistance Exercise in Men Receiving Androgen Deprivation Therapy for Prostate Cancer. JCO 2003, 21, 1653–1659. [Google Scholar] [CrossRef]
- Brady, L.; Hayes, B.; Sheill, G.; Baird, A.-M.; Guinan, E.; Stanfill, B.; Vlajnic, T.; Casey, O.; Murphy, V.; Greene, J.; et al. Platelet Cloaking of Circulating Tumour Cells in Patients with Metastatic Prostate Cancer: Results from ExPeCT, a Randomised Controlled Trial. PLoS ONE 2020, 15, e0243928. [Google Scholar] [CrossRef]
- Katz, R.; Ahmed, M.A.; Safadi, A.; Abu Nasra, W.; Visoki, A.; Huckim, M.; Elias, I.; Nuriel-Ohayon, M.; Neuman, H. Characterization of Fecal Microbiome in Biopsy Positive Prostate Cancer Patients. BJUI Compass 2021, 3, 55–61. [Google Scholar] [CrossRef]
- Li, J.K.M.; Wang, L.L.; Lau, B.S.Y.; Tse, R.T.H.; Cheng, C.K.L.; Leung, S.C.H.; Wong, C.Y.P.; Tsui, S.K.W.; Teoh, J.Y.C.; Chiu, P.K.F.; et al. Oral Antibiotics Perturbation on Gut Microbiota after Prostate Biopsy. Front. Cell. Infect. Microbiol. 2022, 12, 959903. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Wu, K.; Long, Z.; Zhou, X.; Zhong, C.; Wang, S.; Lai, H.; Guo, Y.; Lv, D.; Lu, J.; et al. Gut Dysbiosis Promotes Prostate Cancer Progression and Docetaxel Resistance via Activating NF-ΚB-IL6-STAT3 Axis. Microbiome 2022, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.-Y.; Huang, W.-Y.; Lin, C.-L.; Huang, T.-C.; Wu, Y.-Y.; Chen, J.-H.; Kao, C.-H. Propranolol Reduces Cancer Risk: A Population-Based Cohort Study. Medicine 2015, 94, e1097. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, Q.; Sun, X.; Yin, Q.; Chen, J.; Xu, L.; Xu, C. Β2 -Adrenergic Receptor Signaling Drives Prostate Cancer Progression by Targeting the Sonic Hedgehog-Gli1 Signaling Activation. Prostate 2020, 80, 1328–1340. [Google Scholar] [CrossRef] [PubMed]
- Grytli, H.H.; Fagerland, M.W.; Fosså, S.D.; Taskén, K.A. Association Between Use of β-Blockers and Prostate Cancer–Specific Survival: A Cohort Study of 3561 Prostate Cancer Patients with High-Risk or Metastatic Disease. Eur. Urol. 2014, 65, 635–641. [Google Scholar] [CrossRef] [PubMed]
- McCarty, R. Chapter 4—The Fight-or-Flight Response: A Cornerstone of Stress Research. In Stress: Concepts, Cognition, Emotion, and Behavior; Fink, G., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 33–37. ISBN 978-0-12-800951-2. [Google Scholar]
- Dai, S.; Mo, Y.; Wang, Y.; Xiang, B.; Liao, Q.; Zhou, M.; Li, X.; Li, Y.; Xiong, W.; Li, G.; et al. Chronic Stress Promotes Cancer Development. Front. Oncol. 2020, 10, 1492. [Google Scholar] [CrossRef]
- Mohammadpour, H.; MacDonald, C.R.; Qiao, G.; Chen, M.; Dong, B.; Hylander, B.L.; McCarthy, P.L.; Abrams, S.I.; Repasky, E.A. Β2 Adrenergic Receptor-Mediated Signaling Regulates the Immunosuppressive Potential of Myeloid-Derived Suppressor Cells. J. Clin. Invest. 2019, 129, 5537–5552. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Yang, J.; Zhang, Y.; Han, J.; Yang, Y.; Zhao, Z.; Dai, X.; Wang, H.; Ding, X.; Liu, Y.; et al. Psychologic Stress Drives Progression of Malignant Tumors via DRD2/HIF1α Signaling. Cancer Res. 2021, 81, 5353–5365. [Google Scholar] [CrossRef]
- Cain, D.W.; Cidlowski, J.A. Immune Regulation by Glucocorticoids. Nat. Rev. Immunol. 2017, 17, 233–247. [Google Scholar] [CrossRef]
- Ludolph, P.; Kunzler, A.M.; Stoffers-Winterling, J.; Helmreich, I.; Lieb, K. Interventions to Promote Resilience in Cancer Patients. Dtsch. Arztebl. Int. 2019, 51–52, 865–872. [Google Scholar] [CrossRef]
- Contreras-Rodriguez, O.; Reales-Moreno, M.; Fernández-Barrès, S.; Cimpean, A.; Arnoriaga-Rodríguez, M.; Puig, J.; Biarnés, C.; Motger-Albertí, A.; Cano, M.; Fernández-Real, J.M. Consumption of Ultra-Processed Foods Is Associated with Depression, Mesocorticolimbic Volume, and Inflammation. J. Affect. Disord. 2023, 335, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Mayneris-Perxachs, J.; Castells-Nobau, A.; Arnoriaga-Rodríguez, M.; Martin, M.; de la Vega-Correa, L.; Zapata, C.; Burokas, A.; Blasco, G.; Coll, C.; Escrichs, A.; et al. Microbiota Alterations in Proline Metabolism Impact Depression. Cell. Metab. 2022, 34, 681–701.e10. [Google Scholar] [CrossRef] [PubMed]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C.; et al. The Neuroactive Potential of the Human Gut Microbiota in Quality of Life and Depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef] [PubMed]

| ClinicalTrials.gov ID | Vaccine | Phase | Stage | Reference |
|---|---|---|---|---|
| NCT02049489 | ICT-121 | I | Completed | [11] |
| NCT00323115 | aDCs 1 + RT 2 + TMZ 3 | II | Completed | [12] |
| NCT04277221 | aDCs/tumour antigen + RT + TMZ | III | Unknown | [13] |
| NCT01213407 | Trivax + RT + TMZ | II | Completed | [14] |
| NCT02772094 | aDCs/tumour antigen + TMZ | II | Unknown | [15] |
| NCT01280552 | ICT-107 | II | Completed | [16] |
| NCT01006044 | aDCs | II | Completed | [17] |
| NCT03382977 | VBI-1901 | I/II | Active, not recruiting | [18] |
| NCT02864368 | PEP-CMV + TMZ | I | Terminated | |
| NCT02146066 | DCVax-L + TMZ | E.A. 4 | Available | |
| NCT04968366 | aDCs pulsed with multiple neoantigen peptides | I | Recruiting | |
| NCT02709616 | mRNA-pulsed DCs + RT + TMZ | I | Completed | [19] |
| NCT02808364 | mRNA-pulsed aDCs | I | Completed | [19] |
| NCT02649582 | mRNA-pulsed DCs + RT + TMZ | I/II | Recruiting | [20] |
| NCT02078648 | SL-701 | I/II | Completed | [21] |
| NCT05685004 | TVI-Brain-1 + RT + TMZ | II/III | Not yet recruiting | |
| NCT02510950 | Personalised peptide + Poly-ICLC + TMZ | I | Terminated | |
| NCT02465268 | pp65-shLAMP DC | II | Active, not recruiting | [22] |
| NCT04801147 | aDCs | I/II | Recruiting | |
| NCT01902771 | DCs + tumour lysate + Imiquimod | I | Terminated | |
| NCT04002804 | aDCs + autologous tumour lysate | I | Terminated | |
| NCT03665545 | IMA950 | I/II | Active, not recruiting | [23] |
| NCT04842513 | Multipeptide plus XS15 + RT + TMZ | I | Recruiting | |
| NCT01290692 | TVI-Brain-1 | II | Completed | [24] |
| NCT02366728 | CMV pp65 -LAMP mRNA-pulsed aDCs + TMZ + Basiliximab | II | Completed | [25] |
| NCT04116658 | EO2401 + Nivolumab/Nivolumab + Bevacizumab | Ib/IIa | Active, not recruiting | [26] |
| NCT03916757 | V-Boost | II | Unknown | [27] |
| NCT01567202 | aDCs + Autogeneic glioma stem-like cells (A2B5+) + RT + TMZ | II | Unknown | [28] |
| NCT02010606 | Allogenic GBM stem-like lysate-pulsed aDCs | I | Completed | [29] |
| NCT00643097 | PEP-3-KLH + GM-CSF + TMZ | II | Completed | |
| NCT04963413 | pp65-fLAMP RNA-loaded aDCs + GM-CSF + TMZ | I | Active, not recruiting | |
| NCT01957956 | Allogenic tumour lysate-pulsed aDCs | Early I | Active, not recruiting | [30] |
| NCT01808820 | aDCs + Allogenic tumour lysate + Imiquimod | I | Completed | |
| NCT04015700 | GNOS-PV01 + INO-9012 | I | Active, not recruiting | |
| NCT00639639 | pp65-LAMP mRNA-loaded DCs + Ttd 5 | I | Completed | [31] |
| NCT03360708 | Allogenic tumour lysate-pulsed aDCs | Early I | Active, not recruiting | |
| NCT01081223 | TVI-Brain-1 + IL-2 | I/II | Completed | [24] |
| NCT02722512 | HSPPC-96 + RT | I | Terminated | |
| NCT02455557 | SurVaxM | II | Active, not recruiting | [32] |
| NCT03914768 | Genetically modified tumour cells/antigens-pulsed aDCS | I | Unknown | |
| NCT03615404 | aDCs + CMV RNA + CM-CSF + Ttd | I | Recruiting | |
| NCT02149225 | APVAC + Poly-ICLC + GM-CSF | I | Completed | [33] |
| NCT03223103 | Personalised vaccine + Poly-ICLC | I | Active, not recruiting | [34] |
| NCT05743595 | Personalised neoantigen DNA + Retifanlimab | I | Not yet recruiting | |
| NCT03688178 | aDCs + CMV pp65 + TMZ + Varlilumab | II | Recruiting | |
| NCT04888611 | GSC-DCV + Camrelizumab | II | Recruiting | |
| NCT00890032 | aDCs + Autologous tumour mRNA | I | Completed | |
| NCT00589875 | AdV-tk + Valacyclovir | II | Completed | [35] |
| NCT01403285 | IMA950 + Cyclophosphamide + GM-CSF | I | Terminated | |
| NCT03927222 | aDCs + pp65-LAMP CMV mRNA + GM-CSF + Ttd | II | Terminated | |
| NCT00576537 | aDCs + Allogenic tumour lysate | II | Completed | [36] |
| NCT04573140 | RNA-LP | I | Recruiting | |
| NCT00751270 | GliAtak | I | Completed | [37] |
| NCT03422094 | NeoVax + Nivolumab + Ipilimumab | I | Terminated | |
| NCT04642937 | GBM6-AD + Hp1a8 + Imiquimod | I | Active, not recruiting | [38] |
| NCT02052648 | Indoximod + TMZ | I/II | Completed | [39] |
| NCT01222221 | IMA950 + TMZ + RT | I | Completed | |
| NCT02529072 | DCs + Nivolumab | I | Completed | |
| NCT00846456 | CSC-mRNA transfected DCs | I | Completed | [40] |
| NCT01814813 | MDNA-55 | II | Terminated | [41] |
| NCT02287428 | NeoVax + Pembrolizumab | I | Recruiting | |
| NCT03018288 | HSPCC-96 + Pembrolizumab + RT + TMZ | I | Completed | |
| NCT04201873 | aDCs + Pembrolizumab + Poly-ICLC | I | Recruiting | |
| NCT04280848 | UCPvax | II | Recruiting | [42] |
| NCT01967758 | ADU-623 | I | Completed | [43] |
| NCT02820584 | aDCs + GSC | I | Completed | |
| NCT04552886 | aDCs | I | Recruiting | |
| NCT02718443 | VXM01 | I | Completed | [44] |
| NCT04523688 | aDCs | II | Recruiting | |
| NCT01759810 | DCs | I | Unknown | |
| NCT03750071 | VXM01 + Avelumab | I/II | Active, not recruiting | [45] |
| NCT03879512 | aDCs + Tumour lysate + Cyclophosphamide + Nivolumab + Ipilimumab | I/II | Recruiting | |
| NCT03395587 | aDCs + SoC 6 | II | Recruiting | |
| NCT00045968 | DCVax-L | III | Active, not recruiting | [46] |
| NCT01498328 | CDX-110 + Bevacizumab | II | Completed | [47] |
| NCT05698199 | ITI-1001 | I | Not yet recruiting | |
| NCT03149003 | DSP-7888 + Bevacizumab | III | Completed | [48] |
| NCT00905060 | HSPPC-96 + TMZ + Surgery | II | Completed | [49] |
| NCT00003185 | Autologous tumour cells + Sargramostim | II | Completed | [50] |
| NCT00626015 | PEP3-KLH + Daclizumab + TMZ | I | Completed | [51] |
| NCT00626483 | DCs loaded with CMV pp65-LAMP mRNA | I | Completed | [52] |
| NCT00458601 | CDX-110 + TMZ + GM-CSF | II | Complete | [53] |
| NCT05100641 | AV-GBM-1 | III | Not recruiting yet | |
| NCT01250470 | SurVaxM | I | Complete | [54] |
| NCT03400917 | AV-GBM-1 | II | Active, not recruiting | [55] |
| NCT05163080 | SurVaxM + Montanide + Sargramostim | II | Recruiting | |
| NCT01480479 | CDX-110 + TMZ + GM-CSF | III | Completed | [56] |
| NCT00576641 | aDCs + Autologous tumour lysate | I | Completed | [36] |
| NCT01204684 | Resiquimod + Poly-ICLC | II | Active, not recruiting | |
| NCT01491893 | PVSRIPO | I | Completed | [57] |
| NCT05557240 | NPVAC1/2 + Poly-ICLC | I | Recruiting | |
| NCT04388033 | aDCs + IL-12 | I/II | Recruiting | |
| NCT05356312 | Personalised neoantigen vaccine | E.A. 4 | Available | |
| NCT05283109 | P30-EPS + Hiltonol | I | Not yet recruiting | |
| NCT02498665 | DSP-7888 | I | Completed | [58] |
| NCT02800486 | Cetuximab | II | Recruiting | |
| NCT04978727 | SurVaxM | I | Recruiting | |
| NCT01920191 | IMA950 + Poly-ICLC + TMZ | I/II | Completed | [59] |
| NCT00612001 | aDCs | I | Completed | [60] |
| NCT00068510 | aDCs loaded with tumour lysate | I | Completed | [61] |
| NCT04214392 | CAR T-Cells | I | Recruiting | |
| NCT00293423 | GP96 | I | Completed | [62] |
| NCT01522820 | DEC-205/NY-ESO-1 Fusion Protein CDX-1401 + Sirolimus | I | Completed | |
| NCT04808245 | H3K27M peptide + Imiquimod | I | Recruiting | |
| NCT00069940 | Telomerase: 540–548 peptide + GM-CSF | I | Completed | |
| NCT03043391 | PVSRIPO | Ib | Active, not recruiting | [63] |
| NCT00014573 | Surgery + Paclitaxel + Cyclophosphamide + Filgrastim + Autologous tumour cells + Sargramostim + Cisplatin + Carmustine + IL-2 + Autologous bone marrow/PBMC transplantation | II | Completed | |
| NCT01621542 | WT2725 | I | Completed | [64] |
| NCT00004024 | Autologous tumour cells + Muromonab-CD3 + GM-CSF + IL-2 | II | Completed | |
| ClinicalTrials.gov ID | Intervention | Phase | Stage | Reference |
|---|---|---|---|---|
| NCT03390569 | Exercise | N/A 1 | Completed | |
| NCT05015543 | Personal training programme | N/A | Recruiting | |
| NCT02129335 | Impact of exercise on stress | N/A | Terminated | [70] |
| NCT05431348 | Impact of stress and exercise on chemoradiation outcome | N/A | Recruiting | [71] |
| NCT05116137 | Circuit-based resistance exercise | N/A | Enrolling by invitation | [72] |
| NCT03501134 | NovoTTF 2 device | N/A | Completed | |
| NCT01865162 | Ketogenic diet | I | Completed | [73] |
| NCT05708352 | Ketogenic diet | II | Not yet recruiting | [74] |
| NCT02286167 | Atkins-based diet | N/A | Completed | [75] |
| NCT02939378 | Ketogenic diet | I/II | Unknown | |
| NCT03075514 | Ketogenic diet | N/A | Completed | [76] |
| NCT02302235 | Ketogenic diet | II | Completed | [73] |
| NCT00508456 | Methionine-restricted diet | I | Terminated | |
| NCT04730869 | Metabolic therapy programme | N/A | Recruiting | [77] |
| NCT00575146 | Ketogenic diet | I | Completed | [78] |
| NCT04691960 | Ketogenic diet + Metformin | II | Recruiting | |
| NCT01535911 | Metabolic nutritional therapy | N/A | Active, not recruiting | [79] |
| NCT02046187 | Ketogenic diet | I/II | Terminated | [80] |
| NCT03451799 | Ketogenic diet | I | Active, not recruiting | [81] |
| NCT05183204 | Ketogenic diet + Metformin + Paxalisib | II | Recruiting | |
| NCT03160599 | Ketogenic diet | N/A | Unknown | |
| NCT03278249 | Modified Atkins ketogenic diet | N/A | Active, not recruiting | |
| NCT02768389 | Modified Atkins diet + Bevacizumab | Early I | Completed | |
| NCT01754350 | Calorie-restricted ketogenic diet + Transient fasting | N/A | Completed | [82] |
| NCT00243022 | Boswellia serrata extract + Vitamin B12 | II | Terminated | [83] |
| NCT05326334 | Chemoradiation + Chemotherapy + Microbiome evaluation | N/A | Recruiting | |
| NCT00003751 | Penicillamine + Low copper diet | II | Completed | [84] |
| NCT03631823 | Chemotherapy and/or radiotherapy + Correlation between microbiome and prognosis | N/A | Unknown |
| ClinicalTrials.gov ID | Vaccine | Phase | Stage | Reference |
|---|---|---|---|---|
| NCT04674306 | α-lactalbumin + Zymosan | Early I | Recruiting | [97] |
| NCT04024800 | AE37 peptide + Pembrolizumab | II | Active, not recruiting | [98] |
| NCT03199040 | Neoantigen DNA + Durvalumab | I | Active, not recruiting | |
| NCT04348747 | HER2/HER3 DCs + Pembrolizumab | IIa | Recruiting | [99] |
| NCT02348320 | Polyepitope DNA | I | Completed | |
| NCT02938442 | P10s-PADRE with MONTANIDE ISA 51 VG + Doxorubicin + Cyclophosphamide + Paclitaxel + Surgery | II | Completed | [100] |
| NCT03362060 | PVX-410 + Pembrolizumab | Ib | Active, not recruiting | [101] |
| NCT02826434 | PVX-410 + Durvalumab + Poly-ICLC | Ib | Active, not recruiting | [102] |
| NCT05455658 | STEMVAC + Sargramostim | II | Recruiting | [103] |
| NCT03606967 | Personalised neoantigen peptide + Carboplatin + Gemcitabine + Nab-Paclitaxel + Durvalumab + Tremelimumab + Poly-ICLC | II | Recruiting | |
| NCT03387085 | N-803 + ETBX-011 + ETBX-051 + ETBX-061 + GI-4000 + GI-6207 + GI-6301 + HaNK + Avelumab + Bevacizumab + Aldoxorubicin + Capecitabine + Cisplatin + Cyclophosphamide + 5-Fluorouracil + Leucovorin + Nab-Paclitaxel | Ib/II | Active, not recruiting | |
| NCT03012100 | Multi-epitope folate receptor alpha + Cyclophosphamide + GM-CSF | II | Active, not recruiting | [104] |
| NCT00986609 | MUC1 + Poly-ICLC | Early I | Completed | [105] |
| NCT02593227 | Folate receptor alpha + Cyclophosphamide + GM-CSF | II | Completed | [104] |
| NCT05504707 | HER2-/HER3-primed DC1 | I | Recruiting | |
| NCT04105582 | Neoantigen-pulsed aDCs 1 | I | Completed | |
| NCT02018458 | Cyclin B1/WT1/CEF-pulsed DCs + Doxorubicin + Cyclophosphamide + Paclitaxel + Carboplatin | I | Completed | [106] |
| NCT02316457 | RNA for shared tumour associated antigens + RNA for tumour specific antigens | I | Active, not recruiting | [107] |
| NCT03562637 | OBI-822 + OBI-821 | III | Recruiting | [108] |
| NCT04634747 | PVX-410 + Pembrolizumab + Chemotherapy | II | Not yet recruiting | |
| NCT05269381 | Personalised neoantigen + Pembrolizumab + Cyclophosphamide + GM-CSF | I | Recruiting | |
| NCT03761914 | Galinpepimut-S + Pembrolizumab | I/II | Active, not recruiting | [109] |
| NCT02432963 | P53MVA + Pembrolizumab | I | Active, not recruiting | [110] |
| NCT05329532 | Modi-1/Modi-1v + Pembrolizumab | I/II | Recruiting | [111] |
| NCT00640861 | MUC1 + HER2/neu + CpG + GM-CSF + IFA | Early I | Completed | [112] |
| NCT04879888 | Peptide-pulsed aDCs | I | Completed | |
| NCT05035407 | KK-LC-1 TCR + Aldesleukin + Cyclophosphamide + Fludarabine | I | Recruiting |
| ClinicalTrials.gov ID | Intervention | Phase | Stage | Reference |
|---|---|---|---|---|
| NCT01498536 | Aerobic exercise | N/A 1 | Completed | [147] |
| NCT03733119 | Methionine-restricted diet + ONC201 | II | Terminated | [148] |
| NCT04248998 | Fasting-mimicking diet + Metformin | II | Active, not recruiting | [149] |
| NCT05763992 | Fasting-like approach + SoC 2 | II | Recruiting | |
| NCT03186937 | Methionine-restricted diet | II | Terminated | |
| NCT02348320 | Caloric restriction diet + SABR 3 | II | Recruiting | |
| NCT04677816 | Vitamin D3 + SoC | II | Recruiting | |
| NCT05198843 | Icosapent ethyl + Dasatinib | Ib/II | Recruiting | |
| NCT05037825 | ICI 4 + Microbiome evaluation | N/A | Recruiting | |
| NCT03586297 | SoC + Correlation between microbiome composition and pCR 5 | N/A | Recruiting | |
| NCT04638751 | Chemotherapy + Correlation between microbiome, PFS, 6 and OS 7 | N/A | Recruiting | |
| NCT05916755 | Pembrolizumab and/or chemotherapy + microbiome analysis to establish predictive biomarkers | N/A | Recruiting | |
| NCT03289819 | Pembrolizumab + Nab-Paclitaxel + Epirubicin + Cyclophosphamide + Correlation between microbiome and clinical outcome | II | Completed | [150] |
| ClinicalTrials.gov ID | Vaccine | Phase | Stage | Reference |
|---|---|---|---|---|
| NCT00003871 | Fowlpox prostate specific antigen | II | Completed | [156] |
| NCT00374049 | MUC1 + Poly-ICLC + GM-CSF | I | Completed | |
| NCT00122005 | GVAX | I/II | Unknown | |
| NCT03815942 | ChAdOx1-MVA 5T4 + Nivolumab | I/II | Unknown | [157] |
| NCT02234921 | Cyclophosphamide + Dribble + Imiquimod + Cervarix | I | Completed | |
| NCT01867333 | Enzalutamide + PROSTVAC-F/TRICOM + PROSTVAC-V/TRICOM | II | Completed | [158] |
| NCT04914195 | Leuprolide acetate | III | Recruiting | |
| NCT01420965 | Sipuleucel-T + CT-011 + Cyclophosphamide | II | Terminated | |
| NCT00292045 | NY-ESO-1 protein + CpG 7909 | I | Completed | [159] |
| NCT00140348 | GVAX | I/II | Completed | |
| NCT00140400 | GVAX | I/II | Completed | |
| NCT01095848 | DPX-0907 | I | Completed | [160] |
| NCT00089856 | GVAX | III | Terminated | [161] |
| NCT00133224 | GVAX | III | Terminated | [162] |
| NCT00005039 | Fowlpox prostate specific antigen | II | Terminated | |
| NCT00906243 | CV9103 | I/II | Terminated | [163] |
| NCT05104515 | OVM-200 | I | Recruiting | |
| NCT03384316 | ETBX-051 + ETBX-061 + ETBX-011 | I | Completed | [164] |
| NCT03338790 | Nivolumab + Rucaparib Nivolumab + Docetaxel + Prednisone Novilumab + Enzalutamide | II | Active, not recruiting | [156,157] |
| NCT03879122 | ADT + Docetaxel ADT + Docetaxel + Nivolumab ADT + Ipilimumab/Docetaxel + Nivolumab | II/III | Active, not recruiting | |
| NCT04382898 | BNT112 +/− Cemiplimab | I/II | Recruiting | [158] |
| NCT04077021 | CCW702 | I | Terminated | [159] |
| NCT03805594 | [177Lu]-PSMA-617 + Pembrolizumab | Ib | Active, not recruiting | [160] |
| NCT04100018 | Nivolumab + Docetaxel + Prednisone | III | Recruiting | |
| NCT03637543 | Nivolumab | II | Recruiting | [161] |
| NCT05580107 | MDPK67b | I | Recruiting |
| ClinicalTrials.gov ID | Intervention | Phase | Stage | Reference |
|---|---|---|---|---|
| NCT03658486 | Exercise | N/A 1 | Recruiting | |
| NCT03880422 | Aerobic and resistance exercise + diet | N/A | Recruiting | |
| NCT02233608 | Advanced pelvic floor muscle exercise | I/II | Completed | [168] |
| NCT01973673 | Bone health educational materials | N/A | Completed | [169] |
| NCT05612880 | Physical function assessment following androgen receptor signalling inhibitors | N/A | Recruiting | |
| NCT03397030 * | Exercise | N/A | Completed | [170] |
| NCT00660686 * | Resistance exercise + Flexibility training | N/A | Completed | [171] |
| NCT01696539 | SoC 2 + Walking intervention | N/A | Completed | [172] |
| NCT00658229 | Strength training group | III | Completed | [173] |
| NCT00253916 * | Aerobic cardiovascular exercise Resistance exercise | N/A | Completed | [174] |
| NCT02453139 | Aerobic exercise | N/A | Completed | [175] |
| NCT00329797 | Zoledronic acid and/or Calcium + Vitamin D | III | Completed | |
| NCT02710721 | Fasting | N/A | Completed | |
| NCT02946996 | Metformin + oligomeric procyanidin complex | II | Recruiting | |
| NCT03709485 * | Correlation between microbiota and development of prostate cancer | N/A | Unknown | [176] |
| NCT04687709 * | Correlation between microbiome and ADT-related metabolic changes | N/A | Recruiting | [177] |
| NCT04638049 * | Correlation between microbiota/metabolome and radiation-induced gastrointestinal toxicities | N/A | Completed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puig-Saenz, C.; Pearson, J.R.D.; Thomas, J.E.; McArdle, S.E.B. A Holistic Approach to Hard-to-Treat Cancers: The Future of Immunotherapy for Glioblastoma, Triple Negative Breast Cancer, and Advanced Prostate Cancer. Biomedicines 2023, 11, 2100. https://doi.org/10.3390/biomedicines11082100
Puig-Saenz C, Pearson JRD, Thomas JE, McArdle SEB. A Holistic Approach to Hard-to-Treat Cancers: The Future of Immunotherapy for Glioblastoma, Triple Negative Breast Cancer, and Advanced Prostate Cancer. Biomedicines. 2023; 11(8):2100. https://doi.org/10.3390/biomedicines11082100
Chicago/Turabian StylePuig-Saenz, Carles, Joshua R. D. Pearson, Jubini E. Thomas, and Stéphanie E. B. McArdle. 2023. "A Holistic Approach to Hard-to-Treat Cancers: The Future of Immunotherapy for Glioblastoma, Triple Negative Breast Cancer, and Advanced Prostate Cancer" Biomedicines 11, no. 8: 2100. https://doi.org/10.3390/biomedicines11082100