Effects of Sex on the Susceptibility for Atrial Fibrillation in Pigs with Ischemic Heart Failure
Abstract
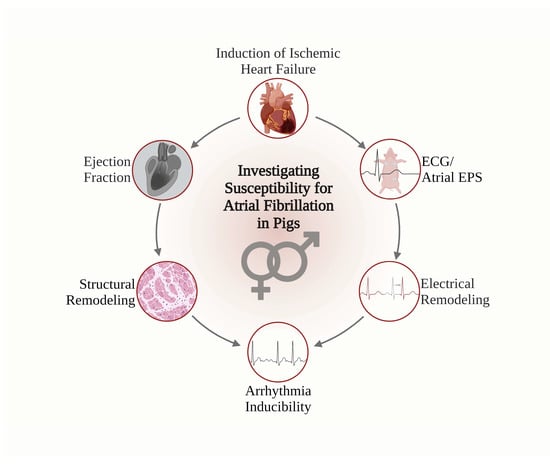
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Anesthesia and Surgical Preparation
2.3. Assessment of the Ejection Fraction
2.4. 12-Lead Electrocardiogram and Electrophysiological Study
2.5. Assessment of Structural Remodeling
2.6. Statistical Analysis
3. Results
3.1. Induction of Ischemic Heart Failure
3.2. ECG
3.3. Arrhythmia Inducibility
3.4. Electrophysiological Studies (EPS)
3.5. Assessment of Structural Remodeling
4. Discussion
5. Limitations and Outlook
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lippi, G.; Sanchis-Gomar, F.; Cervellin, G. Global Epidemiology of Atrial Fibrillation: An Increasing Epidemic and Public Health Challenge. Int. J. Stroke 2021, 16, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Jabre, P.; Jouven, X.; Adnet, F.; Thabut, G.; Bielinski, S.J.; Weston, S.A.; Roger, V.L. Atrial Fibrillation and Death After Myocardial Infarction: A Community Study. Circulation 2011, 123, 2094–2100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, R.E.; Rush, K.L.; Reid, R.C.; Laberge, C.G. Gender and the Symptom Experience Before an Atrial Fibrillation Diagnosis. West. J. Nurs. Res. 2021, 43, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Melloni, C.; Berger, J.S.; Wang, T.Y.; Gunes, F.; Stebbins, A.; Pieper, K.S.; Dolor, R.J.; Douglas, P.S.; Mark, D.B.; Newby, L.K. Representation of Women in Randomized Clinical Trials of Cardiovascular Disease Prevention. Circ. Cardiovasc. Qual. Outcomes 2010, 3, 135–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuehnemund, L.; Koeppe, J.; Feld, J.; Wiederhold, A.; Illner, J.; Makowski, L.; Gerß, J.; Reinecke, H.; Freisinger, E. Gender Differences in Acute Myocardial Infarction-A Nationwide German Real-Life Analysis from 2014 to 2017. Clin. Cardiol. 2021, 44, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Alipour, P.; Azizi, Z.; Norris, C.M.; Raparelli, V.; McMurtry, M.S.; Macle, L.; Andrade, J.; Pilote, L. Representation of Women in Atrial Fibrillation Clinical Practice Guidelines. Can. J. Cardiol. 2022, 38, 729–735. [Google Scholar] [CrossRef]
- Regitz-Zagrosek, V.; Gebhard, C. Gender medicine: Effects of Sex and Gender on Cardiovascular Disease Manifestation and Outcomes. Nat. Rev. Cardiol. 2022, 20, 236–247. [Google Scholar] [CrossRef]
- Westerman, S.; Wenger, N. Gender Differences in Atrial Fibrillation: A Review of Epidemiology, Management, and Outcomes. Curr. Cardiol. Rev. 2019, 15, 136–144. [Google Scholar] [CrossRef] [PubMed]
- König, S.; Ueberham, L.; Schuler, E.; Wiedemann, M.; Reithmann, C.; Seyfarth, M.; Sause, A.; Tebbenjohanns, J.; Schade, A.; Shin, D.-I.; et al. In-Hospital Mortality of Patients with Atrial Arrhythmias: Insights from the German-Wide Helios Hospital Network of 161 502 Patients and 34 025 Arrhythmia-Related Procedures. Eur. Heart J. 2018, 39, 3947–3957. [Google Scholar] [CrossRef] [Green Version]
- Patten, R.D. Models of Gender Differences in Cardiovascular Disease. Drug Discov. Today. Dis. Model. 2007, 4, 227–232. [Google Scholar] [CrossRef] [Green Version]
- Chang, D.H.; Dumanski, S.M.; Ahmed, S.B. Female Sex-Specific Considerations to Improve Rigor and Reproducibility in Cardiovascular Research. Am. J. Physiol.-Heart Circ. Physiol. 2023, 324, H279–H287. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, F.D.; Motazedian, P.; Jung, R.G.; Santo, P.D.; MacDonald, Z.; Simard, T.; Clancy, A.A.; Russo, J.J.; Welch, V.; Wells, G.A.; et al. Sex Bias Is Increasingly Prevalent in Preclinical Cardiovascular Research: Implications for Translational Medicine and Health Equity for Women. Circulation 2017, 135, 625–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clauss, S.; Schüttler, D.; Bleyer, C.; Vlcek, J.; Shakarami, M.; Tomsits, P.; Schneider, S.; Maderspacher, F.; Chataut, K.; Trebo, A.; et al. Characterization of a Porcine Model of Atrial Arrhythmogenicity in the Context of Ischaemic Heart Failure. PLoS ONE 2020, 15, e0232374. [Google Scholar] [CrossRef] [PubMed]
- Schüttler, D.; Tomsits, P.; Bleyer, C.; Vlcek, J.; Pauly, V.; Hesse, N.; Sinner, M.; Merkus, D.; Hamers, J.; Kääb, S.; et al. A Practical Guide to Setting Up Pig Models for Cardiovascular Catheterization, Electrophysiological Assessment and Heart Disease Research. Lab Anim. 2022, 51, 46–67. [Google Scholar] [CrossRef] [PubMed]
- Tadros, R.; Ton, A.-T.; Fiset, C.; Nattel, S. Sex Differences in Cardiac Electrophysiology and Clinical Arrhythmias: Epidemiology, Therapeutics, and Mechanisms. Can. J. Cardiol. 2014, 30, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Moolman, J.A. Unravelling the Cardioprotective Mechanism of Action of Estrogens. Cardiovasc. Res. 2006, 69, 777–780. [Google Scholar] [CrossRef]
- Luo, T.; Kim, J.K. The Role of Estrogen and Estrogen Receptors on Cardiomyocytes: An Overview. Can. J. Cardiol. 2016, 32, 1017–1025. [Google Scholar] [CrossRef] [Green Version]
- Garcia, M.; Mulvagh, S.L.; Merz, C.N.B.; Buring, J.E.; Manson, J.E. Cardiovascular Disease in Women. Circulation 2016, 118, 1273–1293. [Google Scholar] [CrossRef] [Green Version]
- Frederiksen, H.; Johannsen, T.H.; Andersen, S.E.; Albrethsen, J.; Landersoe, S.K.; Petersen, J.H.; Andersen, A.N.; Vestergaard, E.T.; Schorring, M.E.; Linneberg, A.; et al. Sex-Specific Estrogen Levels and Reference Intervals from Infancy to Late Adulthood Determined by LC-MS/MS. J. Clin. Endocrinol. Metab. 2020, 105, 754–768. [Google Scholar] [CrossRef]
- Pham, T.V.; Rosen, M.R. Sex, Hormones, and Repolarization. Cardiovasc. Res. 2002, 53, 740–751. [Google Scholar] [CrossRef]
- Regitz-Zagrosek, V. Sex and Gender Differences in Heart Failure. Int. J. Heart Fail. 2020, 2, 157–181. [Google Scholar] [CrossRef]
- Costa, S.; Saguner, A.M.; Gasperetti, A.; Akdis, D.; Brunckhorst, C.; Duru, F. The Link Between Sex Hormones and Susceptibility to Cardiac Arrhythmias: From Molecular Basis to Clinical Implications. Front. Cardiovasc. Med. 2021, 8, 644279. [Google Scholar] [CrossRef] [PubMed]
- Linde, C.; Bongiorni, M.G.; Birgersdotter-Green, U.; Curtis, A.B.; Deisenhofer, I.; Furokawa, T.; Gillis, A.M.; Haugaa, K.H.; Lip, G.Y.H.; Van Gelder, I.; et al. Sex Differences in Cardiac Arrhythmia: A Consensus Document of the European Heart Rhythm Association, Endorsed By The Heart Rhythm Society and Asia Pacific Heart Rhythm Society. Europace 2018, 20, 1565–1565ao. [Google Scholar] [CrossRef] [PubMed]
- Pecha, S.; Ismaili, D.; Geelhoed, B.; Knaut, M.; Reichenspurner, H.; Eschenhagen, T.; Schnabel, R.B.; Christ, T.; Ravens, U. Resting Membrane Potential is Less Negative in Trabeculae from Right Atrial Appendages of Women, but Action Potential Duration Does Not Shorten with Age. J. Mol. Cell. Cardiol. 2023, 176, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Salama, G.; Bett, G.C.L. Sex Differences in the Mechanisms Underlying Long QT Syndrome. Am. J. Physiol.-Heart Circ. Physiol. 2014, 307, H640–H648. [Google Scholar] [CrossRef] [Green Version]
- Ko, D.; Rahman, F.; Schnabel, R.B.; Yin, X.; Benjamin, E.J.; Christophersen, I.E. Atrial Fibrillation in Women: Epidemiology, Pathophysiology, Presentation, and Prognosis. Nat. Rev. Cardiol. 2016, 13, 321–332. [Google Scholar] [CrossRef]
- Clauss, S.; Bleyer, C.; Schüttler, D.; Tomsits, P.; Renner, S.; Klymiuk, N.; Wakili, R.; Massberg, S.; Wolf, E.; Kääb, S. Animal Models of Arrhythmia: Classic Electrophysiology to Genetically Modified Large Animals. Nat. Rev. Cardiol. 2019, 16, 457–475. [Google Scholar] [CrossRef]
- Berul, C.I.; Christe, M.E.; Aronovitz, M.J.; Maguire, C.T.; Seidman, C.E.; Seidman, J.G.; Mendelsohn, M.E. Familial Hypertrophic Cardiomyopathy Mice Display Gender Differences in Electrophysiological Abnormalities. J. Interv. Card. Electrophysiol. 1997, 2, 7–14. [Google Scholar] [CrossRef]
- Maguire, C.T.; Bevilacqua, L.M.; Wakimoto, H.; Gehrmann, J.; Berul, C.I. Maturational Atrioventricular Nodal Physiology in the Mouse. J. Cardiovasc. Electrophysiol. 2000, 11, 557–564. [Google Scholar] [CrossRef]
- Jeevaratnam, K.; Zhang, Y.; Guzadhur, L.; Duehmke, R.M.; Lei, M.; Grace, A.A.; Huang, C.L. Differences in Sino-Atrial and Atrio-Ventricular Function with Age and Sex Attributable to the Scn5a+/- Mutation in a Murine Cardiac Model. Acta Physiol. 2010, 200, 23–33. [Google Scholar] [CrossRef]
- Thibault, S.; Long, V.; Fiset, C. Higher Na(+)-Ca(2+) Exchanger Function and Triggered Activity Contribute to Male Predisposition to Atrial Fibrillation. Int. J. Mol. Sci. 2022, 23, 10724. [Google Scholar] [CrossRef]
- Thibault, S.; Ton, A.T.; Huynh, F.; Fiset, C. Connexin Lateralization Contributes to Male Susceptibility to Atrial Fibrillation. Int. J. Mol. Sci. 2022, 23, 10696. [Google Scholar] [CrossRef]
- Brouillette, J.; Rivard, K.; Lizotte, E.; Fiset, C. Sex and Strain Differences in Adult Mouse Cardiac Repolarization: Importance of Androgens. Cardiovasc. Res. 2005, 65, 148–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, S.; Tsaih, S.W.; Yuan, R.; Svenson, K.L.; Jorgenson, L.M.; So, M.; Paigen, B.J.; Korstanje, R. Genetic Influence on Electrocardiogram Time Intervals and Heart Rate in Aging Mice. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H1907–H1913. [Google Scholar] [CrossRef]
- Appleton, G.O.; Li, Y.; Taffet, G.E.; Hartley, C.J.; Michael, L.H.; Entman, M.L.; Roberts, R.; Khoury, D.S. Determinants of cardiac Electrophysiological Properties in Mice. J. Interv. Card. Electrophysiol. 2004, 11, 5–14. [Google Scholar] [CrossRef] [PubMed]
- James, A.F.; Choisy, S.C.; Hancox, J.C. Recent Advances in Understanding Sex Differences in Cardiac Repolarization. Prog. Biophys. Mol. Biol. 2007, 94, 265–319. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.C.; Chen, Y.C.; Kao, Y.H.; Lu, Y.Y.; Chen, S.A.; Chen, Y.J. Distinctive Sodium and Calcium Regulation Associated with Sex Differences in Atrial Electrophysiology of Rabbits. Int. J. Cardiol. 2013, 168, 4658–4666. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.C.; Chen, Y.C.; Lin, Y.K.; Chen, S.A.; Chen, Y.J. Sex Differences in the Electrophysiological Characteristics of Pulmonary Veins and Left Atrium and Their Clinical Implication in Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2011, 4, 550–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schüttler, D.; Bapat, A.; Kääb, S.; Lee, K.; Tomsits, P.; Clauss, S.; Hucker, W.J. Animal Models of Atrial Fibrillation. Circ. Res. 2020, 127, 91–110. [Google Scholar] [CrossRef]
- Dixon, J.A.; Spinale, F.G. Large animal models of heart failure: A Critical Link in the Translation of Basic Science to Clinical Practice. Circ. Heart Fail. 2009, 2, 262–271. [Google Scholar] [CrossRef] [Green Version]
- White, F.C.; Roth, D.M.; Bloor, C.M. The pig as a Model for Myocardial Ischemia and Exercise. Lab. Anim. Sci. 1986, 36, 351–356. [Google Scholar] [PubMed]
- Silva, K.A.S.; Emter, C.A. Large Animal Models of Heart Failure: A Translational Bridge to Clinical Success. JACC Basic Transl. Sci. 2020, 5, 840–856. [Google Scholar] [CrossRef] [PubMed]
- Belkouche, A.; Yao, H.; Putot, A.; Chagué, F.; Rochette, L.; Danchin, N.; Fauchier, L.; Zeller, M.; Cottin, Y. The Multifaceted Interplay between Atrial Fibrillation and Myocardial Infarction: A Review. J. Clin. Med. 2021, 10, 198. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, S.J.; Cuijpers, I.; Heymans, S.; Jones, E.A.V. Cellular and Molecular Differences between HFpEF and HFrEF: A Step Ahead in an Improved Pathological Understanding. Cells 2020, 9, 242. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Yuan, S.; Liu, Z.; Fang, Q. Oxidation- and CaMKII-Mediated Sarcoplasmic Reticulum Ca(2+) Leak Triggers Atrial Fibrillation in Aging. J. Cardiovasc. Electrophysiol. 2014, 25, 645–652. [Google Scholar] [CrossRef]
- Tan, J.L.; Johnson, L.; Dziubinski, M.; Napiorkowski, N.; Witkowska, O.; Slusarczyk, M.E.; Healey, J.S.; Russo, A.M. Sex Differences in Presentation of Atrial Fibrillation: Findings from 30-Day Ambulatory Monitoring in Real-World Practice. Am. Heart J. Plus: Cardiol. Res. Pract. 2022, 22, 100208. [Google Scholar] [CrossRef]
- Rao, A.C.A.; Ng, A.C.C.; Sy, R.W.; Chia, K.K.M.; Hansen, P.S.; Chiha, J.; Kilian, J.; Kanagaratnam, L.B. Electrocardiographic QRS Duration is Influenced by Body Mass Index and Sex. IJC Heart Vasc. 2021, 37, 100884. [Google Scholar] [CrossRef]
- Swindle, M.M.; Smith, A.C. Swine in the Laboratory, 3rd ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2016; p. 593. [Google Scholar]
- Prabhavathi, K.; Selvi, K.T.; Poornima, K.N.; Sarvanan, A. Role of Biological Sex in Normal Cardiac Function and in Its Disease Outcome—A Review. J. Clin. Diagn. Res. JCDR 2014, 8, Be01–Be04. [Google Scholar] [CrossRef]
- Andršová, I.; Hnatkova, K.; Helánová, K.; Šišáková, M.; Novotný, T.; Kala, P.; Malik, M. Individually Rate Corrected QTc Intervals in Children and Adolescents. Front. Physiol. 2019, 10, 994. [Google Scholar] [CrossRef] [Green Version]
- Richard, N.; Fogoros, J.M.M. The Electrophysiology Study in the Evaluation of Bradycardia: The SA Node, AV Node, and His–Purkinje System. In Fogoros’ Electrophysiologic Testing, 6th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 63–106. [Google Scholar]
- Taneja, T.; Mahnert, B.W.; Passman, R.; Goldberger, J.; Kadish, A. Effects of Sex and Age on Electrocardiographic and Cardiac Electrophysiological Properties in Adults. Pacing Clin. Electrophysiol. 2001, 24, 16–21. [Google Scholar] [CrossRef]
- Ozcan, C.; Curtis, A.B. 107–Sex Differences in Arrhythmias. In Cardiac Electrophysiology: From Cell to Bedside, 7th ed.; Zipes, D.P., Jalife, J., Stevenson, W.G., Eds.; Elsevier: Philadelphia, PA, USA, 2018; pp. 1011–1019. [Google Scholar]
- Baker, H.E.; Kiel, A.M.; Luebbe, S.T.; Simon, B.R.; Earl, C.C.; Regmi, A.; Roell, W.C.; Mather, K.J.; Tune, J.D.; Goodwill, A.G. Inhibition of Sodium-Glucose Cotransporter-2 Preserves Cardiac Function During Regional Myocardial Ischemia Independent of Alterations in Myocardial Substrate Utilization. Basic Res. Cardiol. 2019, 114, 25. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-S.; Tan, H.-W.; Song, H.-M.; Xu, W.-J.; Liu, X.-B. Impact of Corrected Sinus Node Recovery Time in Predicting Recurrence in Patients with Paroxysmal Atrial Fibrillation. J. Int. Med. Res. 2021, 49, 03000605211010103. [Google Scholar] [CrossRef] [PubMed]
- Noszczyk-Nowak, A.; Cepiel, A.; Janiszewski, A.; Pasławski, R.; Gajek, J.; Pasławska, U.; Nicpoń, J. Normal Values for Heart Electrophysiology Parameters of Healthy Swine Determined on Electrophysiology Study. Adv. Clin. Exp. Med. 2016, 25, 1249–1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaballos, M.; Del Blanco, B.; Sevilla, R.; De Diego, C.; Anadon, M.J.; Jimeno, C.; Almendral, J. Differential effects of Sevoflurane and Propofol on Swine Cardiac Conduction System. Vet. Anaesth. Analg. 2019, 46, 344–351. [Google Scholar] [CrossRef]
- Akoum, N.; Mahnkopf, C.; Kholmovski, E.G.; Brachmann, J.; Marrouche, N.F. Age and Sex Differences in Atrial Fibrosis Among Patients with Atrial Fibrillation. EP Europace 2017, 20, 1086–1092. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Z.; Yin, Z.; Zhang, Y.; Xue, X.; Han, J.; Zhu, Y.; Zhang, J.; Emmert, M.Y.; Wang, H. Gender Differences in Fibrosis Remodeling in Patients with Long-Standing Persistent Atrial Fibrillation. Oncotarget 2017, 8, 53714–53729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aigner, B.; Renner, S.; Kessler, B.; Klymiuk, N.; Kurome, M.; Wünsch, A.; Wolf, E. Transgenic Pigs as Models for Translational Biomedical Research. J. Mol. Med. 2010, 88, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Prather, R.S.; Lorson, M.; Ross, J.W.; Whyte, J.J.; Walters, E. Genetically Engineered Pig Models for Human Diseases. Annu. Rev. Anim. Biosci. 2013, 1, 203–219. [Google Scholar] [CrossRef] [Green Version]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pauly, V.; Vlcek, J.; Zhang, Z.; Hesse, N.; Xia, R.; Bauer, J.; Loy, S.; Schneider, S.; Renner, S.; Wolf, E.; et al. Effects of Sex on the Susceptibility for Atrial Fibrillation in Pigs with Ischemic Heart Failure. Cells 2023, 12, 973. https://doi.org/10.3390/cells12070973
Pauly V, Vlcek J, Zhang Z, Hesse N, Xia R, Bauer J, Loy S, Schneider S, Renner S, Wolf E, et al. Effects of Sex on the Susceptibility for Atrial Fibrillation in Pigs with Ischemic Heart Failure. Cells. 2023; 12(7):973. https://doi.org/10.3390/cells12070973
Chicago/Turabian StylePauly, Valerie, Julia Vlcek, Zhihao Zhang, Nora Hesse, Ruibing Xia, Julia Bauer, Simone Loy, Sarah Schneider, Simone Renner, Eckhard Wolf, and et al. 2023. "Effects of Sex on the Susceptibility for Atrial Fibrillation in Pigs with Ischemic Heart Failure" Cells 12, no. 7: 973. https://doi.org/10.3390/cells12070973