Evaluation of the Effectiveness of an Innovative Polycomponent Formulation on Adult and Aged Human Dermal Fibroblasts
Abstract
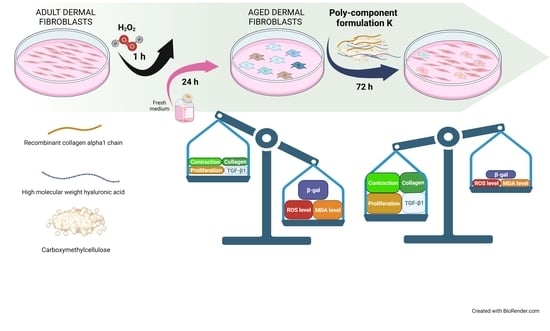
:1. Introduction
2. Materials and Methods
2.1. Preparation of K Polycomponent Formulation for Cell Treatments
2.2. Cell Systems
2.3. Treatments and Assessment of Cell Viability and Growth Rate
2.4. Staining Cells for β-Galactosidase Activity
2.5. Fluorometric Measurement of Reactive Oxygen Species (ROS) with DCFH-DA
2.6. Malondialdehyde (MDA) ELISA Kit
2.7. Western Blot Analysis
2.8. Immunofluorescence Assay for Type I Collagen
2.9. Extracellular Collagen Quantification
2.10. In Vitro Scratch Assay
2.11. Collagen Gel Retraction Assay
2.12. TGF-β1 ELISA
2.13. Statistical Analysis
3. Results
3.1. Effect of K Formulation on Cell Proliferation of Human Adult and Aged Dermal Fibroblasts
3.2. Effect of K Formulation on β-Galactosidase Activity and Oxidative Stress in Aged HDFs
3.3. Effect of K Formulation on Type I Collagen Synthesis in Adult and Aged HDFs
3.4. Effect of K Formulation on Fibroblast Contraction and Migration Activity
3.5. Effect of K Formulation on Extracellular Secretion of TGF-β1 by Adult and Aged HDFs
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galvez-Martin, P.; Soto-Fernandez, C.; Romero-Rueda, J.; Cabanas, J.; Torrent, A.; Castells, G.; Martinez-Puig, D. A Novel Hyaluronic Acid Matrix Ingredient with Regenerative, Anti-Aging and Antioxidant Capacity. Int. J. Mol. Sci. 2023, 24, 4774. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Mazini, L.; Meloux, A.; Zeller, M.; Cottin, Y.; Vergely, C.; Malka, G. Anti-Aging Effects of GDF11 on Skin. Int. J. Mol. Sci. 2020, 21, 2598. [Google Scholar] [CrossRef] [PubMed]
- Haydont, V.; Bernard, B.A.; Fortunel, N.O. Age-related evolutions of the dermis: Clinical signs, fibroblast and extracellular matrix dynamics. Mech. Ageing Dev. 2019, 177, 150–156. [Google Scholar] [CrossRef]
- Fisher, G.J.; Wang, B.; Cui, Y.L.; Shi, M.; Zhao, Y.; Quan, T.H.; Voorhees, J.J. Skin aging from the perspective of dermal fibroblasts: The interplay between the adaptation to the extracellular matrix microenvironment and cell autonomous processes. J. Cell Commun. Signal. 2023, 17, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Wlaschek, M.; Maity, P.; Makrantonaki, E.; Scharffetter-Kochanek, K. Connective Tissue and Fibroblast Senescence in Skin Aging. J. Investig. Dermatol. 2021, 141, 985–992. [Google Scholar] [CrossRef]
- Rittie, L.; Fisher, G.J. Natural and Sun-Induced Aging of Human Skin. Cold Spring Harb. Perspect. Med. 2015, 5, a015370. [Google Scholar] [CrossRef]
- Quan, T.H.; Fisher, G.J. Role of Age-Associated Alterations of the Dermal Extracellular Matrix Microenvironment in Human Skin Aging: A Mini-Review. Gerontology 2015, 61, 427–434. [Google Scholar] [CrossRef]
- Yasin, A.; Ren, Y.; Li, J.A.; Sheng, Y.L.; Cao, C.; Zhang, K. Advances in Hyaluronic Acid for Biomedical Applications. Front. Bioeng. Biotechnol. 2022, 10, 910290. [Google Scholar] [CrossRef]
- Juncan, A.M.; Moisa, D.G.; Santini, A.; Morgovan, C.; Rus, L.L.; Vonica-Tincu, A.L.; Loghin, F. Advantages of Hyaluronic Acid and Its Combination with Other Bioactive Ingredients in Cosmeceuticals. Molecules 2021, 26, 4429. [Google Scholar] [CrossRef]
- Landau, M.; Fagien, S. Science of Hyaluronic Acid Beyond Filling: Fibroblasts and Their Response to the Extracellular Matrix. Plast. Reconstr. Surg. 2015, 136, 188s–195s. [Google Scholar] [CrossRef]
- Adachi, T.; Wang, X.B.; Murata, T.; Obara, M.; Akutsu, H.; Machida, M.; Umezawa, A.; Tomita, M. Production of a Non-Triple Helical Collagen alpha Chain in Transgenic Silkworms and Its Evaluation as a Gelatin Substitute for Cell Culture. Biotechnol. Bioeng. 2010, 106, 860–870. [Google Scholar] [CrossRef]
- Rodriguez, M.I.A.; Barroso, L.G.R.; Sanchez, M.L. Collagen: A review on its sources and potential cosmetic applications. J. Cosmet. Dermatol. 2018, 17, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.N.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019, 31, 1801651. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, L.; Natali, M.L.; Brunetti, C.; Sannino, A.; Gallo, N. An Update on the Clinical Efficacy and Safety of Collagen Injectables for Aesthetic and Regenerative Medicine Applications. Polymers 2023, 15, 1020. [Google Scholar] [CrossRef] [PubMed]
- Varma, D.M.; Gold, G.T.; Taub, P.J.; Nicoll, S.B. Injectable carboxymethylcellulose hydrogels for soft tissue filler applications. Acta Biomater. 2014, 10, 4996–5004. [Google Scholar] [CrossRef] [PubMed]
- Lamanna, L.; Rizzi, F.; Demitri, C.; Pisanello, M.; Scarpa, E.; Qualtieri, A.; Sannino, A.; De Vittorio, M. Determination of absorption and structural properties of cellulose-based hydrogel via ultrasonic pulse-echo time-of-flight approach. Cellulose 2018, 25, 4331–4343. [Google Scholar] [CrossRef]
- Rahman, M.S.; Hasan, M.S.; Nitai, A.S.; Nam, S.; Karmakar, A.K.; Ahsan, M.S.; Shiddiky, M.J.A.; Ahmed, M.B. Recent Developments of Carboxymethyl Cellulose. Polymers 2021, 13, 1345. [Google Scholar] [CrossRef]
- Zhang, W.L.; Liu, Y.N.; Xuan, Y.; Zhang, S.B.A. Synthesis and Applications of Carboxymethyl Cellulose Hydrogels. Gels 2022, 8, 529. [Google Scholar] [CrossRef]
- Sundaram, H.; Voigts, B.; Beer, K.; Meland, M. Comparison of the Rheological Properties of Viscosity and Elasticity in Two Categories of Soft Tissue Fillers: Calcium Hydroxylapatite and Hyaluronic Acid. Dermatol. Surg. 2010, 36, 1859–1865. [Google Scholar] [CrossRef]
- UNI EN ISO 10993-5:2009; Biological Evaluation of Medical Devices–Part 5: In Vitro Cytotoxicity Testing. International Organization for Standardization: Geneva, Switzerland, 2009.
- La Torre, C.; Cinque, B.; Lombardi, F.; Miconi, G.; Palumbo, P.; Evtoski, Z.; Placidi, G.; Fanini, D.; Cimini, A.M.; Benedetti, E.; et al. Nitric Oxide Chemical Donor Affects the Early Phases of In Vitro Wound Healing Process. J. Cell. Physiol. 2016, 231, 2185–2195. [Google Scholar] [CrossRef]
- Lombardi, F.; Augello, F.R.; Artone, S.; Bahiti, B.; Sheldon, J.M.; Giuliani, M.; Cifone, M.G.; Palumbo, P.; Cinque, B. Efficacy of probiotic Streptococcus thermophilus in counteracting TGF-beta 1-induced fibrotic response in normal human dermal fibroblasts. J. Inflamm. 2022, 19, 27. [Google Scholar] [CrossRef] [PubMed]
- Gerasymchuk, M.; Robinson, G.I.; Kovalchuk, O.; Kovalchuk, I. Modeling of the Senescence-Associated Phenotype in Human Skin Fibroblasts. Int. J. Mol. Sci. 2022, 23, 7124. [Google Scholar] [CrossRef]
- Papaccio, F.; D’Arino, A.; Caputo, S.; Bellei, B. Focus on the Contribution of Oxidative Stress in Skin Aging. Antioxidants 2022, 11, 1121. [Google Scholar] [CrossRef] [PubMed]
- Tobin, D.J. Introduction to skin aging. J. Tissue Viability 2017, 26, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Kwon, S.H.; Choi, J.Y.; Na, J.I.; Huh, C.H.; Choi, H.R.; Park, K.C. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef]
- Varani, J.; Dame, M.K.; Rittie, L.; Fligiel, S.E.G.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Decreased collagen production in chronologically aged skin–Roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am. J. Pathol. 2006, 168, 1861–1868. [Google Scholar] [CrossRef]
- Shinde, A.V.; Humeres, C.; Frangogiannis, N.G. The role of alpha-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling. Biochim. Biophys. Acta-Mol. Basis Dis. 2017, 1863, 298–309. [Google Scholar] [CrossRef]
- Lombardi, F.; Palumbo, P.; Augello, F.R.; Giusti, I.; Dolo, V.; Guerrini, L.; Cifone, M.G.; Giuliani, M.; Cinque, B. Type I Collagen Suspension Induces Neocollagenesis and Myodifferentiation in Fibroblasts In Vitro. BioMed Res. Int. 2020, 2020, 6093974. [Google Scholar] [CrossRef]
- Kim, Y.I.; Kim, K.S.; Ahn, H.J.; Kang, I.H.; Shin, M.K. Reduced matrix metalloproteinase and collagen transcription mediated by the TGF-β/Smad pathway in passaged normal human dermal fibroblasts. J. Cosmet. Dermatol. 2020, 19, 1211–1218. [Google Scholar] [CrossRef]
- Semkova, M.E.; Hsuan, J.J. TGF beta-1 Induced Cross-Linking of the Extracellular Matrix of Primary Human Dermal Fibroblasts. Int. J. Mol. Sci. 2021, 22, 984. [Google Scholar] [CrossRef]
- Yuan, L.F.; Alexander, P.B.; Wang, X.F. Cellular senescence: From anti-cancer weapon to anti-aging target. Sci. China-Life Sci. 2020, 63, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Kamal, N.S.M.; Safuan, S.; Shamsuddin, S.; Foroozandeh, P. Aging of the cells: Insight into cellular senescence and detection Methods. Eur. J. Cell Biol. 2020, 99, 151108. [Google Scholar] [CrossRef] [PubMed]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative Stress in Aging Human Skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Shan, Y.H.; Gong, R.C.; Lin, D.F.; Zhang, M.W.; Wang, C.; Wang, L. Mechanism of action and therapeutic effects of oxidative stress and stem cell-based materials in skin aging: Current evidence and future perspectives. Front. Bioeng. Biotechnol. 2023, 10, 1082403. [Google Scholar] [CrossRef]
- Jove, M.; Mota-Martorell, N.; Pradas, I.; Martin-Gari, M.; Ayala, V.; Pamplona, R. The Advanced Lipoxidation End-Product Malondialdehyde-Lysine in Aging and Longevity. Antioxidants 2020, 9, 1132. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Rappu, P.; Salo, A.M.; Myllyharju, J.; Heino, J. Role of prolyl hydroxylation in the molecular interactions of collagens. Extracell. Matrix 2019, 63, 325–335. [Google Scholar] [CrossRef]
- Tsuji-Naito, K.; Ishikura, S.; Akagawa, M.; Saeki, H. alpha-Lipoic acid induces collagen biosynthesis involving prolyl hydroxylase expression via activation of TGF-beta-Smad signaling in human dermal fibroblasts. Connect. Tissue Res. 2010, 51, 378–387. [Google Scholar] [CrossRef]
- Kim, D.; Kim, S.Y.; Mun, S.K.; Rhee, S.; Kim, B.J. Epidermal growth factor improves the migration and contractility of aged fibroblasts cultured on 3D collagen matrices. Int. J. Mol. Med. 2015, 35, 1017–1025. [Google Scholar] [CrossRef]
- Yu, Z.N.; Smith, M.J.; Siow, R.C.M.; Liu, K.K. Ageing modulates human dermal fibroblast contractility: Quantification using nano-biomechanical testing. Biochim. Biophys. Acta-Mol. Cell Res. 2021, 1868, 118972. [Google Scholar] [CrossRef]
- Feru, J.; Delobbe, E.; Ramont, L.; Brassart, B.; Terryn, C.; Dupont-Deshorgue, A.; Garbar, C.; Monboisse, J.C.; Maquart, F.X.; Brassart-Pasco, S. Aging decreases collagen IV expression in vivo in the dermo-epidermal junction and in vitro in dermal fibroblasts: Possible involvement of TGF-beta 1. Eur. J. Dermatol. 2016, 26, 350–360. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Quan, T.; Shao, Y.; Voorhees, J.; Fisher, G. Oxidative exposure impairs TGF-β pathway via reduction of type II receptor and SMAD3 in human skin fibroblasts. Age 2014, 36, 1079–1094. [Google Scholar] [CrossRef] [PubMed]
- Cavinato, M.; Jansen-Durr, P. Molecular mechanisms of UVB-induced senescence of dermal fibroblasts and its relevance for photoaging of the human skin. Exp. Gerontol. 2017, 94, 78–82. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Augello, F.R.; Lombardi, F.; Artone, S.; Ciafarone, A.; Altamura, S.; Di Marzio, L.; Cifone, M.G.; Palumbo, P.; Giuliani, M.; Cinque, B. Evaluation of the Effectiveness of an Innovative Polycomponent Formulation on Adult and Aged Human Dermal Fibroblasts. Biomedicines 2023, 11, 2410. https://doi.org/10.3390/biomedicines11092410
Augello FR, Lombardi F, Artone S, Ciafarone A, Altamura S, Di Marzio L, Cifone MG, Palumbo P, Giuliani M, Cinque B. Evaluation of the Effectiveness of an Innovative Polycomponent Formulation on Adult and Aged Human Dermal Fibroblasts. Biomedicines. 2023; 11(9):2410. https://doi.org/10.3390/biomedicines11092410
Chicago/Turabian StyleAugello, Francesca Rosaria, Francesca Lombardi, Serena Artone, Alessia Ciafarone, Serena Altamura, Luisa Di Marzio, Maria Grazia Cifone, Paola Palumbo, Maurizio Giuliani, and Benedetta Cinque. 2023. "Evaluation of the Effectiveness of an Innovative Polycomponent Formulation on Adult and Aged Human Dermal Fibroblasts" Biomedicines 11, no. 9: 2410. https://doi.org/10.3390/biomedicines11092410