Advancements and Insights in Exosome-Based Therapies for Wound Healing: A Comprehensive Systematic Review (2018–June 2023)
Abstract
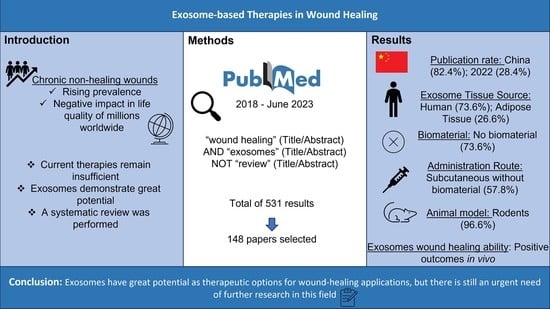
:1. Introduction
2. Data and Methods
3. Results
3.1. Retrieved Data
3.2. Scientific Data Production and Publication Distribution between 2018 and June 2023
3.3. Cell Source and Type
3.4. Biomaterials and Administration Route
3.5. Animal Models
4. Discussion
4.1. Scientific Data Production and Publication Distribution between 2018 and June 2023
4.2. Cell Source and Type
4.3. Biomaterials and Administration Route
4.4. Animal Models
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADSC | Adipose-tissue-derived mesenchymal stem cells |
| BMSC | Bone-marrow-derived mesenchymal stem cells |
| DPs | Dental-pulp-derived mesenchymal stem cells |
| EV | Endovenous |
| ID | Intradermal |
| IP | Intraperitoneal |
| iPSCs | Induced pluripotent stem cells |
| MSCs | Mesenchymal stem cells |
| PMID | PubMed identification |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| SC | Subcutaneous |
| UCMSC | Umbilical cord mesenchymal stem cells |
| UVEC | Umbilical vein endothelial cells |
References
- Carolina, M.; Bruna, L.; Patrícia, S.; Mariana, B.; Ana Catarina, S.; Ana Lúcia, L.; Rui, A.; Ana Colette, M. Application of Cell-Based Therapies in Veterinary Dermatology. In Wound Healing—Recent Advances and Future Opportunities; Ana Colette, M., Rui Damásio, A., Müzeyyen, G., Eds.; IntechOpen: Rijeka, Croatia, 2023; Chapter 1. [Google Scholar]
- Lopes, B.; Sousa, P.; Alvites, R.; Branquinho, M.; Sousa, A.; Mendonça, C.; Atayde, L.M.; Maurício, A.C. The Application of Mesenchymal Stem Cells on Wound Repair and Regeneration. Appl. Sci. 2021, 11, 3000. [Google Scholar] [CrossRef]
- Al-Masawa, M.E.; Alshawsh, M.A.; Ng, C.Y.; Ng, A.M.H.; Foo, J.B.; Vijakumaran, U.; Subramaniam, R.; Ghani, N.A.A.; Witwer, K.W.; Law, J.X. Efficacy and safety of small extracellular vesicle interventions in wound healing and skin regeneration: A systematic review and meta-analysis of animal studies. Theranostics 2022, 12, 6455–6508. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, Z.; Wang, Y.; Zhou, K.; Li, H.; Bi, S.; Wang, Y.; Wu, W.; Huang, Y.; Peng, B.; et al. Bioengineered MSC-derived exosomes in skin wound repair and regeneration. Front. Cell Dev. Biol. 2023, 11, 1029671. [Google Scholar] [CrossRef]
- Wu, J.; Chen, L.H.; Sun, S.Y.; Li, Y.; Ran, X.W. Mesenchymal stem cell-derived exosomes: The dawn of diabetic wound healing. World J. Diabetes 2022, 13, 1066–1095. [Google Scholar] [CrossRef] [PubMed]
- Bailey, A.J.M.; Li, H.; Kirkham, A.M.; Tieu, A.; Maganti, H.B.; Shorr, R.; Fergusson, D.A.; Lalu, M.M.; Elomazzen, H.; Allan, D.S. MSC-Derived Extracellular Vesicles to Heal Diabetic Wounds: A Systematic Review and Meta-Analysis of Preclinical Animal Studies. Stem Cell Rev. Rep. 2022, 18, 968–979. [Google Scholar] [CrossRef]
- Nikfarjam, S.; Rezaie, J.; Zolbanin, N.M.; Jafari, R. Mesenchymal stem cell derived-exosomes: A modern approach in translational medicine. J. Transl. Med. 2020, 18, 449. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wang, J.; Zhou, X.; Xiong, Z.; Zhao, J.; Yu, R.; Huang, F.; Zhang, H.; Chen, L. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci. Rep. 2016, 6, 32993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, Y.; Lin, S.; Tan, X.; Zhu, S.; Nie, F.; Zhen, Y.; Gu, L.; Zhang, C.; Wang, B.; Wei, W.; et al. Exosomes from adipose-derived stem cells and application to skin wound healing. Cell Prolif. 2021, 54, e12993. [Google Scholar] [CrossRef]
- Prasai, A.; Jay, J.W.; Jupiter, D.; Wolf, S.E.; El Ayadi, A. Role of Exosomes in Dermal Wound Healing: A Systematic Review. J. Investig. Dermatol. 2022, 142, 662–678.e668. [Google Scholar] [CrossRef]
- Hu, J.C.; Zheng, C.X.; Sui, B.D.; Liu, W.J.; Jin, Y. Mesenchymal stem cell-derived exosomes: A novel and potential remedy for cutaneous wound healing and regeneration. World J. Stem Cells 2022, 14, 318–329. [Google Scholar] [CrossRef]
- Wang, C.; Wang, M.; Xu, T.; Zhang, X.; Lin, C.; Gao, W.; Xu, H.; Lei, B.; Mao, C. Engineering Bioactive Self-Healing Antibacterial Exosomes Hydrogel for Promoting Chronic Diabetic Wound Healing and Complete Skin Regeneration. Theranostics 2019, 9, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, Z.; Pan, D.; Li, H.; Shen, J. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomes Combined Pluronic F127 Hydrogel Promote Chronic Diabetic Wound Healing and Complete Skin Regeneration. Int. J. Nanomed. 2020, 15, 5911–5926. [Google Scholar] [CrossRef]
- Hu, Y.; Rao, S.S.; Wang, Z.X.; Cao, J.; Tan, Y.J.; Luo, J.; Li, H.M.; Zhang, W.S.; Chen, C.Y.; Xie, H. Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics 2018, 8, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yu, M.; Xie, D.; Wang, L.; Ye, C.; Zhu, Q.; Liu, F.; Yang, L. Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res. Ther. 2020, 11, 259. [Google Scholar] [CrossRef]
- Yuan, M.; Liu, K.; Jiang, T.; Li, S.; Chen, J.; Wu, Z.; Li, W.; Tan, R.; Wei, W.; Yang, X.; et al. GelMA/PEGDA microneedles patch loaded with HUVECs-derived exosomes and Tazarotene promote diabetic wound healing. J. Nanobiotechnol. 2022, 20, 147. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, X.L.; Lu, S.T.; Zhang, N.Y.; Zhang, H.J.; Zhang, J.; Zhang, J. Human adipose-derived mesenchymal stem cells-derived exosomes encapsulated in pluronic F127 hydrogel promote wound healing and regeneration. Stem Cell Res. Ther. 2022, 13, 407. [Google Scholar] [CrossRef]
- Wu, D.; Kang, L.; Tian, J.; Wu, Y.; Liu, J.; Li, Z.; Wu, X.; Huang, Y.; Gao, B.; Wang, H.; et al. Exosomes Derived from Bone Mesenchymal Stem Cells with the Stimulation of Fe3O4 Nanoparticles and Static Magnetic Field Enhance Wound Healing Through Upregulated miR-21-5p. Int. J. Nanomed. 2020, 15, 7979–7993. [Google Scholar] [CrossRef]
- Hu, Y.; Tao, R.; Chen, L.; Xiong, Y.; Xue, H.; Hu, L.; Yan, C.; Xie, X.; Lin, Z.; Panayi, A.C.; et al. Exosomes derived from pioglitazone-pretreated MSCs accelerate diabetic wound healing through enhancing angiogenesis. J. Nanobiotechnol. 2021, 19, 150. [Google Scholar] [CrossRef]
- Wang, J.; Wu, H.; Peng, Y.; Zhao, Y.; Qin, Y.; Zhang, Y.; Xiao, Z. Hypoxia adipose stem cell-derived exosomes promote high-quality healing of diabetic wound involves activation of PI3K/Akt pathways. J. Nanobiotechnol. 2021, 19, 202. [Google Scholar] [CrossRef]
- Li, X.; Xie, X.; Lian, W.; Shi, R.; Han, S.; Zhang, H.; Lu, L.; Li, M. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.; Chen, L.; Liu, P.; Yu, T.; Lin, C.; Yan, C.; Hu, Y.; Zhou, W.; Sun, Y.; Panayi, A.C.; et al. All-in-One: Multifunctional Hydrogel Accelerates Oxidative Diabetic Wound Healing through Timed-Release of Exosome and Fibroblast Growth Factor. Small 2022, 18, e2104229. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Theocharidis, G.; Vlachos, I.S.; Kounas, K.; Lobao, A.; Shu, B.; Wu, B.; Xie, J.; Hu, Z.; Qi, S.; et al. Exosomes Derived from Epidermal Stem Cells Improve Diabetic Wound Healing. J. Investig. Dermatol. 2022, 142, 2508–2517.e2513. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Brown, B.A.; Siegel, A.P.; El Masry, M.S.; Zeng, X.; Song, W.; Das, A.; Khandelwal, P.; Clark, A.; Singh, K.; et al. Exosome-Mediated Crosstalk between Keratinocytes and Macrophages in Cutaneous Wound Healing. ACS Nano 2020, 14, 12732–12748. [Google Scholar] [CrossRef]
- Shi, R.; Jin, Y.; Zhao, S.; Yuan, H.; Shi, J.; Zhao, H. Hypoxic ADSC-derived exosomes enhance wound healing in diabetic mice via delivery of circ-Snhg11 and induction of M2-like macrophage polarization. Biomed. Pharmacother. 2022, 153, 113463. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Liu, W.; Li, J.; Lu, J.; Lu, H.; Jia, W.; Liu, F. Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway. Stem Cell Res. Ther. 2020, 11, 350. [Google Scholar] [CrossRef]
- Geng, X.; Qi, Y.; Liu, X.; Shi, Y.; Li, H.; Zhao, L. A multifunctional antibacterial and self-healing hydrogel laden with bone marrow mesenchymal stem cell-derived exosomes for accelerating diabetic wound healing. Biomater. Adv. 2022, 133, 112613. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, L.; Yan, C.; Zhou, W.; Endo, Y.; Liu, J.; Hu, L.; Hu, Y.; Mi, B.; Liu, G. Circulating Exosomal miR-20b-5p Inhibition Restores Wnt9b Signaling and Reverses Diabetes-Associated Impaired Wound Healing. Small 2020, 16, e1904044. [Google Scholar] [CrossRef] [Green Version]
- Shiekh, P.A.; Singh, A.; Kumar, A. Exosome laden oxygen releasing antioxidant and antibacterial cryogel wound dressing OxOBand alleviate diabetic and infectious wound healing. Biomaterials 2020, 249, 120020. [Google Scholar] [CrossRef]
- Xie, Y.; Yu, L.; Cheng, Z.; Peng, Y.; Cao, Z.; Chen, B.; Duan, Y.; Wang, Y. SHED-derived exosomes promote LPS-induced wound healing with less itching by stimulating macrophage autophagy. J. Nanobiotechnol. 2022, 20, 239. [Google Scholar] [CrossRef]
- Wang, M.; Wang, C.; Chen, M.; Xi, Y.; Cheng, W.; Mao, C.; Xu, T.; Zhang, X.; Lin, C.; Gao, W.; et al. Efficient Angiogenesis-Based Diabetic Wound Healing/Skin Reconstruction through Bioactive Antibacterial Adhesive Ultraviolet Shielding Nanodressing with Exosome Release. ACS Nano 2019, 13, 10279–10293. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, Y.; Liu, Y.; Li, X.; Tang, L.; Duan, M.; Li, J.; Zhang, G. Exosomes derived from human umbilical cord blood mesenchymal stem cells stimulate regenerative wound healing via transforming growth factor-β receptor inhibition. Stem Cell Res. Ther. 2021, 12, 434. [Google Scholar] [CrossRef]
- He, X.; Dong, Z.; Cao, Y.; Wang, H.; Liu, S.; Liao, L.; Jin, Y.; Yuan, L.; Li, B. MSC-Derived Exosome Promotes M2 Polarization and Enhances Cutaneous Wound Healing. Stem Cells Int. 2019, 2019, 7132708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwak, G.; Cheng, J.; Kim, H.; Song, S.; Lee, S.J.; Yang, Y.; Jeong, J.H.; Lee, J.E.; Messersmith, P.B.; Kim, S.H. Sustained Exosome-Guided Macrophage Polarization Using Hydrolytically Degradable PEG Hydrogels for Cutaneous Wound Healing: Identification of Key Proteins and MiRNAs, and Sustained Release Formulation. Small 2022, 18, e2200060. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Jin, Y.; Hu, W.; Lian, W.; Cao, C.; Han, S.; Zhao, S.; Yuan, H.; Yang, X.; Shi, J.; et al. Exosomes derived from mmu_circ_0000250-modified adipose-derived mesenchymal stem cells promote wound healing in diabetic mice by inducing miR-128-3p/SIRT1-mediated autophagy. Am. J. Physiol. Cell Physiol. 2020, 318, C848–C856. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Liu, J.; Zheng, C.; Su, Y.; Bao, L.; Zhu, B.; Liu, S.; Wang, L.; Wang, X.; Wang, Y.; et al. Exosomes released from educated mesenchymal stem cells accelerate cutaneous wound healing via promoting angiogenesis. Cell Prolif. 2020, 53, e12830. [Google Scholar] [CrossRef]
- Qian, L.; Pi, L.; Fang, B.R.; Meng, X.X. Adipose mesenchymal stem cell-derived exosomes accelerate skin wound healing via the lncRNA H19/miR-19b/SOX9 axis. Lab. Investig. 2021, 101, 1254–1266. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, Z.; Wei, Q.; Ma, K.; Hu, W.; Huang, Q.; Su, J.; Li, H.; Zhang, C.; Fu, X. VH298-loaded extracellular vesicles released from gelatin methacryloyl hydrogel facilitate diabetic wound healing by HIF-1α-mediated enhancement of angiogenesis. Acta Biomater. 2022, 147, 342–355. [Google Scholar] [CrossRef]
- Heo, J.S. Selenium-Stimulated Exosomes Enhance Wound Healing by Modulating Inflammation and Angiogenesis. Int. J. Mol. Sci. 2022, 23, 11543. [Google Scholar] [CrossRef]
- Ren, S.; Chen, J.; Guo, J.; Liu, Y.; Xiong, H.; Jing, B.; Yang, X.; Li, G.; Kang, Y.; Wang, C.; et al. Exosomes from Adipose Stem Cells Promote Diabetic Wound Healing through the eHSP90/LRP1/AKT Axis. Cells 2022, 11, 3229. [Google Scholar] [CrossRef]
- Kim, H.; Wang, S.Y.; Kwak, G.; Yang, Y.; Kwon, I.C.; Kim, S.H. Exosome-Guided Phenotypic Switch of M1 to M2 Macrophages for Cutaneous Wound Healing. Adv. Sci. 2019, 6, 1900513. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Luan, S.; Chen, J.; Zhou, Y.; Wang, T.; Li, Z.; Fu, Y.; Zhai, A.; Bi, C. The MSC-Derived Exosomal lncRNA H19 Promotes Wound Healing in Diabetic Foot Ulcers by Upregulating PTEN via MicroRNA-152-3p. Mol. Ther. Nucleic Acids 2020, 19, 814–826. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Cai, Z.; Jiang, X.; Wang, C.; Tang, T.; Xu, T.; Chen, H.; Li, X.; Du, X.; Cui, W. Hypoxia-pretreated ADSC-derived exosome-embedded hydrogels promote angiogenesis and accelerate diabetic wound healing. Acta Biomater. 2023, 157, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Xiao, C.; Miao, Y.; Wang, J.; Chen, R.; Fan, Z.; Hu, Z. Human acellular amniotic membrane incorporating exosomes from adipose-derived mesenchymal stem cells promotes diabetic wound healing. Stem Cell Res. Ther. 2021, 12, 255. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Qin, L.; Chen, C.; Hu, Q.; Wang, J.; Shen, J. Serum exosomes accelerate diabetic wound healing by promoting angiogenesis and ECM formation. Cell Biol. Int. 2021, 45, 1976–1985. [Google Scholar] [CrossRef]
- Xia, W.; Li, M.; Jiang, X.; Huang, X.; Gu, S.; Ye, J.; Zhu, L.; Hou, M.; Zan, T. Young fibroblast-derived exosomal microRNA-125b transfers beneficial effects on aged cutaneous wound healing. J. Nanobiotechnol. 2022, 20, 144. [Google Scholar] [CrossRef]
- Zhang, W.; Bai, X.; Zhao, B.; Li, Y.; Zhang, Y.; Li, Z.; Wang, X.; Luo, L.; Han, F.; Zhang, J.; et al. Cell-free therapy based on adipose tissue stem cell-derived exosomes promotes wound healing via the PI3K/Akt signaling pathway. Exp. Cell Res. 2018, 370, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Chen, J.; Duscher, D.; Liu, Y.; Guo, G.; Kang, Y.; Xiong, H.; Zhan, P.; Wang, Y.; Wang, C.; et al. Microvesicles from human adipose stem cells promote wound healing by optimizing cellular functions via AKT and ERK signaling pathways. Stem Cell Res. Ther. 2019, 10, 47. [Google Scholar] [CrossRef]
- Li, Q.; Hu, W.; Huang, Q.; Yang, J.; Li, B.; Ma, K.; Wei, Q.; Wang, Y.; Su, J.; Sun, M.; et al. MiR146a-loaded engineered exosomes released from silk fibroin patch promote diabetic wound healing by targeting IRAK1. Signal Transduct. Target. Ther. 2023, 8, 62. [Google Scholar] [CrossRef]
- Chen, B.; Sun, Y.; Zhang, J.; Zhu, Q.; Yang, Y.; Niu, X.; Deng, Z.; Li, Q.; Wang, Y. Human embryonic stem cell-derived exosomes promote pressure ulcer healing in aged mice by rejuvenating senescent endothelial cells. Stem Cell Res. Ther. 2019, 10, 142. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Mi, T.; Jin, L.; Li, M.; Zhanghuang, C.; Wang, J.; Tan, X.; Lu, H.; Shen, L.; Long, C.; et al. Comprehensive proteomic analysis of exosome mimetic vesicles and exosomes derived from human umbilical cord mesenchymal stem cells. Stem Cell Res. Ther. 2022, 13, 312. [Google Scholar] [CrossRef]
- Liang, Z.H.; Pan, N.F.; Lin, S.S.; Qiu, Z.Y.; Liang, P.; Wang, J.; Zhang, Z.; Pan, Y.C. Exosomes from mmu_circ_0001052-modified adipose-derived stem cells promote angiogenesis of DFU via miR-106a-5p and FGF4/p38MAPK pathway. Stem Cell Res. Ther. 2022, 13, 336. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, Y.; Xie, Z.; Ye, X.; Liu, X.; Xu, B.; Mao, J. Serum-derived exosomes accelerate scald wound healing in mice by optimizing cellular functions and promoting Akt phosphorylation. Biotechnol. Lett. 2021, 43, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhou, R.; Liang, Y.; Fu, X.; Wang, D.; Wang, C. Blockade of lncRNA-ASLNCS5088-enriched exosome generation in M2 macrophages by GW4869 dampens the effect of M2 macrophages on orchestrating fibroblast activation. FASEB J. 2019, 33, 12200–12212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.; Zhang, X.; Hao, H.; Xu, H.; Shu, J.; Hou, Q.; Wang, M. Exosomes Derived From Umbilical Cord Mesenchymal Stem Cells Treat Cutaneous Nerve Damage and Promote Wound Healing. Front. Cell Neurosci. 2022, 16, 913009. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Peng, L.; Ming, X.; Wang, X.; Cui, A.; Li, Y.; Wang, X.; Meng, D.; Sun, N.; Xiang, M.; et al. Enhanced wound healing promotion by immune response-free monkey autologous iPSCs and exosomes vs. their allogeneic counterparts. EBioMedicine 2019, 42, 443–457. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Quan, H. Adipose-derived stem cells-derived exosomes facilitate cutaneous wound healing by delivering XIST and restoring discoidin domain receptor 2. Cytokine 2022, 158, 155981. [Google Scholar] [CrossRef]
- Yan, C.; Xv, Y.; Lin, Z.; Endo, Y.; Xue, H.; Hu, Y.; Hu, L.; Chen, L.; Cao, F.; Zhou, W.; et al. Human Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes Accelerate Diabetic Wound Healing via Ameliorating Oxidative Stress and Promoting Angiogenesis. Front. Bioeng. Biotechnol. 2022, 10, 829868. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, F.; Gu, L.; Ji, P.; Yang, X.; Liu, M.; Tao, K.; Hu, D. Adipose mesenchymal stem cell exosomes promote wound healing through accelerated keratinocyte migration and proliferation by activating the AKT/HIF-1α axis. J. Mol. Histol. 2020, 51, 375–383. [Google Scholar] [CrossRef]
- Teng, L.; Maqsood, M.; Zhu, M.; Zhou, Y.; Kang, M.; Zhou, J.; Chen, J. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Accelerate Diabetic Wound Healing via Promoting M2 Macrophage Polarization, Angiogenesis, and Collagen Deposition. Int. J. Mol. Sci. 2022, 23, 10421. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, F.; Liu, Z.; Zuo, K.; Wang, B.; Zhang, Y.; Han, X.; Lian, A.; Wang, Y.; Liu, M.; et al. MSC-derived exosomes attenuate cell death through suppressing AIF nucleus translocation and enhance cutaneous wound healing. Stem Cell Res. Ther. 2020, 11, 174. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, Z.; Li, Y.; Wang, Y.; Li, Q.; Han, D. GelMA combined with sustained release of HUVECs derived exosomes for promoting cutaneous wound healing and facilitating skin regeneration. J. Mol. Histol. 2020, 51, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gao, H.; Xiong, Y.; Wang, L.; Zhang, H.; He, F.; Zhao, J.; Liu, S.; Gao, L.; Guo, Y.; et al. Enhancing Cutaneous Wound Healing Based on Human Induced Neural Stem Cell-derived Exosomes. Int. J. Nanomed. 2022, 17, 5991–6006. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Wang, K.; Lin, H.; Tao, E.; Xia, W.; Wang, F.; Mao, C.; Feng, Y. Engineered exosomes derived from miR-132-overexpresssing adipose stem cells promoted diabetic wound healing and skin reconstruction. Front. Bioeng. Biotechnol. 2023, 11, 1129538. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Zhong, C.; Yang, X.; Shu, F.; Xiao, S.; Gong, T.; Luo, P.; Li, L.; Chen, Z.; Zheng, Y.; et al. Exosomes derived from human amniotic epithelial cells accelerate diabetic wound healing via PI3K-AKT-mTOR-mediated promotion in angiogenesis and fibroblast function. Burn. Trauma 2020, 8, tkaa020. [Google Scholar] [CrossRef] [PubMed]
- Lyu, L.; Cai, Y.; Zhang, G.; Jing, Z.; Liang, J.; Zhang, R.; Dang, X.; Zhang, C. Exosomes derived from M2 macrophages induce angiogenesis to promote wound healing. Front. Mol. Biosci. 2022, 9, 1008802. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhao, B.; Zhang, X.L.; Lu, Y.J.; Lu, S.T.; Cheng, J.; Fu, Y.; Lin, L.; Zhang, N.Y.; Li, P.X.; et al. Combined topical and systemic administration with human adipose-derived mesenchymal stem cells (hADSC) and hADSC-derived exosomes markedly promoted cutaneous wound healing and regeneration. Stem Cell Res. Ther. 2021, 12, 257. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Liu, F.; Zhang, Y.; Chen, W.; Luo, W.; Ding, F.; Lu, L.; Wu, C.; Li, Y. Human Umbilical Cord Mesenchymal Stem Cell Derived Exosomes Delivered Using Silk Fibroin and Sericin Composite Hydrogel Promote Wound Healing. Front. Cardiovasc. Med. 2021, 8, 713021. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, T.; Tian, H.; Wei, G.; Zhao, L.; Shi, Y. Macrophage-derived exosomes accelerate wound healing through their anti-inflammation effects in a diabetic rat model. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3793–3803. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Gan, J.; Fan, L.; Luo, Z.; Zhao, Y. Bioinspired Adaptable Indwelling Microneedles for Treatment of Diabetic Ulcers. Adv. Mater. 2023, 35, e2210903. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, P.; Gao, X.; Chang, L.; Chen, Z.; Mei, X. Preparation of exosomes encapsulated nanohydrogel for accelerating wound healing of diabetic rats by promoting angiogenesis. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111671. [Google Scholar] [CrossRef]
- Shen, Y.F.; Huang, J.H.; Wang, K.Y.; Zheng, J.; Cai, L.; Gao, H.; Li, X.L.; Li, J.F. PTH Derivative promotes wound healing via synergistic multicellular stimulating and exosomal activities. Cell Commun. Signal 2020, 18, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Cai, Y.; Zhang, Q.; Zhang, Y. Pharmaceutical Activation of Nrf2 Accelerates Diabetic Wound Healing by Exosomes from Bone Marrow Mesenchymal Stem Cells. Int. J. Stem Cells 2022, 15, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; An, Y.; Sun, Y.; Yang, F.; Xu, Q.; Wang, Z. Adipose Mesenchymal Stem Cell-Derived Exosomes Promote Wound Healing Through the WNT/β-catenin Signaling Pathway in Dermal Fibroblasts. Stem Cell Rev. Rep. 2022, 18, 2059–2073. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, M.; Liao, Y.; Chen, H.; Su, D.; Tao, Y.; Li, J.; Luo, K.; Wu, L.; Zhang, X.; et al. Human umbilical cord mesenchymal stem cell-derived exosomes promote murine skin wound healing by neutrophil and macrophage modulations revealed by single-cell RNA sequencing. Front. Immunol. 2023, 14, 1142088. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Zhao, Y.; Wu, M.; Mao, S.; Cong, P.; Zou, R.; Hou, M.; Jin, H.; Bao, Y. Application of adipose mesenchymal stem cell-derived exosomes-loaded β-chitin nanofiber hydrogel for wound healing. Folia Histochem. Cytobiol. 2022, 60, 167–178. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, X.; Shen, K.; Luo, L.; Zhao, M.; Xu, C.; Jia, Y.; Xiao, D.; Li, Y.; Gao, X.; et al. Exosomes Derived from Adipose Mesenchymal Stem Cells Promote Diabetic Chronic Wound Healing through SIRT3/SOD2. Cells 2022, 11, 2568. [Google Scholar] [CrossRef]
- Sjöqvist, S.; Ishikawa, T.; Shimura, D.; Kasai, Y.; Imafuku, A.; Bou-Ghannam, S.; Iwata, T.; Kanai, N. Exosomes derived from clinical-grade oral mucosal epithelial cell sheets promote wound healing. J. Extracell. Vesicles 2019, 8, 1565264. [Google Scholar] [CrossRef]
- Zhou, Z.; Zheng, J.; Lin, D.; Xu, R.; Chen, Y.; Hu, X. Exosomes derived from dental pulp stem cells accelerate cutaneous wound healing by enhancing angiogenesis via the Cdc42/p38 MAPK pathway. Int. J. Mol. Med. 2022, 50, 143. [Google Scholar] [CrossRef]
- Han, X.; Wu, P.; Li, L.; Sahal, H.M.; Ji, C.; Zhang, J.; Wang, Y.; Wang, Q.; Qian, H.; Shi, H.; et al. Exosomes derived from autologous dermal fibroblasts promote diabetic cutaneous wound healing through the Akt/β-catenin pathway. Cell Cycle 2021, 20, 616–629. [Google Scholar] [CrossRef]
- Camões, S.P.; Bulut, O.; Yazar, V.; Gaspar, M.M.; Simões, S.; Ferreira, R.; Vitorino, R.; Santos, J.M.; Gursel, I.; Miranda, J.P. 3D-MSCs A151 ODN-loaded exosomes are immunomodulatory and reveal a proteomic cargo that sustains wound resolution. J. Adv. Res. 2022, 41, 113–128. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, L.; Li, X.; Liu, Y.; Zhang, G.; Wang, Y. Placental stem cells-derived exosomes stimulate cutaneous wound regeneration via engrailed-1 inhibition. Front. Bioeng. Biotechnol. 2022, 10, 1044773. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Liu, Z.; Wang, L.; Jin, Q.; Zhao, Y.; Du, A.; Ding, N.; Wang, Y.; Jiang, H.; Zhu, L. Exosome Mimetics-Loaded Hydrogel Accelerates Wound Repair by Transferring Functional Mitochondrial Proteins. Front. Bioeng. Biotechnol. 2022, 10, 866505. [Google Scholar] [CrossRef] [PubMed]
- Chen Md, G.; Wu Md, Y.; Zou Md, L.; Zeng Md, Y. Effect of MicroRNA-146a Modified Adipose-Derived Stem Cell Exosomes on Rat Back Wound Healing. Int. J. Low. Extrem. Wounds 2021, 1–9. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, L.; Zhang, X.; Chang, H.; Ma, S.; Xie, Z.; Tang, S.; Ju, X.; Zhu, H.; Shen, B.; et al. MiR-221-3p targets HIPK2 to promote diabetic wound healing. Microvasc. Res. 2022, 140, 104306. [Google Scholar] [CrossRef]
- Wang, X.; Jiao, Y.; Pan, Y.; Zhang, L.; Gong, H.; Qi, Y.; Wang, M.; Gong, H.; Shao, M.; Wang, X.; et al. Fetal Dermal Mesenchymal Stem Cell-Derived Exosomes Accelerate Cutaneous Wound Healing by Activating Notch Signaling. Stem Cells Int. 2019, 2019, 2402916. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Bai, S.; Cao, Y.; Liu, L.; Fang, Y.; Du, J.; Luo, L.; Chen, M.; Shen, B.; Zhang, Q. miRNA-221-3p in Endothelial Progenitor Cell-Derived Exosomes Accelerates Skin Wound Healing in Diabetic Mice. Diabetes Metab. Syndr. Obes. 2020, 13, 1259–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmadpour, F.; Rasouli, H.R.; Talebi, S.; Golchin, D.; Esmailinejad, M.R.; Razie, A. Effects of exosomes derived from fibroblast cells on skin wound healing in Wistar rats. Burns 2023. [Google Scholar] [CrossRef]
- Hsu, H.H.; Wang, A.Y.L.; Loh, C.Y.Y.; Pai, A.A.; Kao, H.K. Therapeutic Potential of Exosomes Derived from Diabetic Adipose Stem Cells in Cutaneous Wound Healing of db/db Mice. Pharmaceutics 2022, 14, 1206. [Google Scholar] [CrossRef]
- Parvanian, S.; Yan, F.; Su, D.; Coelho-Rato, L.S.; Venu, A.P.; Yang, P.; Zou, X.; Jiu, Y.; Chen, H.; Eriksson, J.E.; et al. Exosomal vimentin from adipocyte progenitors accelerates wound healing. Cytoskeleton 2020, 77, 399–413. [Google Scholar] [CrossRef]
- Born, L.J.; Chang, K.H.; Shoureshi, P.; Lay, F.; Bengali, S.; Hsu, A.T.W.; Abadchi, S.N.; Harmon, J.W.; Jay, S.M. HOTAIR-Loaded Mesenchymal Stem/Stromal Cell Extracellular Vesicles Enhance Angiogenesis and Wound Healing. Adv. Healthc. Mater. 2022, 11, e2002070. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, Y.; Liu, T.; Wang, X.; Wang, H.; Song, H.; Wang, W. Exosomes derived from TSG-6 modified mesenchymal stromal cells attenuate scar formation during wound healing. Biochimie 2020, 177, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yan, Z.; Yang, F.; Huang, Y.; Yu, Y.; Zhou, L.; Sun, Z.; Cui, D.; Yan, Y. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Accelerate Cutaneous Wound Healing by Enhancing Angiogenesis through Delivering Angiopoietin-2. Stem Cell Rev. Rep. 2021, 17, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ju, Y.; Fang, B. Exosomes from human adipose-derived mesenchymal stromal/stem cells accelerate angiogenesis in wound healing: Implication of the EGR-1/lncRNA-SENCR/DKC1/VEGF-A axis. Hum. Cell 2022, 35, 1375–1390. [Google Scholar] [CrossRef] [PubMed]
- Shiekh, P.A.; Singh, A.; Kumar, A. Data supporting exosome laden oxygen releasing antioxidant and antibacterial cryogel wound dressing OxOBand alleviate diabetic and infectious wound healing. Data Brief. 2020, 31, 105671. [Google Scholar] [CrossRef]
- Wang, C.; Liang, C.; Wang, R.; Yao, X.; Guo, P.; Yuan, W.; Liu, Y.; Song, Y.; Li, Z.; Xie, X. The fabrication of a highly efficient self-healing hydrogel from natural biopolymers loaded with exosomes for the synergistic promotion of severe wound healing. Biomater. Sci. 2019, 8, 313–324. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, Z.; Sun, J. Human bone marrow mesenchymal stem cell-derived exosomes stimulate cutaneous wound healing mediates through TGF-β/Smad signaling pathway. Stem Cell Res. Ther. 2020, 11, 198. [Google Scholar] [CrossRef]
- Bakadia, B.M.; Qaed Ahmed, A.A.; Lamboni, L.; Shi, Z.; Mutu Mukole, B.; Zheng, R.; Pierre Mbang, M.; Zhang, B.; Gauthier, M.; Yang, G. Engineering homologous platelet-rich plasma, platelet-rich plasma-derived exosomes, and mesenchymal stem cell-derived exosomes-based dual-crosslinked hydrogels as bioactive diabetic wound dressings. Bioact. Mater. 2023, 28, 74–94. [Google Scholar] [CrossRef]
- Parvanian, S.; Zha, H.; Su, D.; Xi, L.; Jiu, Y.; Chen, H.; Eriksson, J.E.; Cheng, F. Exosomal Vimentin from Adipocyte Progenitors Protects Fibroblasts against Osmotic Stress and Inhibits Apoptosis to Enhance Wound Healing. Int. J. Mol. Sci. 2021, 22, 4678. [Google Scholar] [CrossRef]
- Zhang, X.F.; Wang, T.; Wang, Z.X.; Huang, K.P.; Zhang, Y.W.; Wang, G.L.; Zhang, H.J.; Chen, Z.H.; Wang, C.Y.; Zhang, J.X.; et al. Hypoxic ucMSC-secreted exosomal miR-125b promotes endothelial cell survival and migration during wound healing by targeting TP53INP1. Mol. Ther. Nucleic Acids 2021, 26, 347–359. [Google Scholar] [CrossRef]
- Duan, M.; Zhang, Y.; Zhang, H.; Meng, Y.; Qian, M.; Zhang, G. Epidermal stem cell-derived exosomes promote skin regeneration by downregulating transforming growth factor-β1 in wound healing. Stem Cell Res. Ther. 2020, 11, 452. [Google Scholar] [CrossRef]
- Lv, Q.; Deng, J.; Chen, Y.; Wang, Y.; Liu, B.; Liu, J. Engineered Human Adipose Stem-Cell-Derived Exosomes Loaded with miR-21-5p to Promote Diabetic Cutaneous Wound Healing. Mol. Pharm. 2020, 17, 1723–1733. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Wang, L.; Guan, J.; Tang, C.; He, N.; Zhang, W.; Fu, S. Wound healing effects of a Curcuma zedoaria polysaccharide with platelet-rich plasma exosomes assembled on chitosan/silk hydrogel sponge in a diabetic rat model. Int. J. Biol. Macromol. 2018, 117, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Su, X.; Xiao, X.; Yu, H.; Li, X.; Keating, A.; Wang, S.; Zhao, R.C. Hydrogen Peroxide-Induced Senescence Reduces the Wound Healing-Promoting Effects of Mesenchymal Stem Cell-Derived Exosomes Partially via miR-146a. Aging Dis. 2021, 12, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Dalirfardouei, R.; Jamialahmadi, K.; Jafarian, A.H.; Mahdipour, E. Promising effects of exosomes isolated from menstrual blood-derived mesenchymal stem cell on wound-healing process in diabetic mouse model. J. Tissue Eng. Regen. Med. 2019, 13, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Mi, B.; Chen, L.; Xiong, Y.; Yan, C.; Xue, H.; Panayi, A.C.; Liu, J.; Hu, L.; Hu, Y.; Cao, F.; et al. Saliva exosomes-derived UBE2O mRNA promotes angiogenesis in cutaneous wounds by targeting SMAD6. J. Nanobiotechnol. 2020, 18, 68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, M.; Wang, Y.; Han, F.; Shen, K.; Luo, L.; Li, Y.; Jia, Y.; Zhang, J.; Cai, W.; et al. Exosome/metformin-loaded self-healing conductive hydrogel rescues microvascular dysfunction and promotes chronic diabetic wound healing by inhibiting mitochondrial fission. Bioact. Mater. 2023, 26, 323–336. [Google Scholar] [CrossRef]
- Chen, K.; Yu, T.; Wang, X. Inhibition of Circulating Exosomal miRNA-20b-5p Accelerates Diabetic Wound Repair. Int. J. Nanomed. 2021, 16, 371–381. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, Z.; He, L.; Qu, Y.; Ouyang, L.; Han, Y.; Xu, C.; Duan, D. Platelet-Rich Plasma-Derived Exosomal USP15 Promotes Cutaneous Wound Healing via Deubiquitinating EIF4A1. Oxid. Med. Cell Longev. 2021, 2021, 9674809. [Google Scholar] [CrossRef]
- Kobayashi, H.; Ebisawa, K.; Kambe, M.; Kasai, T.; Suga, H.; Nakamura, K.; Narita, Y.; Ogata, A.; Kamei, Y. Effects of exosomes derived from the induced pluripotent stem cells on skin wound healing. Nagoya J. Med. Sci. 2018, 80, 141–153. [Google Scholar] [CrossRef]
- Abdelsaid, K.; Sudhahar, V.; Harris, R.A.; Das, A.; Youn, S.W.; Liu, Y.; McMenamin, M.; Hou, Y.; Fulton, D.; Hamrick, M.W.; et al. Exercise improves angiogenic function of circulating exosomes in type 2 diabetes: Role of exosomal SOD3. FASEB J. 2022, 36, e22177. [Google Scholar] [CrossRef]
- Li, P.; Hong, G.; Zhan, W.; Deng, M.; Tu, C.; Wei, J.; Lin, H. Endothelial progenitor cell derived exosomes mediated miR-182-5p delivery accelerate diabetic wound healing via down-regulating PPARG. Int. J. Med. Sci. 2023, 20, 468–481. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Chen, T.; Hao, Y.; Zhang, F.; Tang, X.; Wang, D.; Wei, Z.; Qi, J. Exosomal miR-135a derived from human amnion mesenchymal stem cells promotes cutaneous wound healing in rats and fibroblast migration by directly inhibiting LATS2 expression. Stem Cell Res. Ther. 2020, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Liang, D.; Wu, X.; Chen, H.; Hong, X.; Wang, J.; Zhu, T.; Zeng, T.; Lin, W.; Chen, S.; et al. Long noncoding RNA LINC01435 impedes diabetic wound healing by facilitating YY1-mediated HDAC8 expression. iScience 2022, 25, 104006. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Wu, M.; Zou, R.; Mao, S.; Cong, P.; Hou, M.; Jin, H.; Zhao, Y.; Bao, Y. Adipose-derived mesenchymal stem cell-loaded β-chitin nanofiber hydrogel promote wound healing in rats. J. Mater. Sci. Mater. Med. 2022, 33, 12. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.H.; Yu, N.N.; Jin, Y.H.; Mao, Y.Y.; Feng, L.; Liu, Y.; Wang, A.G.; Sun, H.N.; Kwon, T.; Han, Y.H. Peroxiredoxin II with dermal mesenchymal stem cells accelerates wound healing. Aging 2021, 13, 13926–13940. [Google Scholar] [CrossRef]
- Park, D.J.; Duggan, E.; Ho, K.; Dorschner, R.A.; Dobke, M.; Nolan, J.P.; Eliceiri, B.P. Serpin-loaded extracellular vesicles promote tissue repair in a mouse model of impaired wound healing. J. Nanobiotechnol. 2022, 20, 474. [Google Scholar] [CrossRef]
- Li, Q.; Guo, L.; Wang, J.; Tao, S.; Jin, P. Exosomes derived from Nr-CWS pretreated MSCs facilitate diabetic wound healing by promoting angiogenesis via the circIARS1/miR-4782-5p/VEGFA axis. Chin. J. Nat. Med. 2023, 21, 172–184. [Google Scholar] [CrossRef]
- Ding, J.; Wang, X.; Chen, B.; Zhang, J.; Xu, J. Exosomes Derived from Human Bone Marrow Mesenchymal Stem Cells Stimulated by Deferoxamine Accelerate Cutaneous Wound Healing by Promoting Angiogenesis. Biomed. Res. Int. 2019, 2019, 9742765. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Yan, J.; Liu, Y.; Chen, Z.; Li, X.; Tang, L.; Li, J.; Duan, M.; Zhang, G. Human Amniotic Fluid Stem Cell-Derived Exosomes as a Novel Cell-Free Therapy for Cutaneous Regeneration. Front. Cell Dev. Biol. 2021, 9, 685873. [Google Scholar] [CrossRef]
- Pi, L.; Yang, L.; Fang, B.R.; Meng, X.X.; Qian, L. Exosomal microRNA-125a-3p from human adipose-derived mesenchymal stem cells promotes angiogenesis of wound healing through inhibiting PTEN. Mol. Cell Biochem. 2022, 477, 115–127. [Google Scholar] [CrossRef]
- Zhao, B.; Li, X.; Shi, X.; Shi, X.; Zhang, W.; Wu, G.; Wang, X.; Su, L.; Hu, D. Exosomal MicroRNAs Derived from Human Amniotic Epithelial Cells Accelerate Wound Healing by Promoting the Proliferation and Migration of Fibroblasts. Stem Cells Int. 2018, 2018, 5420463. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Shu, C.; Li, X.; Ye, C.; Zhang, W.C. Exosomes from linc00511-overexpressing ADSCs accelerates angiogenesis in diabetic foot ulcers healing by suppressing PAQR3-induced Twist1 degradation. Diabetes Res. Clin. Pract. 2021, 180, 109032. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Chen, L.; Yu, T.; Yan, C.; Zhou, W.; Cao, F.; You, X.; Zhang, Y.; Sun, Y.; Liu, J.; et al. Inhibition of circulating exosomal microRNA-15a-3p accelerates diabetic wound repair. Aging 2020, 12, 8968–8986. [Google Scholar] [CrossRef]
- Shi, M.; Gao, Y.; Lee, L.; Song, T.; Zhou, J.; Yan, L.; Li, Y. Adaptive Gelatin Microspheres Enhanced Stem Cell Delivery and Integration With Diabetic Wounds to Activate Skin Tissue Regeneration. Front. Bioeng. Biotechnol. 2022, 10, 813805. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Y.; Shi, L.; Li, B.; Li, J.; Wei, Z.; Lv, H.; Wu, L.; Zhang, H.; Yang, B.; et al. Magnetic targeting enhances the cutaneous wound healing effects of human mesenchymal stem cell-derived iron oxide exosomes. J. Nanobiotechnol. 2020, 18, 113. [Google Scholar] [CrossRef]
- Kim, J.; Kim, E.H.; Lee, H.; Sung, J.H.; Bang, O.Y. Clinical-Scale Mesenchymal Stem Cell-Derived Extracellular Vesicle Therapy for Wound Healing. Int. J. Mol. Sci. 2023, 24, 4273. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, S.; Li, X.; Wang, S.; Zhang, T.; Huo, N.; Duan, R.; Shi, Q.; Zhang, J.; Xu, J. Local transplantation of GMSC-derived exosomes to promote vascularized diabetic wound healing by regulating the Wnt/β-catenin pathways. Nanoscale Adv. 2023, 5, 916–926. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Gong, S.; Yao, W.; Yang, Z.; Wang, R.; Yu, Z.; Wei, M. Exosome loaded genipin crosslinked hydrogel facilitates full thickness cutaneous wound healing in rat animal model. Drug Deliv. 2021, 28, 884–893. [Google Scholar] [CrossRef]
- Han, Z.F.; Cao, J.H.; Liu, Z.Y.; Yang, Z.; Qi, R.X.; Xu, H.L. Exosomal lncRNA KLF3-AS1 derived from bone marrow mesenchymal stem cells stimulates angiogenesis to promote diabetic cutaneous wound healing. Diabetes Res. Clin. Pract. 2022, 183, 109126. [Google Scholar] [CrossRef]
- Kuang, L.; Zhang, C.; Li, B.; Deng, H.; Chen, R.; Li, G. Human Keratinocyte-Derived Exosomal MALAT1 Promotes Diabetic Wound Healing by Upregulating MFGE8 via microRNA-1914-3p. Int. J. Nanomed. 2023, 18, 949–970. [Google Scholar] [CrossRef]
- Liu, Y.; Zhuang, X.; Yu, S.; Yang, N.; Zeng, J.; Liu, X.; Chen, X. Exosomes derived from stem cells from apical papilla promote craniofacial soft tissue regeneration by enhancing Cdc42-mediated vascularization. Stem Cell Res. Ther. 2021, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Bai, Y.; Zhou, J.; Li, L.; Na, J.; Fan, Y.; Guo, X.; Liu, H. A moisturizing chitosan-silk fibroin dressing with silver nanoparticles-adsorbed exosomes for repairing infected wounds. J. Mater. Chem. B 2020, 8, 7197–7212. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.; Huang, X.; Zhao, L.; Wang, T.; Zhang, D.; Xu, T.; Du, L.; Li, Y.; Zhang, W.; Xiao, F.; et al. Exosomal miR-17-92 derived from human mesenchymal stem cells promotes wound healing by enhancing angiogenesis and inhibiting endothelial cell ferroptosis. Tissue Cell 2023, 83, 102124. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yu, M.; Yin, W.; Liang, B.; Li, A.; Li, J.; Li, X.; Zhao, S.; Liu, F. Development of a novel RNAi therapy: Engineered miR-31 exosomes promoted the healing of diabetic wounds. Bioact. Mater. 2021, 6, 2841–2853. [Google Scholar] [CrossRef]
- Liu, M.; Liu, Z.; Chen, Y.; Peng, S.; Yang, J.; Chen, C.; Wang, J.; Shang, R.; Tang, Y.; Huang, Y.; et al. Dendritic epidermal T cells secreting exosomes promote the proliferation of epidermal stem cells to enhance wound re-epithelialization. Stem Cell Res. Ther. 2022, 13, 121. [Google Scholar] [CrossRef]
- Qian, J.; Park, D.J.; Perrott, S.; Patel, P.; Eliceiri, B.P. Genetic Background and Kinetics Define Wound Bed Extracellular Vesicles in a Mouse Model of Cutaneous Injury. Int. J. Mol. Sci. 2021, 22, 3551. [Google Scholar] [CrossRef]
- Yang, H.; Xu, H.; Wang, Z.; Li, X.; Wang, P.; Cao, X.; Xu, Z.; Lv, D.; Rong, Y.; Chen, M.; et al. Analysis of miR-203a-3p/SOCS3-mediated induction of M2 macrophage polarization to promote diabetic wound healing based on epidermal stem cell-derived exosomes. Diabetes Res. Clin. Pract. 2023, 197, 110573. [Google Scholar] [CrossRef]
- Wang, S.; Shi, M.; Zhou, J.; Wang, W.; Zhang, Y.; Li, Y. Circulating Exosomal miR-181b-5p Promoted Cell Senescence and Inhibited Angiogenesis to Impair Diabetic Foot Ulcer via the Nuclear Factor Erythroid 2-Related Factor 2/Heme Oxygenase-1 Pathway. Front. Cardiovasc. Med. 2022, 9, 844047. [Google Scholar] [CrossRef]
- Henriques-Antunes, H.; Cardoso, R.M.S.; Zonari, A.; Correia, J.; Leal, E.C.; Jiménez-Balsa, A.; Lino, M.M.; Barradas, A.; Kostic, I.; Gomes, C.; et al. The Kinetics of Small Extracellular Vesicle Delivery Impacts Skin Tissue Regeneration. ACS Nano 2019, 13, 8694–8707. [Google Scholar] [CrossRef]
- Zeng, J.; Sun, Z.; Zeng, F.; Gu, C.; Chen, X. M2 macrophage-derived exosome-encapsulated microneedles with mild photothermal therapy for accelerated diabetic wound healing. Mater. Today Bio 2023, 20, 100649. [Google Scholar] [CrossRef]
- Cardoso, R.M.S.; Rodrigues, S.C.; Gomes, C.F.; Duarte, F.V.; Romao, M.; Leal, E.C.; Freire, P.C.; Neves, R.; Simões-Correia, J. Development of an optimized and scalable method for isolation of umbilical cord blood-derived small extracellular vesicles for future clinical use. Stem Cells Transl. Med. 2021, 10, 910–921. [Google Scholar] [CrossRef]
- Zhao, X.; Fu, L.; Zou, H.; He, Y.; Pan, Y.; Ye, L.; Huang, Y.; Fan, W.; Zhang, J.; Ma, Y.; et al. Optogenetic engineered umbilical cord MSC-derived exosomes for remodeling of the immune microenvironment in diabetic wounds and the promotion of tissue repair. J. Nanobiotechnol. 2023, 21, 176. [Google Scholar] [CrossRef]
- Zhang, L.; Ouyang, P.; He, G.; Wang, X.; Song, D.; Yang, Y.; He, X. Exosomes from microRNA-126 overexpressing mesenchymal stem cells promote angiogenesis by targeting the PIK3R2-mediated PI3K/Akt signalling pathway. J. Cell Mol. Med. 2021, 25, 2148–2162. [Google Scholar] [CrossRef]
- Kou, X.; Xu, X.; Chen, C.; Sanmillan, M.L.; Cai, T.; Zhou, Y.; Giraudo, C.; Le, A.; Shi, S. The Fas/Fap-1/Cav-1 complex regulates IL-1RA secretion in mesenchymal stem cells to accelerate wound healing. Sci. Transl. Med. 2018, 10, eaai8524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, W.; Liu, Y.; Jiang, X.; Li, M.; Zheng, S.; Zhang, Z.; Huang, X.; Luo, S.; Khoong, Y.; Hou, M.; et al. Lean adipose tissue macrophage derived exosome confers immunoregulation to improve wound healing in diabetes. J. Nanobiotechnol. 2023, 21, 128. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Song, P.; He, M.; Rui, S.; Duan, X.; Ma, Y.; Armstrong, D.G.; Deng, W. Sphingosine-1-phosphate derived from PRP-Exos promotes angiogenesis in diabetic wound healing via the S1PR1/AKT/FN1 signalling pathway. Burn. Trauma 2023, 11, tkad003. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, Y.; Du, Z.; Wu, T.; Yang, C. Hair follicle mesenchymal stem cell exosomal lncRNA H19 inhibited NLRP3 pyroptosis to promote diabetic mouse skin wound healing. Aging 2023, 15, 791–809. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Chen, B.; Zhang, X.; Chen, H. Human Adipose-Derived Mesenchymal Stem Cells-Derived Exosomal microRNA-19b Promotes the Healing of Skin Wounds Through Modulation of the CCL1/TGF-β Signaling Axis. Clin. Cosmet. Investig. Dermatol. 2020, 13, 957–971. [Google Scholar] [CrossRef]
- Wu, M.; Tu, J.; Huang, J.; Wen, H.; Zeng, Y.; Lu, Y. Exosomal IRF1-loaded rat adipose-derived stem cell sheet contributes to wound healing in the diabetic foot ulcers. Mol. Med. 2023, 29, 60. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, M.; Xie, W.; Zhang, Y.A.; Jiang, C.; Li, N.; Li, L.; Tian, J.; Zhou, C. Sprayable alginate hydrogel dressings with oxygen production and exosome loading for the treatment of diabetic wounds. Int. J. Biol. Macromol. 2023, 242, 125081. [Google Scholar] [CrossRef]
- Xu, Y.; Ouyang, L.; He, L.; Qu, Y.; Han, Y.; Duan, D. Inhibition of exosomal miR-24-3p in diabetes restores angiogenesis and facilitates wound repair via targeting PIK3R3. J. Cell Mol. Med. 2020, 24, 13789–13803. [Google Scholar] [CrossRef]
- Deng, D.; Li, X.; Zhang, J.J.; Yin, Y.; Tian, Y.; Gan, D.; Wu, R.; Wang, J.; Tian, B.M.; Chen, F.M.; et al. Biotin-Avidin System-Based Delivery Enhances the Therapeutic Performance of MSC-Derived Exosomes. ACS Nano 2023, 17, 8530–8550. [Google Scholar] [CrossRef]
- Bae, Y.U.; Son, Y.; Kim, C.H.; Kim, K.S.; Hyun, S.H.; Woo, H.G.; Jee, B.A.; Choi, J.H.; Sung, H.K.; Choi, H.C.; et al. Embryonic Stem Cell-Derived mmu-miR-291a-3p Inhibits Cellular Senescence in Human Dermal Fibroblasts Through the TGF-β Receptor 2 Pathway. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Luo, L.; Bai, X.; Shen, K.; Liu, K.; Wang, J.; Hu, D. Highly-expressed micoRNA-21 in adipose derived stem cell exosomes can enhance the migration and proliferation of the HaCaT cells by increasing the MMP-9 expression through the PI3K/AKT pathway. Arch. Biochem. Biophys. 2020, 681, 108259. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.C.; Balaji, S.; Martin, W.B.; Siegmund, N.; Poland, L.; Sanders Hanna, M.; Wei, D.; Kaliada, H.; Littlejohn, S.; Ganey, T. Protecting human amnion and chorion matrices (HACM) during processing: Performance enhancement in a Diabetic Mouse Model and Human Co-culture System. Wound Repair Regen. 2023, 31, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Qian, L.; Pi, L.; Meng, X. A therapeutic role of exosomal lncRNA H19 from adipose mesenchymal stem cells in cutaneous wound healing by triggering macrophage M2 polarization. Cytokine 2023, 165, 156175. [Google Scholar] [CrossRef] [PubMed]
- Bahr, M.M.; Amer, M.S.; Abo-El-Sooud, K.; Abdallah, A.N.; Shehab, G.G.; El-Tookhy, O.S. Proficiency of Carboxymethylcellulose as a Cryoprotectant. Clinical and Histological Evaluation of Cryopreserved Heterogenous Mesenchymal Stem Cell-Exosomal Hydrogel on Critical Size Skin Wounds in Dogs. Int. J. Hematol. Oncol. Stem Cell Res. 2021, 15, 178–191. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Shi, J.; Liu, K.; Wang, X.; Jia, Y.; He, T.; Shen, K.; Wang, Y.; Liu, J.; et al. Exosomes derived from human adipose mesenchymal stem cells attenuate hypertrophic scar fibrosis by miR-192-5p/IL-17RA/Smad axis. Stem Cell Res. Ther. 2021, 12, 221. [Google Scholar] [CrossRef]
- Huayllani, M.T.; Sarabia-Estrada, R.; Restrepo, D.J.; Boczar, D.; Sisti, A.; Nguyen, J.H.; Rinker, B.D.; Moran, S.L.; Quiñones-Hinojosa, A.; Forte, A.J. Adipose-derived stem cells in wound healing of full-thickness skin defects: A review of the literature. J. Plast. Surg. Hand Surg. 2020, 54, 263–279. [Google Scholar] [CrossRef]
- Pang, C.; Ibrahim, A.; Bulstrode, N.W.; Ferretti, P. An overview of the therapeutic potential of regenerative medicine in cutaneous wound healing. Int. Wound J. 2017, 14, 450–459. [Google Scholar] [CrossRef]
- Shojaei, F.; Rahmati, S.; Banitalebi Dehkordi, M. A review on different methods to increase the efficiency of mesenchymal stem cell-based wound therapy. Wound Repair Regen. 2019, 27, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Golchin, A.; Shams, F.; Basiri, A.; Ranjbarvan, P.; Kiani, S.; Sarkhosh-Inanlou, R.; Ardeshirylajimi, A.; Gholizadeh-Ghaleh Aziz, S.; Sadigh, S.; Rasmi, Y. Combination Therapy of Stem Cell-derived Exosomes and Biomaterials in the Wound Healing. Stem Cell Rev. Rep. 2022, 18, 1892–1911. [Google Scholar] [CrossRef] [PubMed]
- Rustad, K.C.; Wong, V.W.; Sorkin, M.; Glotzbach, J.P.; Major, M.R.; Rajadas, J.; Longaker, M.T.; Gurtner, G.C. Enhancement of mesenchymal stem cell angiogenic capacity and stemness by a biomimetic hydrogel scaffold. Biomaterials 2012, 33, 80–90. [Google Scholar] [CrossRef] [Green Version]
- Nilforoushzadeh, M.A.; Khodadadi Yazdi, M.; Baradaran Ghavami, S.; Farokhimanesh, S.; Mohammadi Amirabad, L.; Zarrintaj, P.; Saeb, M.R.; Hamblin, M.R.; Zare, M.; Mozafari, M. Mesenchymal Stem Cell Spheroids Embedded in an Injectable Thermosensitive Hydrogel: An In Situ Drug Formation Platform for Accelerated Wound Healing. ACS Biomater. Sci. Eng. 2020, 6, 5096–5109. [Google Scholar] [CrossRef]
- Grada, A.; Mervis, J.; Falanga, V. Research Techniques Made Simple: Animal Models of Wound Healing. J. Investig. Dermatol. 2018, 138, 2095–2105.e1. [Google Scholar] [CrossRef] [Green Version]
- Sami, D.G.; Heiba, H.H.; Abdellatif, A. Wound healing models: A systematic review of animal and non-animal models. Wound Med. 2019, 24, 8–17. [Google Scholar] [CrossRef]
- Saeed, S.; Martins-Green, M. Animal models for the study of acute cutaneous wound healing. Wound Repair Regen. 2023, 31, 6–16. [Google Scholar] [CrossRef]
- Peric, M.; Dumic-Cule, I.; Grcevic, D.; Matijasic, M.; Verbanac, D.; Paul, R.; Grgurevic, L.; Trkulja, V.; Bagi, C.M.; Vukicevic, S. The rational use of animal models in the evaluation of novel bone regenerative therapies. Bone 2015, 70, 73–86. [Google Scholar] [CrossRef] [Green Version]
- De Vries, R.B.M.; Wever, K.E.; Avey, M.T.; Stephens, M.L.; Sena, E.S.; Leenaars, M. The Usefulness of Systematic Reviews of Animal Experiments for the Design of Preclinical and Clinical Studies. ILAR J. 2014, 55, 427–437. [Google Scholar] [CrossRef] [Green Version]
- Hanson, S.E.; Kleinbeck, K.R.; Cantu, D.; Kim, J.; Bentz, M.L.; Faucher, L.D.; Kao, W.J.; Hematti, P. Local delivery of allogeneic bone marrow and adipose tissue-derived mesenchymal stromal cells for cutaneous wound healing in a porcine model. J. Tissue Eng. Regen. Med. 2016, 10, E90–E100. [Google Scholar] [CrossRef] [Green Version]
- Ochiai, H.; Kishi, K.; Kubota, Y.; Oka, A.; Hirata, E.; Yabuki, H.; Iso, Y.; Suzuki, H.; Umezawa, A. Transplanted mesenchymal stem cells are effective for skin regeneration in acute cutaneous wounds of pigs. Regen. Ther. 2017, 7, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Martinello, T.; Gomiero, C.; Perazzi, A.; Iacopetti, I.; Gemignani, F.; DeBenedictis, G.M.; Ferro, S.; Zuin, M.; Martines, E.; Brun, P.; et al. Allogeneic mesenchymal stem cells improve the wound healing process of sheep skin. BMC Vet. Res. 2018, 14, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, J.; Pereira, T.; Amorim, I.; Caseiro, A.R.; Lopes, M.A.; Lima, J.; Gartner, A.; Santos, J.D.; Bártolo, P.J.; Rodrigues, J.M.; et al. Cell therapy with human MSCs isolated from the umbilical cord Wharton jelly associated to a PVA membrane in the treatment of chronic skin wounds. Int. J. Med. Sci. 2014, 11, 979–987. [Google Scholar] [CrossRef] [Green Version]
- Enciso, N.; Avedillo, L.; Fermín, M.L.; Fragío, C.; Tejero, C. Cutaneous wound healing: Canine allogeneic ASC therapy. Stem Cell Res. Ther. 2020, 11, 261. [Google Scholar] [CrossRef] [PubMed]
- Cibelli, J.; Emborg, M.E.; Prockop, D.J.; Roberts, M.; Schatten, G.; Rao, M.; Harding, J.; Mirochnitchenko, O. Strategies for Improving Animal Models for Regenerative Medicine. Cell Stem Cell 2013, 12, 271–274. [Google Scholar] [CrossRef] [Green Version]
- Qiao, Z.; Wang, X.; Zhao, H.; Deng, Y.; Zeng, W.; Yang, K.; Chen, H.; Yan, Q.; Li, C.; Wu, J.; et al. The effectiveness of cell-derived exosome therapy for diabetic wound: A systematic review and meta-analysis. Ageing Res. Rev. 2023, 85, 101858. [Google Scholar] [CrossRef]







| Ref | Year | Country | Cell Source | Cell Type | Biomaterial | Administration Route | Animal Models |
|---|---|---|---|---|---|---|---|
| [12] | 2019 | China | Human | ADSC | Hydrogel | SC Injection | Mice |
| [13] | 2020 | China | Human | UCMSC | Hydrogel | SC Injection | Rat |
| [14] | 2018 | China | Human | UCMSC | x | SC Injection | Mice |
| [15] | 2020 | China | Human | BMSC | x | SC Injection | Rat |
| [16] | 2022 | China | Human | UVEC | Patch | Patch | Mice |
| [17] | 2022 | China | Human | ADSC | Hydrogel | Topical | Mice |
| [18] | 2020 | China | Human | BMSC | x | SC Injection | Rat |
| [19] | 2021 | China | Rat | BMSC | x | SC Injection | Rat |
| [20] | 2021 | China | Human | ADSC | x | SC Injection | Mice |
| [21] | 2018 | China | Human | ADSC | x | SC Injection | Rat |
| [22] | 2022 | China | Mice | BMSC | Hydrogel | SC Injection | Mice |
| [23] | 2022 | USA | Human | Epidermal | x | SC Injection | Mice |
| [24] | 2020 | USA | Mice | Keratinocyte | x | SC Injection | Mice |
| [25] | 2022 | China | Human | ADSC | x | SC Injection | Mice |
| [26] | 2020 | China | Human | BMSC | x | SC Injection | Rat |
| [27] | 2022 | China | Rat | BMSC | Hydrogel | Topical | Rat |
| [28] | 2020 | China | Human | Peripheral Blood | x | SC Injection | Mice |
| [29] | 2020 | India | Rat | ADSC | Scaffold | Scaffold | Rat |
| [30] | 2022 | China | Human | DPs | x | SC Injection | Mice |
| [31] | 2019 | China | Human | ADSC | Scaffold | Scaffold | Mice |
| [32] | 2021 | China | Human | UCMSC | x | EV Injection | Rat |
| [33] | 2019 | China | Human | BMSC | x | SC Injection | Mice |
| [34] | 2022 | Republic of Korea | Mice | BMSC | Hydrogel | SC Injection | Mice |
| [35] | 2020 | China | Human | ADSC | x | SC Injection | Rat |
| [36] | 2020 | China | Mice | BMSC | x | ID Injection | Mice |
| [37] | 2021 | China | Human | ADSC | x | SC Injection | Mice |
| [38] | 2022 | China | Human | Epidermal | Hydrogel | SC Injection | Mice |
| [39] | 2022 | Republic of Korea | Human | ADSC | x | SC Injection | Mice |
| [40] | 2022 | China | Human | ADSC | x | SC Injection | Mice |
| [41] | 2019 | Republic of Korea | Mice | BMSC | x | SC Injection | Mice |
| [42] | 2020 | China | Human | BMSC | x | SC Injection | Mice |
| [43] | 2023 | China | Mice | ADSC | Hydrogel | SC Injection | Mice |
| [44] | 2021 | China | Human | ADSC | Scaffold | Scaffold | Mice |
| [45] | 2021 | China | Mice | Serum | x | SC Injection | Mice |
| [46] | 2022 | China | Mice | Fibroblast | x | ID Injection | Mice |
| [47] | 2018 | China | Human | ADSC | x | SC and ID Injection | Mice |
| [48] | 2019 | China | Human | ADSC | x | SC Injection | Mice |
| [49] | 2023 | China | Human | Placenta | Patch | Patch | Mice |
| [50] | 2019 | China | Human | Embryonic | x | Topical | Mice |
| [51] | 2022 | China | Human | UCMSC | x | SC Injection | Mice |
| [52] | 2022 | China | Mice | ADSC | x | SC Injection | Mice |
| [53] | 2021 | China | Rat and Mice | Serum | x | SC Injection | Mice |
| [54] | 2019 | China | Human | Macrophage | x | SC Injection | Mice |
| [55] | 2022 | China | Human | UCMSC | x | Topical | Mice |
| [56] | 2019 | China | Macaque | iPSCs | x | Topical | Macaque |
| [57] | 2022 | China | Mice | ADSC | x | SC Injection | Mice |
| [58] | 2022 | China | Human | UCMSC | x | SC Injection | Mice |
| [59] | 2020 | China | Human | ADSC | x | SC Injection | Mice |
| [60] | 2022 | China | Human | UCMSC | x | SC Injection | Rat |
| [61] | 2020 | China | Human | UCMSC | x | SC Injection | Mice |
| [62] | 2020 | China | Human | UVEC | Hydrogel | Topical | Rat |
| [63] | 2022 | China | Human | iPSCs | Hydrogel | Topical | Mice |
| [64] | 2023 | China | Mice | ADSC | x | SC Injection | Mice |
| [65] | 2020 | China | Human | Amniotic Membrane | x | SC Injection | Mice |
| [66] | 2022 | China | Human | UVEC | x | SC Injection | Mice |
| [67] | 2021 | China | Human | ADSC | x | Injection and Topical | Mice |
| [68] | 2021 | China | Human | UCMSC | Hydrogel | Topical | Mice |
| [69] | 2019 | China | Human | UVEC | x | SC Injection | Rat |
| [70] | 2023 | China | Human | UVEC | Hydrogel | Microneedle | Rat |
| [71] | 2021 | China | Human | UCMSC | Hydrogel | SC Injection | Rat |
| [72] | 2020 | China | Human | UVEC | Hydrogel | SC Injection | Rat |
| [73] | 2022 | China | Rat | BMSC | x | SC Injection | Rat |
| [74] | 2022 | China | Human | ADSC | x | SC Injection | Rat |
| [75] | 2023 | China | Human | UCMSC | x | SC Injection | Mice |
| [76] | 2022 | China | Mice | ADSC | Hydrogel | Topical | Rat |
| [77] | 2022 | China | Human | ADSC | x | SC Injection | Mice |
| [78] | 2019 | Japan | Human | Epithelial | x | Topical | Rat |
| [79] | 2022 | China | Human | DPs | x | SC Injection | Mice |
| [80] | 2021 | China | Rat | Dermal | x | SC Injection | Rat |
| [81] | 2022 | Portugal | Human | UCMSC | x | SC Injection | Rat |
| [82] | 2022 | China | Rat | Placenta and ADSC | x | SC Injection | Rat |
| [83] | 2022 | China | Human | UCMSC | Hydrogel | SC Injection | Mice |
| [84] | 2021 | China | Human | ADSC | x | SC Injection | Rat |
| [85] | 2022 | China | Human | UVEC | x | SC Injection | Mice |
| [86] | 2019 | China | Human | Fetal dermal | x | SC Injection | Mice |
| [87] | 2020 | China | Mice | BMSC | x | Topical | Mice |
| [88] | 2023 | Iran | Human | Fetal dermal | x | Topical | Rat |
| [89] | 2022 | Taiwan | Mice | ADSC and dermal | x | Topical | Mice |
| [90] | 2020 | China and Finland | Human | ADSC | x | IP Injection | Mice |
| [91] | 2022 | USA | Human | BMSC | x | SC Injection | Mice and Rat |
| [92] | 2020 | China | Human | BMSC | x | SC Injection | Mice |
| [93] | 2021 | China | Human | UCMSC | x | SC Injection | Rat |
| [94] | 2022 | China | Human | ADSC | x | SC Injection | Mice |
| [95] | 2020 | India | Rat | ADSC | Scaffold | Scaffold | Rat |
| [96] | 2019 | China | Human | Placenta | Hydrogel | SC Injection | Mice |
| [97] | 2020 | China | Human | BMSC | x | SC Injection | Rat |
| [98] | 2023 | China | Rat | BMSC and plasma | Hydrogel | Topical | Rat |
| [99] | 2021 | China and Finland | Human | ADSC | x | IP Injection | Mice |
| [100] | 2021 | China | Human | UCMSC | x | SC Injection | Mice |
| [101] | 2020 | China | Human | Epidermal | x | SC Injection | Rat |
| [102] | 2020 | China | Human | ADSC | Hydrogel | Topical | Rat |
| [103] | 2018 | China | Human | Plasma | Sponge | Sponge | Rat |
| [104] | 2021 | China | Human | UCMSC and ADSC | x | SC Injection | Mice |
| [105] | 2019 | Iran | Human | Menstrual Blood | x | ID Injection | Mice |
| [106] | 2020 | China | Human | Saliva | x | SC Injection | Mice |
| [107] | 2023 | China | Human | ADSC | Hydrogel | SC Injection | Mice |
| [108] | 2021 | China | Human | Peripheral Blood | x | SC Injection | Mice |
| [109] | 2021 | China | Mice | Plasma | x | SC Injection | Mice |
| [110] | 2018 | Japan | Human | iPSCs | x | SC Injection | Mice |
| [111] | 2022 | USA | Mice and Human | Plasma | x | Topical | Mice |
| [112] | 2023 | China | Human | UCMSC | x | Topical | Mice |
| [113] | 2020 | China | Human | Amnion | x | SC Injection | Rat |
| [114] | 2022 | China | Human | Keratinocyte | x | SC Injection | Mice |
| [115] | 2022 | China | Mice | ADSC | Hydrogel | SC Injection | Rat |
| [116] | 2021 | China | Mice | Dermal | x | ID Injection | Mice |
| [117] | 2022 | USA | Mice | Skin | Sponge | Sponge | Mice |
| [118] | 2023 | China | Human | UCMSC | x | SC Injection | Mice |
| [119] | 2019 | China | Human | BMSC | x | SC Injection | Rat |
| [120] | 2021 | China | Human | Amniotic Fluid | x | SC Injection | Rat |
| [121] | 2022 | China | Human | ADSC | x | SC Injection | Mice |
| [122] | 2018 | China | Human | Amniotic Fluid | x | SC Injection | Mice |
| [123] | 2021 | China | Human | ADSC | x | SC Injection | Rat |
| [124] | 2020 | China | Human | Peripheral Blood | x | Injection | Mice |
| [125] | 2022 | China | Rat | ADSC | Hydrogel | Topical | Rat |
| [126] | 2020 | China | Human | UCMSC | Nanoparticles | EV Injection | Rat |
| [127] | 2023 | Republic of Korea | Human | UCMSC | x | Injection and Topical | Mice and Rat |
| [128] | 2022 | China | Human | Gingival | x | SC Injection | Mice |
| [129] | 2021 | China | Human | UCMSC | Hydrogel | Topical | Rat |
| [130] | 2022 | China | Human | BMSC | x | EV Injection | Mice |
| [131] | 2023 | China | Human | Keratinocyte | x | SC Injection | Mice |
| [132] | 2021 | China | Human | DPs | x | Topical | Mice |
| [133] | 2020 | China | Human | UCMSC | Dressing | Topical | Mice |
| [134] | 2023 | China | Human | UCMSC | x | SC Injection | Mice |
| [135] | 2021 | China | Human | Embryonic | x | SC Injection | Rat |
| [136] | 2022 | China | Mice | Dendritic epidermal T cells | x | SC Injection | Mice |
| [137] | 2021 | USA | Mice | Skin | Sponge | Topical | Mice |
| [138] | 2023 | China | Human | Epidermal | x | SC Injection | Mice |
| [139] | 2022 | China | Human | UVEC | x | SC Injection | Mice |
| [140] | 2019 | Portugal | Human | UCMSC | Hydrogel | Topical | Mice |
| [141] | 2023 | China | Mice | Macrophage | x | Topical | Rat |
| [142] | 2021 | Portugal | Human | UCMSC | Hydrogel | Topical | Mice |
| [143] | 2023 | China | Human | UCMSC | x | SC Injection | Mice |
| [144] | 2021 | China | Human | BMSC | x | SC Injection | Mice |
| [145] | 2018 | USA | Mice and Human | BMSCs, Skin and Gingiva | x | SC Injection | Mice |
| [146] | 2023 | China | Mice | ADSC | x | SC Injection | Mice |
| [147] | 2023 | China | Human | Plasma | x | SC Injection | Mice |
| [148] | 2023 | China | Human | Hair follicle | x | SC Injection | Mice |
| [149] | 2020 | China | Human | ADSC | x | SC Injection | Mice |
| [150] | 2023 | China | Rat | ADSC | x | SC Injection | Rat |
| [151] | 2023 | China | Mice | BMSC | Dressing | Topical | Rat |
| [152] | 2020 | China | Human | Peripheral Blood | x | SC Injection | Mice |
| [153] | 2023 | China | Rat | BMSC | Hydrogel | SC Injection | Mice and Rat |
| [154] | 2019 | Republic of Korea | Human | Fibroblast | x | Topical | Mice |
| [155] | 2020 | China | Human | ADSC | x | SC Injection | Mice |
| [156] | 2023 | USA | Human | Placenta | x | Topical | Mice |
| [157] | 2023 | China | Human | ADSC | x | SC Injection | Mice |
| [158] | 2021 | Egypt | Dog | BMSC | Hydrogel | Topical | Dog |
| [159] | 2021 | China | Human | ADSC | x | SC Injection | Mice |
| Human (109 Studies) | Mice (23 Studies) | Rat (11 Studies) | |
|---|---|---|---|
| BMSC | 11 (10.1%) | 6 (26.1%) | 4 (36.4%) |
| ADSC | 29 (26.6%) | 7 (30.4%) | 4 (36.4%) |
| UCMSC | 25 (22.9%) | X | X |
| UVECS | 8 (7.3%) | X | X |
| DP | 3 (2.8%) | X | X |
| Epidermal | 4 (3.7%) | X | X |
| Peripherical blood | 4 (3.7%) | X | X |
| Placenta | 3 (2.8%) | X | X |
| Others | 22 (20.2%) | 10 (43.5%) | 3 (27.3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, P.; Lopes, B.; Sousa, A.C.; Moreira, A.; Coelho, A.; Alvites, R.; Alves, N.; Geuna, S.; Maurício, A.C. Advancements and Insights in Exosome-Based Therapies for Wound Healing: A Comprehensive Systematic Review (2018–June 2023). Biomedicines 2023, 11, 2099. https://doi.org/10.3390/biomedicines11082099
Sousa P, Lopes B, Sousa AC, Moreira A, Coelho A, Alvites R, Alves N, Geuna S, Maurício AC. Advancements and Insights in Exosome-Based Therapies for Wound Healing: A Comprehensive Systematic Review (2018–June 2023). Biomedicines. 2023; 11(8):2099. https://doi.org/10.3390/biomedicines11082099
Chicago/Turabian StyleSousa, Patrícia, Bruna Lopes, Ana Catarina Sousa, Alícia Moreira, André Coelho, Rui Alvites, Nuno Alves, Stefano Geuna, and Ana Colette Maurício. 2023. "Advancements and Insights in Exosome-Based Therapies for Wound Healing: A Comprehensive Systematic Review (2018–June 2023)" Biomedicines 11, no. 8: 2099. https://doi.org/10.3390/biomedicines11082099