Green Tea Polyphenol (-)-Epigallocatechin-3-Gallate (EGCG): A Time for a New Player in the Treatment of Respiratory Diseases?
Abstract
:1. Introduction
2. Epigallocatechin-Gallate (EGCG)
3. Antioxidant Mechanisms of EGCG
4. Effects of EGCG in Non-Respiratory Diseases
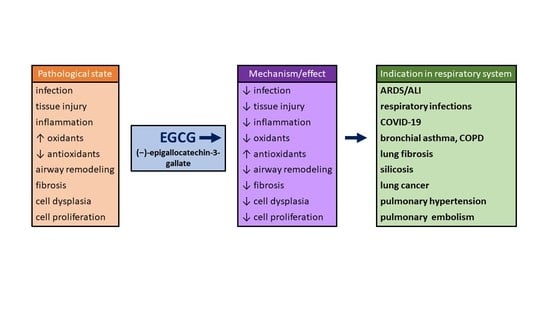
5. Effects of EGCG in Respiratory Diseases
5.1. EGCG in ALI
5.2. EGCG in Bacterial and Viral Respiratory Infections
EGCG in COVID-19
5.3. EGCG in Bronchial Asthma
| Animal Model | Species | EGCG Dose/Way of Delivery | Major Findings | Study |
|---|---|---|---|---|
| OVA-induced model of bronchial asthma | Guinea pigs | EGCG (25 mg/kg s.c.) given 20 min prior to OVA challenge | ↓ bronchoconstriction, ↓ inflammation, ↓ lung injury; ↑ eNOS activity | [99] |
| OVA-induced model of bronchial asthma | Balb/c mice | EGCG (0.5 mg/mL in drinking water) given for 8 weeks, started 1 h after the 1st OVA challenge | ↓ cell counts in BALF, ↓ inflammation and EMT | [138] |
| OVA-induced model of bronchial asthma | Balb/c mice | EGCG (10 or 20 mg/kg/d i.v.) given 3 d after OVA sensibilization and challenge | ↓ bronchoconstriction and inflammation, ↓ TGF-β1 and phosphorylated (p)-Smad2/3 | [37] |
| OVA-induced model of bronchial asthma | Balb/c mice | EGCG (5 or 50 mg/kg i.p.) given 1 h before each OVA challenge, for 30 d | ↓ bronchoconstriction and inflammation | [185] |
| Obesity-associated OVA-induced asthma | C57BL/6 mice | EGCG (10 mg/kg/day, gavage, for 2 weeks) given simultaneously with OVA sensitization | ↓ inflammation, ↓ ROS, ↑ SOD, ↓ iNOS and NOx | [97] |
| Toluene diisocyanate (TDI)-inhalation induced model of bronchial asthma | Balb/c mice | EGCG (0.3% in drinking water) given for 10 d from last sensitization to 2 days after first challenge | ↓ bronchoconstriction, ↓ cells in BALF, ↓ MMP-9 in the lung, ↓ ROS, TNFα, and IL-5 in BALF | [186] |
| Fine particulate matter 2.5 (PM2.5)-induced model of bronchial asthma | Sprague-Dawley rats | EGCG (10 or 50 mg/kg i.p.) given 1 h before 1st atomization of PM2.5 (10 mg/kg, by i.t. atomization done 4-times every other day) | ↓ lung injury and inflammation, ↓ bronchial smooth muscle thickness, ↓ HMGB1 and RAGE | [38] |
| House dust mite (HDM)-induced asthma | C57BL/6 mice | EGCG (50 mg/kg i.p.) given 1 h before HDM challenge | ↓ tissue injury, ↓ inflammation, ↓ mucus production, ↓ collagen deposition, ↓ M2 macrophages in the lung | [187] |
| Cigarette smoke (CS)-induced model of COPD | Sprague-Dawley rats | EGCG (50 mg/kg) given by oral gavage every other day during 56 d of cigarette smoke exposure | ↓ markers of oxidative stress and neutrophil inflammation, ↑ SOD, catalase, GST, ↓ mucus, ↓ airway remodeling | [36] |
| Bleomycin-induced lung fibrosis | Wistar rats | EGCG (20 mg/kg i.p.) given for 28 d, started 6 h after bleomycin (6.5 U/kg i.t.) instillation | ↓ lung injury, inflammation, and fibrosis, ↓ ROS, ↑ antioxidants | [34,35,188,189] |
| Irradiation-induced pulmonary fibrosis | Sprague-Dawley rats | EGCG (25 mg/kg i.p.) given for 30 d, started after (60)Co irradiation (22 Gy) | ↓ mortality, ↓ lung injury, inflammation, and fibrosis | [106] |
| Cyclophosphamide-induced pulmonary fibrosis | Wistar rats | Green tea extract (150 mg/kg i.g.) given for 14 d, before cyclophosphamide (150 mg/kg i.p.) administration in 2 consecutive days | ↓ oxidative stress, inflammation, and fibrosis | [114] |
| Paraquat-induced pulmonary fibrosis | Sprague-Dawley rats | Green tea extract (1% i.g.), after paraquat (0.3 mg/kg i.t.) instillation | ↓ oxidative stress and ET-1 | [190] |
| Particulate silica-induced lung fibrosis | Sprague-Dawley rats | EGCG (50 mg/kg), PBCA-NPs (150 mg/kg) or their combination, given daily by gavage for 28 d, started 2 d after silicosis modeling (SiO2 50 mg/mL, 1 mL i.t.) | ↓ fibrosis, restored body weight | [191] |
| CS-induced model of bronchial cells dysplasia | Sprague-Dawley rats | EGCG (0.3%) in drinking water, given paralelly with inhalation of CS for 4, 8, 12 or 16 weeks | ↓ benzopyrene-DNA adducts, ↓ precancerous lesions of bronchial cells | [192] |
5.4. EGCG in COPD
5.5. EGCG in Lung Fibrosis
5.6. EGCG in Lung Silicosis
5.7. Lung Cancer
5.8. Pulmonary Hypertension
5.9. Pulmonary Embolism
6. Advanced EGCG Delivery Forms
7. Adverse Effects and Drug Interactions of EGCG
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soriano, J.B.; Kendrick, P.; Paulson, K.; Gupta, V.; Vos, T.; GBD Chronic Respiratory Disease Collaborators. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2020, 8, 585–596. [Google Scholar] [CrossRef]
- Causes of Death—Standardised Death Rate by NUTS 2 Region of Residence. Available online: https://ec.europa.eu/eurostat/databrowser/view/hlth_cd_asdr2/default/table?lang=en (accessed on 31 July 2022).
- Labaki, W.W.; Han, M.K. Chronic respiratory diseases: A global view. Lancet Respir. Med. 2020, 8, 531–533. [Google Scholar] [CrossRef]
- Gibson, G.J.; Lodeenkemper, R.; Lundbäck, B.; Sibille, Y. Respiratory health and disease in Europe: The new European Lung White Book. Eur. Respir. J. 2013, 42, 559–563. [Google Scholar] [CrossRef]
- European Lung White Book: The Economic Burden of Lung Disease. Available online: https://www.erswhitebook.org/chapters/the-economic-burden-of-lung-disease/ (accessed on 31 July 2022).
- Kellner, M.; Noonepalle, S.; Lu, Q.; Srivastava, A.; Zemskov, E.; Black, S.M. ROS Signaling in the Pathogenesis of Acute Lung Injury (ALI) and Acute Respiratory Distress Syndrome (ARDS). Adv. Exp. Med. Biol. 2017, 967, 105–137. [Google Scholar] [CrossRef]
- Soto, M.E.; Guarner-Lans, V.; Soria-Castro, E.; Manzano Pech, L.; Pérez-Torres, I. Is Antioxidant Therapy a Useful Complementary Measure for Covid-19 Treatment? An Algorithm for Its Application. Medicina 2020, 56, 386. [Google Scholar] [CrossRef]
- Mokrá, D. Acute lung injury—From pathophysiology to treatment. Physiol. Res. 2020, 69, S353–S366. [Google Scholar] [CrossRef]
- Von Knethen, A.; Heinicke, U.; Laux, V.; Parnham, M.J.; Steinbicker, A.U.; Zacharowski, K. Antioxidants as Therapeutic Agents in Acute Respiratory Distress Syndrome (ARDS) Treatment-From Mice to Men. Biomedicines 2022, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Lodha, R.; Kabra, S.K. Upper respiratory tract infections. Indian J. Pediatr. 2001, 68, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Bakaletz, L.O. Viral-bacterial co-infections in the respiratory tract. Curr. Opin. Microbiol. 2017, 35, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Laforge, M.; Elbim, C.; Frère, C.; Hémadi, M.; Massaad, C.; Nuss, P.; Benoliel, J.J.; Becker, C. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat. Rev. Immunol. 2020, 20, 515–516. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Cellular and molecular mechanisms of asthma and COPD. Clin. Sci. 2017, 131, 1541–1558. [Google Scholar] [CrossRef]
- Michaeloudes, C.; Abubakar-Waziri, H.; Lakhdar, R.; Raby, K.; Dixey, P.; Adcock, I.M.; Mumby, S.; Bhavsar, P.K.; Chung, K.F. Molecular mechanisms of oxidative stress in asthma. Mol. Asp. Med. 2022, 85, 101026. [Google Scholar] [CrossRef]
- Kirkham, P.A.; Barnes, P.J. Oxidative stress in COPD. Chest 2013, 144, 266–273. [Google Scholar] [CrossRef]
- Barnes, P.J. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2016, 138, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Cheresh, P.; Kim, S.J.; Tulasiram, S.; Kamp, D.W. Oxidative stress and pulmonary fibrosis. Biochim. Biophys. Acta 2013, 1832, 1028–1040. [Google Scholar] [CrossRef]
- Phan, T.H.G.; Paliogiannis, P.; Nasrallah, G.K.; Giordo, R.; Eid, A.H.; Fois, A.G.; Zinellu, A.; Mangoni, A.A.; Pintus, G. Emerging cellular and molecular determinants of idiopathic pulmonary fibrosis. Cell. Mol. Life Sci. 2021, 78, 2031–2057. [Google Scholar] [CrossRef] [PubMed]
- Adamcakova, J.; Mokra, D. New Insights into Pathomechanisms and Treatment Possibilities for Lung Silicosis. Int. J. Mol. Sci. 2021, 22, 4162. [Google Scholar] [CrossRef]
- Tan, S.; Chen, S. Macrophage Autophagy and Silicosis: Current Perspective and Latest Insights. Int. J. Mol. Sci. 2021, 22, 453. [Google Scholar] [CrossRef]
- Todoric, J.; Antonucci, L.; Karin, M. Targeting Inflammation in Cancer Prevention and Therapy. Cancer Prev. Res. 2016, 9, 895–905. [Google Scholar] [CrossRef]
- Hayakawa, S.; Ohishi, T.; Miyoshi, N.; Oishi, Y.; Nakamura, Y.; Isemura, M. Anti-Cancer Effects of Green Tea Epigallocatchin-3-Gallate and Coffee Chlorogenic Acid. Molecules 2020, 25, 4553. [Google Scholar] [CrossRef]
- Poch, D.; Mandel, J. Pulmonary Hypertension. Ann. Intern. Med. 2021, 174, ITC49–ITC64. [Google Scholar] [CrossRef] [PubMed]
- Doherty, S. Pulmonary embolism: An update. Aust. Fam. Physician 2017, 46, 816–820. [Google Scholar] [PubMed]
- Kuruvilla, M.E.; Lee, F.E.; Lee, G.B. Understanding Asthma Phenotypes, Endotypes, and Mechanisms of Disease. Clin. Rev. Allergy Immunol. 2019, 56, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Banga, J.; Silveyra, P. Oxidative stress and cellular pathways of asthma and inflammation: Therapeutic strategies and pharmacological targets. Pharmacol. Ther. 2018, 181, 169–182. [Google Scholar] [CrossRef]
- Fischer, B.M.; Voynow, J.A.; Ghio, A.J. COPD: Balancing oxidants and antioxidants. Int. J. Chron. Obstruct. Pulmon. Dis. 2015, 10, 261–276. [Google Scholar] [CrossRef]
- Lee, J.; Jang, J.; Park, S.M.; Yang, S.R. An Update on the Role of Nrf2 in Respiratory Disease: Molecular Mechanisms and Therapeutic Approaches. Int. J. Mol. Sci. 2021, 22, 8406. [Google Scholar] [CrossRef]
- Van der Vliet, A.; Janssen-Heininger, Y.M.W.; Anathy, V. Oxidative stress in chronic lung disease: From mitochondrial dysfunction to dysregulated redox signaling. Mol. Asp. Med. 2018, 63, 59–69. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, S.; Xiang, D.; Ju, L.; Shen, D.; Wang, X.; Wang, Y. Friend or Foe? The Roles of Antioxidants in Acute Lung Injury. Antioxidants 2021, 10, 1956. [Google Scholar] [CrossRef]
- Audousset, C.; McGovern, T.; Martin, J.G. Role of Nrf2 in Disease: Novel Molecular Mechanisms and Therapeutic Approaches—Pulmonary Disease/Asthma. Front. Physiol. 2021, 12, 727806. [Google Scholar] [CrossRef]
- Lago, J.H.; Toledo-Arruda, A.C.; Mernak, M.; Barrosa, K.H.; Martins, M.A.; Tibério, I.F.; Prado, C.M. Structure-activity association of flavonoids in lung diseases. Molecules 2014, 19, 3570–3595. [Google Scholar] [CrossRef]
- Adamcakova, J.; Mokra, D. Herbal compounds in the treatment of pulmonary silicosis. Physiol. Res. 2021, 70, S275–S287. [Google Scholar] [CrossRef]
- Sriram, N.; Kalayarasan, S.; Sudhandiran, G. Epigallocatechin-3-gallate augments antioxidant activities and inhibits inflammation during bleomycin-induced experimental pulmonary fibrosis through Nrf2-Keap1 signaling. Pulm. Pharmacol. Ther. 2009, 22, 221–236. [Google Scholar] [CrossRef]
- Sriram, N.; Kalayarasan, S.; Sudhandiran, G. Epigallocatechin-3-gallate exhibits anti-fibrotic effect by attenuating bleomycin-induced glycoconjugates, lysosomal hydrolases and ultrastructural changes in rat model pulmonary fibrosis. Chem. Biol. Interact. 2009, 180, 271–280. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, K.W.K.; Yeung, S.C.; Li, X.; Ip, M.S.M.; Mak, J.C.W. (−)-Epigallocatechin-3-gallate Reduces Cigarette Smoke-Induced Airway Neutrophilic Inflammation and Mucin Hypersecretion in Rats. Front. Pharmacol. 2017, 8, 618. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Kang, X.; Liu, F.; Cai, X.; Han, X.; Shang, Y. Epigallocatechin gallate improves airway inflammation through TGF-β1 signaling pathway in asthmatic mice. Mol. Med. Rep. 2018, 18, 2088–2096. [Google Scholar] [CrossRef]
- Li, Y.; Chen, L.; Guo, F.; Cao, Y.; Hu, W.; Shi, Y.; Lin, X.; Hou, J.; Li, L.; Ding, X.; et al. Effects of epigallocatechin-3-gallate on the HMGB1/RAGE pathway in PM2.5-exposed asthmatic rats. Biochem. Biophys. Res. Commun. 2019, 513, 898–903. [Google Scholar] [CrossRef]
- Wang, M.; Zhong, H.; Zhang, X.; Huang, X.; Wang, J.; Li, Z.; Chen, M.; Xiao, Z. EGCG promotes PRKCA expression to alleviate LPS-induced acute lung injury and inflammatory response. Sci. Rep. 2021, 11, 11014. [Google Scholar] [CrossRef]
- Liu, J.; Bodnar, B.H.; Meng, F.; Khan, A.I.; Wang, X.; Saribas, S.; Wang, T.; Lohani, S.C.; Wang, P.; Wei, Z.; et al. Epigallocatechin gallate from green tea effectively blocks infection of SARS-CoV-2 and new variants by inhibiting spike binding to ACE2 receptor. Cell. Biosci. 2021, 11, 168. [Google Scholar] [CrossRef]
- Tang, H.; Hao, S.; Khan, M.F.; Zhao, L.; Shi, F.; Li, Y.; Guo, H.; Zou, Y.; Lv, C.; Luo, J.; et al. Epigallocatechin-3-Gallate Ameliorates Acute Lung Damage by Inhibiting Quorum-Sensing-Related Virulence Factors of Pseudomonas aeruginosa. Front. Microbiol. 2022, 13, 874354. [Google Scholar] [CrossRef]
- Carloni, P.; Tiano, L.; Padella, L.; Bacchetti, T.; Customu, C.; Kay, A.; Damiani, E. Antioxidant Activity of White, Green and Black Tea Obtained from the Same Tea Cultivar. Food Res. Int. 2013, 53, 900–908. [Google Scholar] [CrossRef]
- Devkota, H.P.; Gaire, B.P.; Hori, K.; Subedi, L.; Adhikari-Devkota, A.; Belwal, T.; Paudel, K.R.; Jha, N.K.; Singh, S.K.; Chellappan, D.K.; et al. The science of matcha: Bioactive compounds, analytical techniques and biological properties. Trends Food Sci. Technol. 2021, 118, 735–743. [Google Scholar] [CrossRef]
- Mukhtar, H.; Ahmad, N. Tea polyphenols: Prevention of cancer and optimizing health. Am. J. Clin. Nutr. 2000, 71, 1698S–1702S. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Afaq, F.; Saleem, M.; Ahmad, N.; Mukhtar, H. Targeting multiple signaling pathways by green tea polyphenol (−)-epigallocatechin-3-gallate. Cancer Res. 2006, 66, 2500–2505. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef]
- Henning, S.M.; Niu, Y.; Lee, N.H.; Thames, G.D.; Minutti, R.R.; Wang, H.; Go, V.L.; Heber, D. Bioavailability and antioxidant activity of tea flavanols after consumption of green tea, black tea, or a green tea extract supplement. Am. J. Clin. Nutr. 2004, 80, 1558–1564. [Google Scholar] [CrossRef] [PubMed]
- Epigallocatechin Gallate. Available online: https://en.wikipedia.org/wiki/Epigallocatechin_gallate (accessed on 1 August 2022).
- Van Amelsvoort, J.M.; van Hof, K.H.; Mathot, J.N.; Mulder, T.P.; Wiersma, A.; Tijburg, L.B. Plasma concentrations of individual tea catechins after a single oral dose in humans. Xenobiotica 2001, 31, 891–901. [Google Scholar] [CrossRef]
- Lee, M.J.; Maliakal, P.; Chen, L.; Meng, X.; Bondoc, F.Y.; Prabhu, S.; Lambert, G.; Mohr, S.; Yang, C.S. Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans: Formation of different metabolites and individual variability. Cancer Epidemiol. Biomarkers Prev. 2002, 11, 1025–1032. [Google Scholar]
- Kim, H.S.; Quon, M.J.; Kim, J.A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, N.; Yamada, K.; Yamashita, K.; Shoji, K.; Mori, M.; Sugano, M. Inhibitory effect of tea polyphenols on histamine and leukotriene B4 release from rat peritoneal exudate cells. In Vitro Cell. Dev. Biol. Anim. 1996, 32, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, I. Multifunctional effects of green tea catechins on prevention of the metabolic syndrome. Asia Pac. J. Clin. Nutr. 2008, 17, 273–274. [Google Scholar] [PubMed]
- Nanjo, F.; Mori, M.; Goto, K.; Hara, Y. Radical scavenging activity of tea catechins and their related compounds. Biosci. Biotechnol. Biochem. 1999, 63, 1621–1623. [Google Scholar] [CrossRef] [PubMed]
- Higdon, J.V.; Frei, B. Tea catechins and polyphenols: Health effects, metabolism, and antioxidant functions. Crit. Rev. Food Sci. Nutr. 2003, 43, 89–143. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Lee, M.J.; Diamond, L.; Ju, J.; Hong, J.; Bose, M.; Newmark, H.L.; Yang, C.S. Dose-dependent levels of epigallocatechin-3-gallate in human colon cancer cells and mouse plasma and tissues. Drug Metab. Dispos. 2006, 34, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Velalar, C.N.; Ruan, R. Regulating the age-related oxidative damage, mitochondrial integrity, and antioxidative enzyme activity in Fischer 344 rats by supplementation of the antioxidant epigallocatechin-3-gallate. Rejuvenation Res. 2008, 11, 649–660. [Google Scholar] [CrossRef]
- Basu, A.; Sanchez, K.; Leyva, M.J.; Wu, M.; Betts, N.M.; Aston, C.E.; Lyons, T.J. Green tea supplementation affects body weight, lipids, and lipid peroxidation in obese subjects with metabolic syndrome. J. Am. Coll. Nutr. 2010, 29, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, S.; Zhang, W.; Zhao, P.; He, B.; Wu, N.; Han, P. Epigallocatechin-3-O-gallate (EGCG) attenuates FFAs-induced peripheral insulin resistance through AMPK pathway and insulin signaling pathway in vivo. Diabetes Res. Clin. Pract. 2011, 93, 205–214. [Google Scholar] [CrossRef]
- Krupkova, O.; Ferguson, S.J.; Wuertz-Kozak, K. Stability of (−)-epigallocatechin gallate and its activity in liquid formulations and delivery systems. J. Nutr. Biochem. 2016, 37, 1–12. [Google Scholar] [CrossRef]
- Lambert, J.D.; Lee, M.J.; Lu, H.; Meng, X.; Hong, J.J.; Seril, D.N.; Sturgill, M.G.; Yang, C.S. Epigallocatechin-3-gallate is absorbed but extensively glucuronidated following oral administration to mice. J. Nutr. 2003, 133, 4172–4177. [Google Scholar] [CrossRef]
- Cai, Z.Y.; Li, X.M.; Liang, J.P.; Xiang, L.P.; Wang, K.R.; Shi, Y.L.; Yang, R.; Shi, M.; Ye, J.H.; Lu, J.L.; et al. Bioavailability of Tea Catechins and Its Improvement. Molecules 2018, 23, 2346. [Google Scholar] [CrossRef]
- Naumovski, N.; Blades, B.L.; Roach, P.D. Food Inhibits the Oral Bioavailability of the Major Green Tea Antioxidant Epigallocatechin Gallate in Humans. Antioxidants 2015, 4, 373–393. [Google Scholar] [CrossRef]
- Tkaczyk, J.; Vízek, M. Oxidative stress in the lung tissue—Sources of reactive oxygen species and antioxidant defence. Prague Med. Rep. 2007, 108, 105–114. [Google Scholar]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef]
- Fan, Y.; Mao, R.; Yang, J. NF-κB and STAT3 signaling pathways collaboratively link inflammation to cancer. Protein Cell 2013, 4, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Vijayalekshmi, R.V.; Sung, B. Targeting inflammatory pathways for prevention and therapy of cancer: Short-term friend, long-term foe. Clin. Cancer Res. 2009, 15, 425–430. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Moloney, J.N.; Cotter, T.G. ROS signalling in the biology of cancer. Semin. Cell. Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef]
- Dong, Z.; Ma, W.; Huang, C.; Yang, C.S. Inhibition of tumor promoter-induced activator protein 1 activation and cell transformation by tea polyphenols, (−)-epigallocatechin gallate, and theaflavins. Cancer Res. 1997, 57, 4414–4419. [Google Scholar]
- Gupta, S.; Hastak, K.; Afaq, F.; Ahmad, N.; Mukhtar, H. Essential role of caspases in epigallocatechin-3-gallate-mediated inhibition of nuclear factor κB and induction of apoptosis. Oncogene 2004, 23, 2507–2522. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Deguchi, A.; Lim, J.T.; Moriwaki, H.; Kopelovich, L.; Weinstein, I.B. (−)Epigallocatechin gallate and polyphenon E inhibit growth and activation of the epidermal growth factor receptor and human epidermal growth factor receptor-2 signaling pathways in human colon cancer cells. Clin. Cancer Res. 2005, 11, 2735–2746. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Slika, H.; Mansour, H.; Wehbe, N.; Nasser, S.A.; Iratni, R.; Nasrallah, G.; Shaito, A.; Ghaddar, T.; Kobeissy, F.; Eid, A.H. Therapeutic potential of flavonoids in cancer: ROS-mediated mechanisms. Biomed. Pharmacother. 2022, 146, 112442. [Google Scholar] [CrossRef]
- Ferrali, M.; Signorini, C.; Caciotti, B.; Sugherini, L.; Ciccoli, L.; Giachetti, D.; Comporti, M. Protection against oxidative damage of erythrocyte membrane by the flavonoid quercetin and its relation to iron chelating activity. FEBS. Lett. 1997, 416, 123–129. [Google Scholar] [CrossRef]
- Frei, B.; Higdon, J.V. Antioxidant activity of tea polyphenols in vivo: Evidence from animal studies. J. Nutr. 2003, 133, 3275S–3284S. [Google Scholar] [CrossRef]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef]
- Lambert, J.D.; Elias, R.J. The antioxidant and pro-oxidant activities of green tea polyphenols: A role in cancer prevention. Arch. Biochem. Biophys. 2010, 501, 65–72. [Google Scholar] [CrossRef]
- Cos, P.; Ying, L.; Calomme, M.; Hu, J.P.; Cimanga, K.; van Poel, B.; Pieters, L.; Vlietinck, A.J.; Vanden Berghe, D. Structure-activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J. Nat. Prod. 1998, 61, 71–76. [Google Scholar] [CrossRef]
- Yousefian, M.; Shakour, N.; Hosseinzadeh, H.; Hayes, A.W.; Hadizadeh, F.; Karimi, G. The natural phenolic compounds as modulators of NADPH oxidases in hypertension. Phytomedicine 2019, 55, 200–213. [Google Scholar] [CrossRef]
- Sahoo, S.; Meijles, D.N.; Pagano, P.J. NADPH oxidases: Key modulators in aging and age-related cardiovascular diseases? Clin. Sci. 2016, 130, 317–335. [Google Scholar] [CrossRef]
- Buvelot, H.; Jaquet, V.; Krause, K.H. Mammalian NADPH Oxidases. Methods Mol. Biol. 2019, 1982, 17–36. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Dikalov, S.I.; Dikalova, A.E.; Bikineyeva, A.T.; Schmidt, H.H.; Harrison, D.G.; Griendling, K.K. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic. Biol. Med. 2008, 45, 1340–1351. [Google Scholar] [CrossRef]
- Elbatreek, M.H.; Mucke, H.; Schmidt, H.H. NOX Inhibitors: From Bench to Naxibs to Bedside. In Reactive Oxygen Species; Springer: Cham, Switzerland, 2021; Volume 264, pp. 145–168. [Google Scholar] [CrossRef]
- Carnesecchi, S.; Deffert, C.; Donati, Y.; Basset, O.; Hinz, B.; Preynat-Seauve, O.; Guichard, C.; Arbiser, J.L.; Banfi, B.; Pache, J.C.; et al. A key role for NOX4 in epithelial cell death during development of lung fibrosis. Antioxid. Redox Signal. 2011, 15, 607–619. [Google Scholar] [CrossRef]
- Thichanpiang, P.; Wongprasert, K. Green tea polyphenol epigallocatechin-3-gallate attenuates TNF-α-induced intercellular adhesion molecule-1 expression and monocyte adhesion to retinal pigment epithelial cells. Am. J. Chin. Med. 2015, 43, 103–119. [Google Scholar] [CrossRef]
- Sarkar, J.; Chakraborti, T.; Chowdhury, A.; Bhuyan, R.; Chakraborti, S. Protective role of epigallocatechin-3-gallate in NADPH oxidase-MMP2-Spm-Cer-S1P signalling axis mediated ET-1 induced pulmonary artery smooth muscle cell proliferation. J. Cell. Commun. Signal. 2019, 13, 473–489. [Google Scholar] [CrossRef]
- Ramos, M.F.D.P.; de Barros, A.D.C.M.M.; Razvickas, C.V.; Borges, F.T.; Schor, N. Xanthine oxidase inhibitors and sepsis. Int. J. Immunopathol. Pharmacol. 2018, 32, 2058738418772210. [Google Scholar] [CrossRef]
- Faggioni, R.; Gatti, S.; Demitri, M.T.; Delgado, R.; Echtenacher, B.; Gnocchi, P.; Heremans, H.; Ghezzi, P. Role of xanthine oxidase and reactive oxygen intermediates in LPS- and TNF-induced pulmonary edema. J. Lab. Clin. Med. 1994, 123, 394–399. [Google Scholar]
- Zhang, G.; Zhu, M.; Liao, Y.; Gong, D.; Hu, X. Action mechanisms of two key xanthine oxidase inhibitors in tea polyphenols and their combined effect with allopurinol. J. Sci. Food Agric. 2022. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, I.S.; Mar, W. Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 activity by 1,2,3,4,6-penta-O-galloyl-beta-D-glucose in murine macrophage cells. Arch. Pharm. Res. 2003, 26, 832–839. [Google Scholar] [CrossRef]
- Hussain, T.; Gupta, S.; Adhami, V.M.; Mukhtar, H. Green tea constituent epigallocatechin-3-gallate selectively inhibits COX-2 without affecting COX-1 expression in human prostate carcinoma cells. Int. J. Cancer 2005, 113, 660–669. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Shishodia, S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharmacol. 2006, 71, 1397–1421. [Google Scholar] [CrossRef]
- Ricciardolo, F.L.; Sterk, P.J.; Gaston, B.; Folkerts, G. Nitric oxide in health and disease of the respiratory system. Physiol. Rev. 2004, 84, 731–765. [Google Scholar] [CrossRef]
- André, D.M.; Horimoto, C.M.; Calixto, M.C.; Alexandre, E.C.; Antunes, E. Epigallocatechin-3-gallate protects against the exacerbation of allergic eosinophilic inflammation associated with obesity in mice. Int. Immunopharmacol. 2018, 62, 212–219. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Almatroudi, A.; Alsahli, M.A.; Aljasir, M.A.; Syed, M.A.; Rahmani, A.H. Epigallocatechin-3-Gallate (EGCG), an Active Compound of Green Tea Attenuates Acute Lung Injury Regulating Macrophage Polarization and Krüpple-like-Factor 4 (KLF4) Expression. Molecules 2020, 25, 2853. [Google Scholar] [CrossRef]
- Bani, D.; Giannini, L.; Ciampa, A.; Masini, E.; Suzuki, Y.; Menegazzi, M.; Nistri, S.; Suzuki, H. Epigallocatechin-3-gallate reduces allergen-induced asthma-like reaction in sensitized guinea pigs. J. Pharmacol. Exp. Ther. 2006, 317, 1002–1011. [Google Scholar] [CrossRef]
- Yamagata, K. Protective Effect of Epigallocatechin Gallate on Endothelial Disorders in Atherosclerosis. J. Cardiovasc. Pharmacol. 2020, 75, 292–298. [Google Scholar] [CrossRef]
- Na, H.K.; Surh, Y.J. Modulation of Nrf2-mediated antioxidant and detoxifying enzyme induction by the green tea polyphenol EGCG. Food Chem. Toxicol. 2008, 46, 1271–1278. [Google Scholar] [CrossRef]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta 2013, 1830, 3143–3153. [Google Scholar] [CrossRef]
- Lu, S.C. Regulation of glutathione synthesis. Mol. Asp. Med. 2009, 30, 42–59. [Google Scholar] [CrossRef]
- Fernández-Checa, J.C.; Kaplowitz, N.; García-Ruiz, C.; Colell, A.; Miranda, M.; Marí, M.; Ardite, E.; Morales, A. GSH transport in mitochondria: Defense against TNF-induced oxidative stress and alcohol-induced defect. Am. J. Physiol. 1997, 273, G7–G17. [Google Scholar] [CrossRef]
- Simos, Y.V.; Verginadis, I.I.; Toliopoulos, I.K.; Velalopoulou, A.P.; Karagounis, I.V.; Karkabounas, S.C.; Evangelou, A.M. Effects of catechin and epicatechin on superoxide dismutase and glutathione peroxidase activity, in vivo. Redox Rep. 2012, 17, 181–186. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Wei, L.; Sun, W.L.; Wang, L.; Yang, Z.L.; Liu, Y.; Zheng, K.; Wang, Y.; Zhang, W.J. The green tea extract epigallocatechin-3-gallate inhibits irradiation-induced pulmonary fibrosis in adult rats. Int. J. Mol. Med. 2014, 34, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.Z.; Wang, Z.J.; Bai, F.; Qin, X.J.; Cao, J.; Lv, J.Y.; Zhang, M.S. Epigallocatechin-3-gallate protects HUVECs from PM2.5-induced oxidative stress injury by activating critical antioxidant pathways. Molecules 2015, 20, 6626–6639. [Google Scholar] [CrossRef]
- Azambuja, J.H.; Mancuso, R.I.; Via, F.I.D.; Torello, C.O.; Saad, S.T.O. Protective effect of green tea and epigallocatechin-3-gallate in a LPS-induced systemic inflammation model. J. Nutr. Biochem. 2022, 101, 108920. [Google Scholar] [CrossRef] [PubMed]
- Kong, A.N.; Owuor, E.; Yu, R.; Hebbar, V.; Chen, C.; Hu, R.; Mandlekar, S. Induction of xenobiotic enzymes by the MAP kinase pathway and the antioxidant or electrophile response element (ARE/EpRE). Drug Metab. Rev. 2001, 33, 255–271. [Google Scholar] [CrossRef]
- Ishii, T.; Itoh, K.; Takahashi, S.; Sato, H.; Yanagawa, T.; Katoh, Y.; Bannai, S.; Yamamoto, M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J. Biol. Chem. 2000, 275, 16023–16029. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Mochizuki, M.; Ishii, Y.; Ishii, T.; Shibata, T.; Kawamoto, Y.; Kelly, V.; Sekizawa, K.; Uchida, K.; Yamamoto, M. Transcription factor Nrf2 regulates inflammation by mediating the effect of 15-deoxy-Delta(12,14)-prostaglandin j(2). Mol. Cell. Biol. 2004, 24, 36–45. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, J.; Duan, H.; Li, R.; Peng, W.; Wu, C. Activation of Nrf2/HO-1 signaling: An important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress. J. Adv. Res. 2021, 34, 43–63. [Google Scholar] [CrossRef]
- Rushworth, S.A.; O’Connell, M.A. Haem oxygenase-1 in inflammation. Biochem. Soc. Trans. 2004, 32, 1093–1094. [Google Scholar] [CrossRef]
- Hamdy, M.A.; El-Maraghy, S.A.; Kortam, M.A. Modulatory effects of curcumin and green tea extract against experimentally induced pulmonary fibrosis: A comparison with N-acetyl cysteine. J. Biochem. Mol. Toxicol. 2012, 26, 461–468. [Google Scholar] [CrossRef]
- Pullikotil, P.; Chen, H.; Muniyappa, R.; Greenberg, C.C.; Yang, S.; Reiter, C.E.; Lee, J.W.; Chung, J.H.; Quon, M.J. Epigallocatechin gallate induces expression of heme oxygenase-1 in endothelial cells via p38 MAPK and Nrf-2 that suppresses proinflammatory actions of TNF-α. J. Nutr. Biochem. 2012, 23, 1134–1145. [Google Scholar] [CrossRef]
- Satoh, M.; Takemura, Y.; Hamada, H.; Sekido, Y.; Kubota, S. EGCG induces human mesothelioma cell death by inducing reactive oxygen species and autophagy. Cancer Cell. Int. 2013, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Mukhtar, H. Tea Polyphenols in Promotion of Human Health. Nutrients 2018, 11, 39. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Almatroudi, A.; Khan, A.A.; Alhumaydhi, F.A.; Alsahli, M.A.; Rahmani, A.H. Potential Therapeutic Targets of Epigallocatechin Gallate (EGCG), the Most Abundant Catechin in Green Tea, and Its Role in the Therapy of Various Types of Cancer. Molecules 2020, 25, 3146. [Google Scholar] [CrossRef]
- Romano, A.; Martel, F. The Role of EGCG in Breast Cancer Prevention and Therapy. Mini Rev. Med. Chem. 2021, 21, 883–898. [Google Scholar] [CrossRef] [PubMed]
- Cháirez-Ramírez, M.H.; de la Cruz-López, K.G.; García-Carrancá, A. Polyphenols as Antitumor Agents Targeting Key Players in Cancer-Driving Signaling Pathways. Front. Pharmacol. 2021, 12, 710304. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, E.; Bettuzzi, S.; Naponelli, V. The Potential of Epigallocatechin Gallate (EGCG) in Targeting Autophagy for Cancer Treatment: A Narrative Review. Int. J. Mol. Sci. 2022, 23, 6075. [Google Scholar] [CrossRef] [PubMed]
- Maleki Dana, P.; Sadoughi, F.; Asemi, Z.; Yousefi, B. The role of polyphenols in overcoming cancer drug resistance: A comprehensive review. Cell. Mol. Biol. Lett. 2022, 27, 1. [Google Scholar] [CrossRef] [PubMed]
- Unno, K.; Nakamura, Y. Green Tea Suppresses Brain Aging. Molecules 2021, 26, 4897. [Google Scholar] [CrossRef] [PubMed]
- Payne, A.; Nahashon, S.; Taka, E.; Adinew, G.M.; Soliman, K.F.A. Epigallocatechin-3-Gallate (EGCG): New Therapeutic Perspectives for Neuroprotection, Aging, and Neuroinflammation for the Modern Age. Biomolecules 2022, 12, 371. [Google Scholar] [CrossRef]
- Pervin, M.; Unno, K.; Ohishi, T.; Tanabe, H.; Miyoshi, N.; Nakamura, Y. Beneficial Effects of Green Tea Catechins on Neurodegenerative Diseases. Molecules 2018, 23, 1297. [Google Scholar] [CrossRef] [PubMed]
- Unno, K.; Pervin, M.; Taguchi, K.; Konishi, T.; Nakamura, Y. Green Tea Catechins Trigger Immediate-Early Genes in the Hippocampus and Prevent Cognitive Decline and Lifespan Shortening. Molecules 2020, 25, 1484. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, S.; Shimazu, T.; Ohmori, K.; Kikuchi, N.; Nakaya, N.; Nishino, Y.; Tsubono, Y.; Tsuji, I. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: The Ohsaki study. J. Am. Med. Assoc. 2006, 296, 1255–1265. [Google Scholar] [CrossRef]
- Eng, Q.Y.; Thanikachalam, P.V.; Ramamurthy, S. Molecular understanding of Epigallocatechin gallate (EGCG) in cardiovascular and metabolic diseases. J. Ethnopharmacol. 2018, 210, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K. Polyphenols Regulate Endothelial Functions and Reduce the Risk of Cardiovascular Disease. Curr. Pharm. Des. 2019, 25, 2443–2458. [Google Scholar] [CrossRef]
- Suzuki, T.; Pervin, M.; Goto, S.; Isemura, M.; Nakamura, Y. Beneficial Effects of Tea and the Green Tea Catechin Epigallocatechin-3-gallate on Obesity. Molecules 2016, 21, 1305. [Google Scholar] [CrossRef]
- Carrasco-Pozo, C.; Cires, M.J.; Gotteland, M. Quercetin and Epigallocatechin Gallate in the Prevention and Treatment of Obesity: From Molecular to Clinical Studies. J. Med. Food 2019, 22, 753–770. [Google Scholar] [CrossRef] [PubMed]
- Potenza, M.A.; Iacobazzi, D.; Sgarra, L.; Montagnani, M. The Intrinsic Virtues of EGCG, an Extremely Good Cell Guardian, on Prevention and Treatment of Diabesity Complications. Molecules 2020, 25, 3061. [Google Scholar] [CrossRef]
- Shahwan, M.; Alhumaydhi, F.; Ashraf, G.M.; Hasan, P.M.Z.; Shamsi, A. Role of polyphenols in combating Type 2 Diabetes and insulin resistance. Int. J. Biol. Macromol. 2022, 206, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Minnelli, C.; Cianfruglia, L.; Laudadio, E.; Mobbili, G.; Galeazzi, R.; Armeni, T. Effect of Epigallocatechin-3-Gallate on EGFR Signaling and Migration in Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2021, 22, 11833. [Google Scholar] [CrossRef]
- Chatterjee, S.; Bhattacharjee, B. Use of natural molecules as anti-angiogenic inhibitors for vascular endothelial growth factor receptor. Bioinformation 2012, 8, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fan, S.M.; Zhang, J. Epigallocatechin-3-gallate ameliorates lipopolysaccharide-induced acute lung injury by suppression of TLR4/NF-κB signaling activation. Braz. J. Med. Biol. Res. 2019, 52, e8092. [Google Scholar] [CrossRef]
- Bhardwaj, V.; Mandal, A.K.A. Next-Generation Sequencing Reveals the Role of Epigallocatechin-3-Gallate in Regulating Putative Novel and Known microRNAs Which Target the MAPK Pathway in Non-Small-Cell Lung Cancer A549 Cells. Molecules 2019, 24, 368. [Google Scholar] [CrossRef]
- Yang, N.; Zhang, H.; Cai, X.; Shang, Y. Epigallocatechin-3-gallate inhibits inflammation and epithelial-mesenchymal transition through the PI3K/AKT pathway via upregulation of PTEN in asthma. Int. J. Mol. Med. 2018, 41, 818–828. [Google Scholar] [CrossRef]
- Banerjee, S.; Manna, S.; Mukherjee, S.; Pal, D.; Panda, C.K.; Das, S. Black tea polyphenols restrict benzopyrene-induced mouse lung cancer progression through inhibition of Cox-2 and induction of caspase-3 expression. Asian Pac. J. Cancer Prev. 2006, 7, 661–666. [Google Scholar]
- Ranzato, E.; Martinotti, S.; Magnelli, V.; Murer, B.; Biffo, S.; Mutti, L.; Burlando, B. Epigallocatechin-3-gallate induces mesothelioma cell death via H2O2-dependent T-type Ca2+ channel opening. J. Cell. Mol. Med. 2012, 16, 2667–2678. [Google Scholar] [CrossRef]
- Chen, B.H.; Hsieh, C.H.; Tsai, S.Y.; Wang, C.Y.; Wang, C.C. Anticancer effects of epigallocatechin-3-gallate nanoemulsion on lung cancer cells through the activation of AMP-activated protein kinase signaling pathway. Sci. Rep. 2020, 10, 5163. [Google Scholar] [CrossRef]
- Rajagopal, C.; Lankadasari, M.B.; Aranjani, J.M.; Harikumar, K.B. Targeting oncogenic transcription factors by polyphenols: A novel approach for cancer therapy. Pharmacol. Res. 2018, 130, 273–291. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.Y.; Liao, J.; Li, C.; Chung, J.; Yurkow, E.J.; Ho, C.T.; Yang, C.S. Effect of black and green tea polyphenols on c-jun phosphorylation and H2O2 production in transformed and non-transformed human bronchial cell lines: Possible mechanisms of cell growth inhibition and apoptosis induction. Carcinogenesis 2000, 21, 2035–2039. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Dong, M.; Bo, L.; Li, C.; Liu, Q.; Li, Y.; Ma, L.; Xie, Y.; Fu, E.; Mu, D.; et al. Epigallocatechin-3-gallate ameliorates seawater aspiration-induced acute lung injury via regulating inflammatory cytokines and inhibiting JAK/STAT1 pathway in rats. Mediat. Inflamm. 2014, 2014, 612593. [Google Scholar] [CrossRef]
- Liu, W.; Dong, M.; Bo, L.; Li, C.; Liu, Q.; Li, Z.; Jin, F. Epigallocatechin-3-gallate suppresses alveolar epithelial cell apoptosis in seawater aspiration-induced acute lung injury via inhibiting STAT1-caspase-3/p21 associated pathway. Mol. Med. Rep. 2016, 13, 829–836. [Google Scholar] [CrossRef]
- Rawangkan, A.; Wongsirisin, P.; Namiki, K.; Iida, K.; Kobayashi, Y.; Shimizu, Y.; Fujiki, H.; Suganuma, M. Green Tea Catechin Is an Alternative Immune Checkpoint Inhibitor that Inhibits PD-L1 Expression and Lung Tumor Growth. Molecules 2018, 23, 2071. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ren, X.; Deng, C.; Yang, L.; Yan, E.; Guo, T.; Li, Y.; Xu, M.X. Mechanism of the inhibition of the STAT3 signaling pathway by EGCG. Oncol. Rep. 2013, 30, 2691–2696. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.T.; Lin, C.C.; Lee, C.Y.; Hsieh, P.W.; Yang, C.M. Protective effects of (−)-epigallocatechin-3-gallate against TNF-α-induced lung inflammation via ROS-dependent ICAM-1 inhibition. J. Nutr. Biochem. 2013, 24, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, T.; Selvaraj, M.; Poomalai, S. Epigallocatechin gallate potentially abrogates fluoride induced lung oxidative stress, inflammation via Nrf2/Keap1 signaling pathway in rats: An in-vivo and in-silico study. Int. Immunopharmacol. 2016, 39, 128–139. [Google Scholar] [CrossRef]
- Sharma, A.; Vaghasiya, K.; Ray, E.; Gupta, P.; Gupta, U.D.; Singh, A.K.; Verma, R.K. Targeted Pulmonary Delivery of the Green Tea Polyphenol Epigallocatechin Gallate Controls the Growth of Mycobacterium tuberculosis by Enhancing the Autophagy and Suppressing Bacterial Burden. ACS. Biomater. Sci. Eng. 2020, 6, 4126–4140. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.X.; Wei, F.; Li, N.; Li, J.L.; Chen, L.J.; Liu, Y.Y.; Luo, F.; Xiong, H.R.; Hou, W.; Yang, Z.Q. Amelioration of influenza virus-induced reactive oxygen species formation by epigallocatechin gallate derived from green tea. Acta Pharmacol. Sin. 2012, 33, 1533–1541. [Google Scholar] [CrossRef]
- Park, R.; Jang, M.; Park, Y.I.; Park, Y.; Jung, W.; Park, J.; Park, J. Epigallocatechin Gallate (EGCG), a Green Tea Polyphenol, Reduces Coronavirus Replication in a Mouse Model. Viruses 2021, 13, 2533. [Google Scholar] [CrossRef]
- Steinmann, J.; Buer, J.; Pietschmann, T.; Steinmann, E. Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br. J. Pharmacol. 2013, 168, 1059–1073. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Bi, K.; He, Y.; Yan, W.; Yang, C.S.; Zhang, J. Potential protective mechanisms of green tea polyphenol EGCG against COVID-19. Trends Food Sci. Technol. 2021, 114, 11–24. [Google Scholar] [CrossRef]
- Chourasia, M.; Koppula, P.R.; Battu, A.; Ouseph, M.M.; Singh, A.K. EGCG, a Green Tea Catechin, as a Potential Therapeutic Agent for Symptomatic and Asymptomatic SARS-CoV-2 Infection. Molecules 2021, 26, 1200. [Google Scholar] [CrossRef]
- Park, J.; Park, R.; Jang, M.; Park, Y.I. Therapeutic Potential of EGCG, a Green Tea Polyphenol, for Treatment of Coronavirus Diseases. Life 2021, 11, 197. [Google Scholar] [CrossRef]
- Menegazzi, M.; Campagnari, R.; Bertoldi, M.; Crupi, R.; di Paola, R.; Cuzzocrea, S. Protective Effect of Epigallocatechin-3-Gallate (EGCG) in Diseases with Uncontrolled Immune Activation: Could Such a Scenario Be Helpful to Counteract COVID-19? Int. J. Mol. Sci. 2020, 21, 5171. [Google Scholar] [CrossRef]
- Bimonte, S.; Forte, C.A.; Cuomo, M.; Esposito, G.; Cascella, M.; Cuomo, A. An Overview on the Potential Roles of EGCG in the Treatment of COVID-19 Infection. Drug Des. Dev. Ther. 2021, 15, 4447–4454. [Google Scholar] [CrossRef]
- Mendonca, P.; Soliman, K.F.A. Flavonoids Activation of the Transcription Factor Nrf2 as a Hypothesis Approach for the Prevention and Modulation of SARS-CoV-2 Infection Severity. Antioxidants 2020, 9, 659. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Henss, L.; Auste, A.; Schürmann, C.; Schmidt, C.; von Rhein, C.; Mühlebach, M.D.; Schnierle, B.S. The green tea catechin epigallocatechin gallate inhibits SARS-CoV-2 infection. J. Gen. Virol. 2021, 102, 001574. [Google Scholar] [CrossRef]
- Jang, M.; Park, R.; Park, Y.I.; Cha, Y.E.; Yamamoto, A.; Lee, J.I.; Park, J. EGCG, a green tea polyphenol, inhibits human coronavirus replication in vitro. Biochem. Biophys. Res. Commun. 2021, 547, 23–28. [Google Scholar] [CrossRef]
- Du, A.; Zheng, R.; Disoma, C.; Li, S.; Chen, Z.; Li, S.; Liu, P.; Zhou, Y.; Shen, Y.; Liu, S.; et al. Epigallocatechin-3-gallate, an active ingredient of Traditional Chinese Medicines, inhibits the 3CLpro activity of SARS-CoV-2. Int. J. Biol. Macromol. 2021, 176, 1–12. [Google Scholar] [CrossRef]
- Karkhanei, B.; Talebi Ghane, E.; Mehri, F. Evaluation of oxidative stress level: Total antioxidant capacity, total oxidant status and glutathione activity in patients with COVID-19. New Microbes New Infect. 2021, 42, 100897. [Google Scholar] [CrossRef]
- Çakırca, G.; Çakırca, T.D.; Üstünel, M.; Torun, A.; Koyuncu, İ. Thiol level and total oxidant/antioxidant status in patients with COVID-19 infection. Ir. J. Med. Sci. 2022, 191, 1925–1930. [Google Scholar] [CrossRef]
- Moghaddam, A.; Heller, R.A.; Sun, Q.; Seelig, J.; Cherkezov, A.; Seibert, L.; Hackler, J.; Seemann, P.; Diegmann, J.; Pilz, M.; et al. Selenium Deficiency Is Associated with Mortality Risk from COVID-19. Nutrients 2020, 12, 2098. [Google Scholar] [CrossRef]
- Grommes, J.; Soehnlein, O. Contribution of neutrophils to acute lung injury. Mol. Med. 2011, 17, 293–307. [Google Scholar] [CrossRef]
- Liu, Y.; Du, X.; Chen, J.; Jin, Y.; Peng, L.; Wang, H.H.X.; Luo, M.; Chen, L.; Zhao, Y. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J. Infect. 2020, 81, e6–e12. [Google Scholar] [CrossRef]
- Schönrich, G.; Raftery, M.J.; Samstag, Y. Devilishly radical NETwork in COVID-19: Oxidative stress, neutrophil extracellular traps (NETs), and T cell suppression. Adv. Biol. Regul. 2020, 77, 100741. [Google Scholar] [CrossRef]
- Janiuk, K.; Jabłońska, E.; Garley, M. Significance of NETs Formation in COVID-19. Cells 2021, 10, 151. [Google Scholar] [CrossRef]
- Araneda, O.F.; Tuesta, M. Lung oxidative damage by hypoxia. Oxidative Med. Cell. Longev. 2012, 2012, 856918. [Google Scholar] [CrossRef]
- Kumar, P.; Osahon, O.; Vides, D.B.; Hanania, N.; Minard, C.G.; Sekhar, R.V. Severe Glutathione Deficiency, Oxidative Stress and Oxidant Damage in Adults Hospitalized with COVID-19: Implications for GlyNAC (Glycine and N-Acetylcysteine) Supplementation. Antioxidants 2021, 11, 50. [Google Scholar] [CrossRef]
- Oliveira, M.R.; Nabavi, S.F.; Daglia, M.; Rastrelli, L.; Nabavi, S.M. Epigallocatechin gallate and mitochondria-A story of life and death. Pharmacol. Res. 2016, 104, 70–85. [Google Scholar] [CrossRef]
- Gibellini, L.; de Biasi, S.; Paolini, A.; Borella, R.; Boraldi, F.; Mattioli, M.; lo Tartaro, D.; Fidanza, L.; Caro-Maldonado, A.; Meschiari, M.; et al. Altered bioenergetics and mitochondrial dysfunction of monocytes in patients with COVID-19 pneumonia. EMBO Mol. Med. 2020, 12, e13001. [Google Scholar] [CrossRef]
- Köseler, A.; Sabirli, R.; Gören, T.; Türkçüer, I.; Kurt, Ö. Endoplasmic Reticulum Stress Markers in SARS-CoV-2 Infection and Pneumonia: Case-Control Study. In Vivo 2020, 34, 1645–1650. [Google Scholar] [CrossRef]
- Wan, Q.; Song, D.; Li, H.; He, M.L. Stress proteins: The biological functions in virus infection, present and challenges for target-based antiviral drug development. Signal Transduct. Target Ther. 2020, 5, 125. [Google Scholar] [CrossRef]
- Li, W.; Zhu, S.; Li, J.; Assa, A.; Jundoria, A.; Xu, J.; Fan, S.; Eissa, N.T.; Tracey, K.J.; Sama, A.E.; et al. EGCG stimulates autophagy and reduces cytoplasmic HMGB1 levels in endotoxin-stimulated macrophages. Biochem. Pharmacol. 2011, 81, 1152–1163. [Google Scholar] [CrossRef]
- Lu, B.; Antoine, D.J.; Kwan, K.; Lundbäck, P.; Wähämaa, H.; Schierbeck, H.; Robinson, M.; van Zoelen, M.A.; Yang, H.; Li, J.; et al. JAK/STAT1 signaling promotes HMGB1 hyperacetylation and nuclear translocation. Proc. Natl. Acad. Sci. USA 2014, 111, 3068–3073. [Google Scholar] [CrossRef]
- Kang, W.S.; Chung, K.H.; Chung, J.H.; Lee, J.Y.; Park, J.B.; Zhang, Y.H.; Yoo, H.S.; Yun, Y.P. Antiplatelet activity of green tea catechins is mediated by inhibition of cytoplasmic calcium increase. J. Cardiovasc. Pharmacol. 2001, 38, 875–884. [Google Scholar] [CrossRef]
- Holy, E.W.; Stämpfli, S.F.; Akhmedov, A.; Holm, N.; Camici, G.G.; Lüscher, T.F.; Tanner, F.C. Laminin receptor activation inhibits endothelial tissue factor expression. J. Mol. Cell. Cardiol. 2010, 48, 1138–1145. [Google Scholar] [CrossRef]
- Le Bagge, S.; Fotheringham, A.K.; Leung, S.S.; Forbes, J.M. Targeting the receptor for advanced glycation end products (RAGE) in type 1 diabetes. Med. Res. Rev. 2020, 40, 1200–1219. [Google Scholar] [CrossRef]
- Bierhaus, A.; Schiekofer, S.; Schwaninger, M.; Andrassy, M.; Humpert, P.M.; Chen, J.; Hong, M.; Luther, T.; Henle, T.; Klöting, I.; et al. Diabetes-associated sustained activation of the transcription factor nuclear factor-kappaB. Diabetes 2001, 50, 2792–2808. [Google Scholar] [CrossRef]
- Huang, S.M.; Chang, Y.H.; Chao, Y.C.; Lin, J.A.; Wu, C.H.; Lai, C.Y.; Chan, K.C.; Tseng, S.T.; Yen, G.C. EGCG-rich green tea extract stimulates sRAGE secretion to inhibit S100A12-RAGE axis through ADAM10-mediated ectodomain shedding of extracellular RAGE in type 2 diabetes. Mol. Nutr. Food Res. 2013, 57, 2264–2268. [Google Scholar] [CrossRef]
- Choi, Y.S.; Bae, C.H.; Song, S.Y.; Kim, Y.D. The effect of Epigallocatechin-3-gallate in allergic airway inflammation. Rhinology 2014, 52, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Shang, Y.X. Epigallocatechin gallate ameliorates airway inflammation by regulating Treg/Th17 imbalance in an asthmatic mouse model. Int. Immunopharmacol. 2019, 72, 422–428. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, H.J.; Lee, C.M.; Choi, I.W.; Moon, D.O.; Roh, H.J.; Lee, H.K.; Park, Y.M. Epigallocatechin-3-gallate protects toluene diisocyanate-induced airway inflammation in a murine model of asthma. FEBS Lett. 2006, 580, 1883–1890. [Google Scholar] [CrossRef]
- Yang, N.; Li, X. Epigallocatechin gallate relieves asthmatic symptoms in mice by suppressing HIF-1α/VEGFA-mediated M2 skewing of macrophages. Biochem. Pharmacol. 2022, 202, 115112. [Google Scholar] [CrossRef]
- Sriram, N.; Kalayarasan, S.; Sudhandiran, G. Enhancement of antioxidant defense system by epigallocatechin-3-gallate during bleomycin induced experimental pulmonary fibrosis. Biol. Pharm. Bull. 2008, 31, 1306–1311. [Google Scholar] [CrossRef]
- Sriram, N.; Kalayarasan, S.; Manikandan, R.; Arumugam, M.; Sudhandiran, G. Epigallocatechin gallate attenuates fibroblast proliferation and excessive collagen production by effectively intervening TGF-β1 signalling. Clin. Exp. Pharmacol. Physiol. 2015, 42, 849–859. [Google Scholar] [CrossRef]
- Kim, H.R.; Park, B.K.; Oh, Y.M.; Lee, Y.S.; Lee, D.S.; Kim, H.K.; Kim, J.Y.; Shim, T.S.; Lee, S.D. Green tea extract inhibits paraquat-induced pulmonary fibrosis by suppression of oxidative stress and endothelin-l expression. Lung 2006, 184, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.-J.; Ma, Q.-Q.; Shen, W.-W.; Li, L.-C.; Hu, D. Nano-enabled delivery of EGCG ameliorates silica-induced pulmonary fibrosis in rats. Toxicology 2022, 469, 153114. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Chen, F.; Chen, N.; Wang, J.; Li, Z.; Deng, X. Effect of EGCG on bronchial epithelial cell premalignant lesions induced by cigarette smoke and on its CYP1A1 expression. Int. J. Mol. Med. 2021, 48, 220. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.B.; Rodríguez-Roisin, R. Chronic obstructive pulmonary disease overview: Epidemiology, risk factors, and clinical presentation. Proc. Am. Thorac. Soc. 2011, 8, 363–367. [Google Scholar] [CrossRef]
- Salvi, S. Tobacco smoking and environmental risk factors for chronic obstructive pulmonary disease. Clin. Chest Med. 2014, 35, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, S.P.; Reddy, A.T.; Kodidhela, L.D.; Varadacharyulu, N.C. Epigallocatechin gallate diminishes cigarette smoke-induced oxidative stress, lipid peroxidation, and inflammation in human bronchial epithelial cells. Life Sci. 2020, 259, 118260. [Google Scholar] [CrossRef]
- Oh, C.M.; Oh, I.H.; Choe, B.K.; Yoon, T.Y.; Choi, J.M.; Hwang, J. Consuming Green Tea at Least Twice Each Day Is Associated with Reduced Odds of Chronic Obstructive Lung Disease in Middle-Aged and Older Korean Adults. J. Nutr. 2018, 148, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.C. Pulmonary fibrosis, part I: Epidemiology, pathogenesis, and diagnosis. Expert. Rev. Respir. Med. 2017, 11, 343–359. [Google Scholar] [CrossRef] [PubMed]
- Michalski, J.E.; Kurche, J.S.; Schwartz, D.A. From ARDS to pulmonary fibrosis: The next phase of the COVID-19 pandemic? Transl. Res. 2022, 241, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Sodji, Q.H.; Oyelere, A.K. Inflammation, Fibrosis and Cancer: Mechanisms, Therapeutic Options and Challenges. Cancers 2022, 14, 552. [Google Scholar] [CrossRef]
- Liu, G.; Philp, A.M.; Corte, T.; Travis, M.A.; Schilter, H.; Hansbro, N.G.; Burns, C.J.; Eapen, M.S.; Sohal, S.S.; Burgess, J.K.; et al. Therapeutic targets in lung tissue remodelling and fibrosis. Pharmacol. Ther. 2021, 225, 107839. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Dong, W.; Jackson, J.; Ho, T.C.; le Saux, C.J.; Brumwell, A.; Li, X.; Klesney-Tait, J.; Cohen, M.L.; Wolters, P.J.; et al. Blocking LOXL2 and TGFβ1 signalling induces collagen I turnover in precision-cut lung slices derived from patients with idiopathic pulmonary fibrosis. Thorax 2021, 76, 729–732. [Google Scholar] [CrossRef] [PubMed]
- Bellaye, P.S.; Burgy, O.; Bonniaud, P.; Kolb, M. HSP47: A potential target for fibrotic diseases and implications for therapy. Expert. Opin. Ther. Targets 2021, 25, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Mukae, H.; Kakugawa, T.; Iwashita, T.; Kaida, H.; Fujii, T.; Hayashi, T.; Kadota, J.; Kohno, S. Increased expression of collagen-binding heat shock protein 47 in murine bleomycin-induced pneumopathy. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003, 285, L957–L963. [Google Scholar] [CrossRef]
- Kakugawa, T.; Mukae, H.; Hishikawa, Y.; Ishii, H.; Sakamoto, N.; Ishimatsu, Y.; Fujii, T.; Koji, T.; Kohno, S. Localization of HSP47 mRNA in murine bleomycin-induced pulmonary fibrosis. Virchows Arch. 2010, 456, 309–315. [Google Scholar] [CrossRef]
- Iwashita, T.; Kadota, J.; Naito, S.; Kaida, H.; Ishimatsu, Y.; Miyazaki, M.; Ozono, Y.; Kohno, S. Involvement of collagen-binding heat shock protein 47 and procollagen type I synthesis in idiopathic pulmonary fibrosis: Contribution of type II pneumocytes to fibrosis. Hum. Pathol. 2000, 31, 1498–1505. [Google Scholar] [CrossRef] [PubMed]
- Okuno, D.; Sakamoto, N.; Tagod, M.S.O.; Akiyama, Y.; Moriyama, S.; Miyamura, T.; Hara, A.; Kido, T.; Ishimoto, H.; Ishimatsu, Y.; et al. Screening of Inhibitors Targeting Heat Shock Protein 47 Involved in the Development of Idiopathic Pulmonary Fibrosis. ChemMedChem 2021, 16, 2515–2523. [Google Scholar] [CrossRef] [PubMed]
- Adamcakova, J.; Balentova, S.; Hanusrichterova, J.; Mikolka, P.; Adamkov, M.; Kalenska, D.; Kunertova, L.; Mokra, D. Influence of natural polyphenol epigallocatechin-gallate (EGCG) on several markers of inflammation and histopathological changes in a model of lung silicosis in rats. In New Trends and Perspectives in Histology [Electronic Document]: No. 8, 1st ed.; Kovalska, M., Adamkov, M., Eds.; Jessenius Faculty of Medicine Comenius University: Martin, Slovakia, 2022; pp. 13–17. ISBN 978-80-8187-120-7. (In Slovakian) [Google Scholar]
- Schabath, M.B.; Cote, M.L. Cancer Progress and Priorities: Lung Cancer. Cancer Epidemiol. Biomarkers Prev. 2019, 28, 1563–1579. [Google Scholar] [CrossRef] [PubMed]
- Helfinger, V.; Schröder, K. Redox control in cancer development and progression. Mol. Asp. Med. 2018, 63, 88–98. [Google Scholar] [CrossRef]
- Milligan, S.A.; Burke, P.; Coleman, D.T.; Bigelow, R.L.; Steffan, J.J.; Carroll, J.L.; Williams, B.J.; Cardelli, J.A. The green tea polyphenol EGCG potentiates the antiproliferative activity of c-Met and epidermal growth factor receptor inhibitors in non-small cell lung cancer cells. Clin. Cancer Res. 2009, 15, 4885–4894. [Google Scholar] [CrossRef]
- Christensen, J.G.; Burrows, J.; Salgia, R. C-Met as a target for human cancer and characterization of inhibitors for therapeutic intervention. Cancer Lett. 2005, 225, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Sequist, L.V.; Bell, D.W.; Lynch, T.J.; Haber, D.A. Molecular predictors of response to epidermal growth factor receptor antagonists in non-small-cell lung cancer. J. Clin. Oncol. 2007, 25, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Lambert, J.D.; Chin, K.V.; Yang, C.S. Effects of tea polyphenols on signal transduction pathways related to cancer chemoprevention. Mutat. Res. 2004, 555, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Wu, X.; Wang, X.; Huang, W.; Feng, Q. NEAT1 upregulates EGCG-induced CTR1 to enhance cisplatin sensitivity in lung cancer cells. Oncotarget 2016, 7, 43337–43351. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Jiang, P.; Zeb, F.; Wu, X.; Xu, C.; Chen, L.; Feng, Q. EGCG regulates CTR1 expression through its pro-oxidative property in non-small-cell lung cancer cells. J. Cell. Physiol. 2020, 235, 7970–7981. [Google Scholar] [CrossRef]
- Zhou, D.H.; Wang, X.; Yang, M.; Shi, X.; Huang, W.; Feng, Q. Combination of low concentration of (−)-epigallocatechin gallate (EGCG) and curcumin strongly suppresses the growth of non-small cell lung cancer in vitro and in vivo through causing cell cycle arrest. Int. J. Mol. Sci. 2013, 14, 12023–12036. [Google Scholar] [CrossRef]
- Wang, P.; Heber, D.; Henning, S.M. Quercetin increased bioavailability and decreased methylation of green tea polyphenols in vitro and in vivo. Food Funct. 2012, 3, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Leone, M.; Zhai, D.; Sareth, S.; Kitada, S.; Reed, J.C.; Pellecchia, M. Cancer prevention by tea polyphenols is linked to their direct inhibition of antiapoptotic Bcl-2-family proteins. Cancer Res. 2003, 63, 8118–8121. [Google Scholar] [PubMed]
- Li, M.; Li, J.J.; Gu, Q.H.; An, J.; Cao, L.M.; Yang, H.P.; Hu, C.P. EGCG induces lung cancer A549 cell apoptosis by regulating Ku70 acetylation. Oncol. Rep. 2016, 35, 2339–2347. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, S.; Shi, Y.; Li, C.H.; Wang, X.J.; Li, F.J.; Wang, C.H.; Meng, Q.H.; Zhong, J.N.; Liu, M.; et al. Epigallocatechin gallate from green tea exhibits potent anticancer effects in A-549 non-small lung cancer cells by inducing apoptosis, cell cycle arrest and inhibition of cell migration. J. BUON 2017, 22, 1422–1427. [Google Scholar]
- Zhu, J.; Jiang, Y.; Yang, X.; Wang, S.; Xie, C.; Li, X.; Li, Y.; Chen, Y.; Wang, X.; Meng, Y.; et al. Wnt/β-catenin pathway mediates (−)-Epigallocatechin-3-gallate (EGCG) inhibition of lung cancer stem cells. Biochem. Biophys. Res. Commun. 2017, 482, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Gupta, S.; Mukhtar, H. Green tea polyphenol epigallocatechin-3-gallate differentially modulates nuclear factor kappaB in cancer cells versus normal cells. Arch. Biochem. Biophys. 2000, 376, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xie, J.; Gan, R.; Wu, Z.; Luo, H.; Chen, X.; Lu, Y.; Wu, L.; Zheng, D. Synergistic inhibition of lung cancer cells by EGCG and NF-κB inhibitor BAY11-7082. J. Cancer 2019, 10, 6543–6556. [Google Scholar] [CrossRef]
- Syed, D.N.; Afaq, F.; Kweon, M.H.; Hadi, N.; Bhatia, N.; Spiegelman, V.S.; Mukhtar, H. Green tea polyphenol EGCG suppresses cigarette smoke condensate-induced NF-kappaB activation in normal human bronchial epithelial cells. Oncogene 2007, 26, 673–682. [Google Scholar] [CrossRef]
- Li, G.X.; Chen, Y.K.; Hou, Z.; Xiao, H.; Jin, H.; Lu, G.; Lee, M.J.; Liu, B.; Guan, F.; Yang, Z.; et al. Pro-oxidative activities and dose-response relationship of (−)-epigallocatechin-3-gallate in the inhibition of lung cancer cell growth: A comparative study in vivo and in vitro. Carcinogenesis 2010, 31, 902–910. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, W.; Tu, G.; Chen, X.; Lu, Y.; Wu, L.; Zheng, D. Enhanced Chemotherapeutic Efficacy of PLGA-Encapsulated Epigallocatechin Gallate (EGCG) Against Human Lung Cancer. Int. J. Nanomed. 2020, 15, 4417–4429. [Google Scholar] [CrossRef]
- Zhu, W.; Zhao, Y.; Zhang, S.; Li, X.; Xing, L.; Zhao, H.; Yu, J. Evaluation of Epigallocatechin-3-Gallate as a Radioprotective Agent During Radiotherapy of Lung Cancer Patients: A 5-Year Survival Analysis of a Phase 2 Study. Front. Oncol. 2021, 11, 686950. [Google Scholar] [CrossRef] [PubMed]
- Mandras, S.A.; Mehta, H.S.; Vaidya, A. Pulmonary Hypertension: A Brief Guide for Clinicians. Mayo Clin. Proc. 2020, 95, 1978–1988. [Google Scholar] [CrossRef]
- Zhu, T.T.; Zhang, W.F.; Luo, P.; He, F.; Ge, X.Y.; Zhang, Z.; Hu, C.P. Epigallocatechin-3-gallate ameliorates hypoxia-induced pulmonary vascular remodeling by promoting mitofusin-2-mediated mitochondrial fusion. Eur. J. Pharmacol. 2017, 809, 42–51. [Google Scholar] [CrossRef]
- Chowdhury, A.; Nandy, S.K.; Sarkar, J.; Chakraborti, T.; Chakraborti, S. Inhibition of pro-/active MMP-2 by green tea catechins and prediction of their interaction by molecular docking studies. Mol. Cell. Biochem. 2017, 427, 111–122. [Google Scholar] [CrossRef]
- Chelladurai, P.; Seeger, W.; Pullamsetti, S.S. Matrix metalloproteinases and their inhibitors in pulmonary hypertension. Eur. Respir. J. 2012, 40, 766–782. [Google Scholar] [CrossRef]
- Kang, W.S.; Lim, I.H.; Yuk, D.Y.; Chung, K.H.; Park, J.B.; Yoo, H.S.; Yun, Y.P. Antithrombotic activities of green tea catechins and (−)-epigallocatechin gallate. Thromb. Res. 1999, 96, 229–237. [Google Scholar] [CrossRef]
- Rietveld, A.; Wiseman, S. Antioxidant effects of tea: Evidence from human clinical trials. J. Nutr. 2003, 133, 3285S–3292S. [Google Scholar] [CrossRef]
- Munin, A.; Edwards-Lévy, F. Encapsulation of natural polyphenolic compounds: A review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef]
- Lambert, J.D.; Sang, S.; Yang, C.S. Biotransformation of green tea polyphenols and the biological activities of those metabolites. Mol. Pharm. 2007, 4, 819–825. [Google Scholar] [CrossRef]
- Timmel, M.A.; Byl, J.A.; Osheroff, N. Epimerization of green tea catechins during brewing does not affect the ability to poison human type II topoisomerases. Chem. Res. Toxicol. 2013, 26, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhou, W.; Jiang, X. Reaction kinetics of degradation and epimerization of epigallocatechin gallate (EGCG) in aqueous system over a wide temperature range. J. Agric. Food Chem. 2008, 56, 2694–2701. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Teng, C.; Min, Q. Advanced Nanovehicles-Enabled Delivery Systems of Epigallocatechin Gallate for Cancer Therapy. Front. Chem. 2020, 8, 573297. [Google Scholar] [CrossRef] [PubMed]
- Minnelli, C.; Moretti, P.; Laudadio, E.; Gerelli, Y.; Pigozzo, A.; Armeni, T.; Galeazzi, R.; Mariani, P.; Mobbili, G. Tuning curvature and phase behavior of monoolein bilayers by epigallocatechin-3-gallate: Structural insight and cytotoxicity. Colloids Surf. B Biointerfaces 2022, 209, 112171. [Google Scholar] [CrossRef]
- Chavva, S.R.; Deshmukh, S.K.; Kanchanapally, R.; Tyagi, N.; Coym, J.W.; Singh, A.P.; Singh, S. Epigallocatechin Gallate-Gold Nanoparticles Exhibit Superior Antitumor Activity Compared to Conventional Gold Nanoparticles: Potential Synergistic Interactions. Nanomaterials 2019, 9, 396. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Yao, J.; Xue, J.; Li, R.; Bao, B.; Jiang, L.; Zhu, J.J.; He, Z. Tumor-Homing Cell-Penetrating Peptide Linked to Colloidal Mesoporous Silica Encapsulated (−)-Epigallocatechin-3-gallate as Drug Delivery System for Breast Cancer Therapy in Vivo. ACS Appl. Mater. Interfaces 2015, 7, 18145–18155. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Kulhari, H.; Pooja, D.; Gudem, S.; Bhargava, S.; Shukla, R.; Sistla, R. Encapsulation of biophenolic phytochemical EGCG within lipid nanoparticles enhances its stability and cytotoxicity against cancer. Chem. Phys. Lipids 2016, 198, 51–60. [Google Scholar] [CrossRef]
- Dahiya, S.; Rani, R.; Kumar, S.; Dhingra, D.; Dilbaghi, N. Chitosan-gellan gum bipolymeric nanohydrogels-a potential nanocarrier for the delivery of epigallocatechin gallate. BioNanoScience 2017, 7, 508–520. [Google Scholar] [CrossRef]
- Yang, R.; Liu, Y.; Gao, Y.; Wang, Y.; Blanchard, C.; Zhou, Z. Ferritin glycosylated by chitosan as a novel EGCG nano-carrier: Structure, stability, and absorption analysis. Int. J. Biol. Macromol. 2017, 105, 252–261. [Google Scholar] [CrossRef]
- Minnelli, C.; Galeazz, I.R.; Laudadio, E.; Amici, A.; Rusciano, D.; Armeni, T.; Cantarini, M.; Stipa, P.; Mobbili, G. Monoalkylated Epigallocatechin-3-gallate (C18-EGCG) as Novel Lipophilic EGCG Derivative: Characterization and Antioxidant Evaluation. Antioxidants 2020, 9, 208. [Google Scholar] [CrossRef]
- Landis-Piwowar, K.R.; Kuhn, D.J.; Wan, S.B.; Chen, D.; Chan, T.H.; Dou, Q.P. Evaluation of proteasome-inhibitory and apoptosis-inducing potencies of novel (−)-EGCG analogs and their prodrugs. Int. J. Mol. Med. 2005, 15, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Miyake, S.; Kobe, T.; Nakaya, T.; Fuller, S.D.; Kato, N.; Kaihatsu, K. Enhanced anti-influenza A virus activity of (−)-epigallocatechin-3-O-gallate fatty acid monoester derivatives: Effect of alkyl chain length. Bioorg. Med. Chem. Lett. 2008, 18, 4249–4252. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yan, W. Lipophilization of EGCG and effects on antioxidant activities. Food Chem. 2019, 272, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Park, K.D.; Cho, S.J. Synthesis and antimicrobial activities of 3-O-alkyl analogues of (+)-catechin: Improvement of stability and proposed action mechanism. Eur. J. Med. Chem. 2010, 45, 1028–1033. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Hu, J.M.; Huang, Y.W.; Wu, X.Y.; Zi, C.T.; Wang, X.J.; Sheng, J. Synthesis and Biological Testing of Novel Glucosylated Epigallocatechin Gallate (EGCG) Derivatives. Molecules 2016, 21, 620. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, X.J.; Su, F.W.; Xie, Y.R.; Wang, L.X.; Zhang, N.; Wu, Y.L.; Niu, Y.; Zhang, D.Y.; Zi, C.T.; et al. Novel Perbutyrylated Glucose Derivatives of (−)-Epigallocatechin-3-Gallate Inhibit Cancer Cells Proliferation by Decreasing Phosphorylation of the EGFR: Synthesis, Cytotoxicity, and Molecular Docking. Molecules 2021, 26, 4361. [Google Scholar] [CrossRef]
- Peters, C.M.; Green, R.J.; Janle, E.M.; Ferruzzi, M.G. Formulation with ascorbic acid and sucrose modulates catechin bioavailability from green tea. Food Res. Int. 2010, 43, 95–102. [Google Scholar] [CrossRef]
- Tao, L.; Forester, S.C.; Lambert, J.D. The role of the mitochondrial oxidative stress in the cytotoxic effects of the green tea catechin, (−)-epigallocatechin-3-gallate, in oral cells. Mol. Nutr. Food Res. 2014, 58, 665–676. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, P.; Ling, T.; Wang, Y.; Dong, R.; Zhang, C.; Zhang, L.; Han, M.; Wang, D.; Wan, X.; et al. Certain (−)-epigallocatechin-3-gallate (EGCG) auto-oxidation products (EAOPs) retain the cytotoxic activities of EGCG. Food Chem. 2016, 204, 218–226. [Google Scholar] [CrossRef]
- Sun, W.; Liu, X.; Zhang, H.; Song, Y.; Li, T.; Liu, X.; Liu, Y.; Guo, L.; Wang, F.; Yang, T.; et al. Epigallocatechin gallate upregulates NRF2 to prevent diabetic nephropathy via disabling KEAP1. Free Radic. Biol. Med. 2017, 108, 840–857. [Google Scholar] [CrossRef]
- Yang, C.S.; Zhang, J. Studies on the Prevention of Cancer and Cardiometabolic Diseases by Tea: Issues on Mechanisms, Effective Doses, and Toxicities. J. Agric. Food Chem. 2019, 67, 5446–5456. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R.; Genthner, A.; Wolff, A.; Frenzel, C.; Schulze, J.; Eickhoff, A. Herbal hepatotoxicity: Analysis of cases with initially reported positive re-exposure tests. Dig. Liver Dis. 2014, 46, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R.; Zhang, L.; Melzer, L.; Schulze, J.; Eickhoff, A. Green tea extract and the risk of drug-induced liver injury. Expert. Opin. Drug Metab. Toxicol. 2014, 10, 1663–1676. [Google Scholar] [CrossRef]
- Yates, A.A.; Erdman, J.W., Jr.; Shao, A.; Dolan, L.C.; Griffiths, J.C. Bioactive nutrients—Time for tolerable upper intake levels to address safety. Regul. Toxicol. Pharmacol. 2017, 84, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Wang, H.; Sheridan, Z.P. Studies on prevention of obesity, metabolic syndrome, diabetes, cardiovascular diseases and cancer by tea. J. Food Drug Anal. 2018, 26, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Samavat, H.; Dostal, A.M.; Wang, R.; Torkelson, C.J.; Yang, C.S.; Butler, L.M.; Kensler, T.W.; Wu, A.H.; Kurzer, M.S.; et al. Effect of Green Tea Supplements on Liver Enzyme Elevation: Results from a Randomized Intervention Study in the United States. Cancer Prev. Res. 2017, 10, 571–579. [Google Scholar] [CrossRef]
- Dostal, A.M.; Samavat, H.; Bedell, S.; Torkelson, C.; Wang, R.; Swenson, K.; Le, C.; Wu, A.H.; Ursin, G.; Yuan, J.M.; et al. The safety of green tea extract supplementation in postmenopausal women at risk for breast cancer: Results of the Minnesota Green Tea Trial. Food Chem. Toxicol. 2015, 83, 26–35. [Google Scholar] [CrossRef]
- Church, R.J.; Gatti, D.M.; Urban, T.J.; Long, N.; Yang, X.; Shi, Q.; Eaddy, J.S.; Mosedale, M.; Ballard, S.; Churchill, G.A.; et al. Sensitivity to hepatotoxicity due to epigallocatechin gallate is affected by genetic background in diversity outbred mice. Food Chem. Toxicol. 2015, 76, 19–26. [Google Scholar] [CrossRef]
- Inoue-Choi, M.; Yuan, J.M.; Yang, C.S.; van den Berg, D.J.; Lee, M.J.; Gao, Y.T.; Yu, M.C. Genetic Association Between the COMT Genotype and Urinary Levels of Tea Polyphenols and Their Metabolites among Daily Green Tea Drinkers. Int. J. Mol. Epidemiol. Genet. 2010, 1, 114–123. [Google Scholar]
- Zhang, K.; Dong, R.; Sun, K.; Wang, X.; Wang, J.; Yang, C.S.; Zhang, J. Synergistic toxicity of epigallocatechin-3-gallate and diethyldithiocarbamate, a lethal encounter involving redox-active copper. Free Radic. Biol. Med. 2017, 113, 143–156. [Google Scholar] [CrossRef]
- Kaleri, N.A.; Sun, K.; Wang, L.; Li, J.; Zhang, W.; Chen, X.; Li, X. Dietary Copper Reduces the Hepatotoxicity of (−)-epigallocatechin-3-Gallate in Mice. Molecules 2017, 23, 38. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wei, Y.; Wang, T.; Wan, X.; Yang, C.S.; Reiter, R.J.; Zhang, J. Melatonin attenuates (−)-epigallocatehin-3-gallate-triggered hepatotoxicity without compromising its downregulation of hepatic gluconeogenic and lipogenic genes in mice. J. Pineal Res. 2015, 59, 497–507. [Google Scholar] [CrossRef]
- James, K.D.; Forester, S.C.; Lambert, J.D. Dietary pretreatment with green tea polyphenol, (−)-epigallocatechin-3-gallate reduces the bioavailability and hepatotoxicity of subsequent oral bolus doses of (−)-epigallocatechin-3-gallate. Food Chem. Toxicol. 2015, 76, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Veerman, G.D.M.; van der Werff, S.C.; Koolen, S.L.W.; Miedema, J.R.; Oomen-de Hoop, E.; van der Mark, S.C.; Chandoesing, P.P.; de Bruijn, P.; Wijsenbeek, M.S.; Mathijssen, R.H.J. The influence of green tea extract on nintedanib’s bioavailability in patients with pulmonary fibrosis. Biomed. Pharmacother. 2022, 151, 113101. [Google Scholar] [CrossRef] [PubMed]
- Sadzuka, Y.; Sugiyama, T.; Hirota, S. Modulation of cancer chemotherapy by green tea. Clin. Cancer Res. 1998, 4, 153–156. [Google Scholar] [PubMed]



| Targets | Modulation by EGCG | Biological Effects |
|---|---|---|
| Cell surface receptors | ||
| EGFR VEGFR | Inhibition inhibition | inhibited proliferation of lung non-small cancer cells [134] anti-angiogenic action [135] |
| TLR4 | inhibition | anti-inflammatory action [136] |
| SARS-CoV-2 spike receptor binding domain ACE2 | inhibition | inhibition of SARS-CoV-2 from entering into cells [40] |
| Intracellular signaling pathways | ||
| MAPK | inhibition | anti-inflammatory and anti-tumorous action [39,137] |
| PI3K/Akt/eNOS | inhibition | vasorelaxation, anti-inflammatory and anti-tumorous action [138] |
| COX-2 | inhibition | anti-inflammatory and anti-tumorous action [139] |
| Cytosolic calcium | elevation | various biological actions including induction of apoptosis [140] |
| AMPK | activation | anti-tumorous action [141] |
| Nuclear transcription factors | ||
| NF-κB | inhibition | anti-inflammatory action, anti-oxidant action, inhibited proliferation of cancer cells [142] |
| AP-1 | inhibition | anti-inflammatory action, inhibition of cell growth [71,143] |
| Nrf2/HO-1 | activation | anti-oxidant action, anti-inflammatory action [106] |
| STAT1 | inhibition | inhibited apoptosis of lung epithelial cells, anti-inflammatory and anti-tumorous action [144,145,146] |
| STAT3 | inhibition | induction of apoptosis and anti-proliferative effect, anti-inflammatory action [147,148] |
| Animal Model | Species | EGCG Dose/Way of Delivery | Major Findings | Study |
|---|---|---|---|---|
| LPS-induced ALI | BAL/c mice | EGCG 10 mg/kg i.p., given 1 h before LPS (10 mg/kg i.p.) | ↓ inflammation, ↓ injury, ↑ gas exchange | [136] |
| LPS-induced ALI | C57BL/6 mice | EGCG 15 mg/kg i.p., given 1 h before and 3 h after LPS (2 mg/kg i.t.) | ↓ inflammation, ↓ oxidation markers, ↓ lung injury and ↑ regeneration capacity | [98] |
| LPS-induced ALI | BALB/c mice | EGCG 10 mg/kg i.p., given 1 h before LPS (5 mg/kg i.t.) | ↓ inflammation, ↓ lung edema, ↓ MPO and PK Cα | [39] |
| Fluoride-induced ALI | Wistar rats | EGCG (40 mg/kg) administered 90 min before oral fluoride, given for 4 weeks | ↓ markers of oxidative stress, ↑ antioxidants, ↓ inflammation | [149] |
| Pseudomonas aeruginosa-induced pneumonia | ICR mice | EGCG 20, 40 or 80 mg/kg i.g., P. aeruginosa instillation (2.5 × 108 CFU i.t.) | ↓ inflammation, ↓ lung injury, ↓ P. aeruginosa load and virulence | [41] |
| Mycobacterium tuberculosis-induced pneumonia | BAL/c mice | Encapsulated EGCG (10, 20 and 50 mg) given by inhalation or EGCG (2.5 mg) by oral gavage, given 4 weeks after inoculation (2.8 × 106 CFU/mL i.t.) | ↓ inflammation, ↓ bacterial burden | [150] |
| Influenza A-induced pneumonia | BAL/c mice | EGCG (10, 20 or 40 mg/kg/d, p.o.) for 5 d, influenza A infection on 3rd day of EGCG | ↑ survival, ↓ inflammation, ↓ virus yields, ↓ ROS | [151] |
| SARS-CoV-2-induced pneumonia | C57BL/6 mice | EGCG 10 mg/kg daily p.o. for 14 days, given after infection with 10 μL of HCoV-OC43 virus (107 PFU/mL) i.n. | ↓ viral replication | [152] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokra, D.; Adamcakova, J.; Mokry, J. Green Tea Polyphenol (-)-Epigallocatechin-3-Gallate (EGCG): A Time for a New Player in the Treatment of Respiratory Diseases? Antioxidants 2022, 11, 1566. https://doi.org/10.3390/antiox11081566
Mokra D, Adamcakova J, Mokry J. Green Tea Polyphenol (-)-Epigallocatechin-3-Gallate (EGCG): A Time for a New Player in the Treatment of Respiratory Diseases? Antioxidants. 2022; 11(8):1566. https://doi.org/10.3390/antiox11081566
Chicago/Turabian StyleMokra, Daniela, Jana Adamcakova, and Juraj Mokry. 2022. "Green Tea Polyphenol (-)-Epigallocatechin-3-Gallate (EGCG): A Time for a New Player in the Treatment of Respiratory Diseases?" Antioxidants 11, no. 8: 1566. https://doi.org/10.3390/antiox11081566