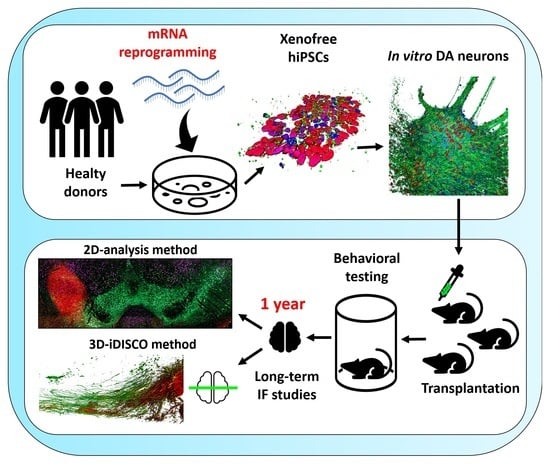
Long-Term Evaluation of Intranigral Transplantation of Human iPSC-Derived Dopamine Neurons in a Parkinson’s Disease Mouse Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fibroblast Reprogramming and hiPSC Culture
2.2. Differentiation of hiPSC-Derived DA Neurons
2.3. Animals
2.4. Lesion and Transplantation Procedures
2.5. Amphetamine-Induced Rotation
2.6. Tissue Processing
2.7. Immunochemistry
2.7.1. Immunofluorescence on Cells
2.7.2. Immunohistochemistry on Brain Sections
2.8. Grafted Brain Clearing
2.9. Data Acquisition and Quantification
2.10. Statistical Analysis
3. Results
3.1. Generation of hiPSCs from Human Fibroblasts via mRNA Reprogramming
3.2. hiPSC Differentiation to Midbrain Floor Plate Progenitor Cells
3.3. Maturation of DA Neurons In Vitro
3.4. Maturation of hiPSC-Derived Neurons Takes Much Longer Than Previously Assumed
3.5. Cellular Composition of the Graft
3.6. Proliferation and Apoptosis in Grafts
3.7. Reconstruction of the Nigrostriatal Pathway and Innervation of the Striatum
3.8. Long-Term Functional Motor Recovery after Transplantation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548. [Google Scholar] [CrossRef]
- Wakeman, D.R.; Dodiya, H.B.; Kordower, J.H. Cell Transplantation and Gene Therapy in Parkinson’s Disease. Mt. Sinai J. Med. 2011, 78, 126–158. [Google Scholar] [CrossRef]
- Peschanski, M.; Defer, G.; N’Guyen, J.P.; Ricolfi, F.; Monfort, J.C.; Remy, P.; Geny, C.; Samson, Y.; Hantraye, P.; Jeny, R. Bilateral Motor Improvement and Alteration of L-Dopa Effect in Two Patients with Parkinson’s Disease Following Intrastriatal Transplantation of Foetal Ventral Mesencephalon. Brain 1994, 117 Pt 3, 487–499. [Google Scholar] [CrossRef]
- Lindvall, O.; Björklund, A. Transplantation Strategies in the Treatment of Parkinson’s Disease: Experimental Basis and Clinical Trials. Acta. Neurol. Scand. Suppl. 1989, 126, 197–210. [Google Scholar] [CrossRef]
- Björklund, A. Dopaminergic Transplants in Experimental Parkinsonism: Cellular Mechanisms of Graft-Induced Functional Recovery. Curr. Opin. Neurobiol. 1992, 2, 683–689. [Google Scholar] [CrossRef]
- Mendez, I.; Hong, M.; Smith, S.; Dagher, A.; Desrosiers, J. Neural Transplantation Cannula and Microinjector System: Experimental and Clinical Experience. Technical Note. J. Neurosurg. 2000, 92, 493–499. [Google Scholar] [CrossRef]
- Gaillard, A.; Jaber, M. Rewiring the Brain with Cell Transplantation in Parkinson’s Disease. Trends Neurosci. 2011, 34, 124–133. [Google Scholar] [CrossRef]
- Lee, D.; Dallapiazza, R.; De Vloo, P.; Lozano, A. Current Surgical Treatments for Parkinson’s Disease and Potential Therapeutic Targets. Neural. Regen Res. 2018, 13, 1342. [Google Scholar] [CrossRef]
- Spencer, D.D.; Robbins, R.J.; Naftolin, F.; Marek, K.L.; Vollmer, T.; Leranth, C.; Roth, R.H.; Price, L.H.; Gjedde, A.; Bunney, B.S.; et al. Unilateral Transplantation of Human Fetal Mesencephalic Tissue into the Caudate Nucleus of Patients with Parkinson’s Disease. N. Engl. J. Med. 1992, 327, 1541–1548. [Google Scholar] [CrossRef]
- Freed, C.R.; Breeze, R.E.; Rosenberg, N.L.; Schneck, S.A.; Kriek, E.; Qi, J.X.; Lone, T.; Zhang, Y.B.; Snyder, J.A.; Wells, T.H. Survival of Implanted Fetal Dopamine Cells and Neurologic Improvement 12 to 46 Months after Transplantation for Parkinson’s Disease. N. Engl. J. Med. 1992, 327, 1549–1555. [Google Scholar] [CrossRef]
- Kordower, J.H.; Freeman, T.B.; Snow, B.J.; Vingerhoets, F.J.; Mufson, E.J.; Sanberg, P.R.; Hauser, R.A.; Smith, D.A.; Nauert, G.M.; Perl, D.P. Neuropathological Evidence of Graft Survival and Striatal Reinnervation after the Transplantation of Fetal Mesencephalic Tissue in a Patient with Parkinson’s Disease. N. Engl. J. Med. 1995, 332, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Sanders, L.M.; Giudice, L.; Raffin, T.A. Ethics of Fetal Tissue Transplantation. West. J. Med. 1993, 9. [Google Scholar]
- Stoker, T.B.; Barker, R.A. Cell Therapies for Parkinson’s Disease: How Far Have We Come? Regen. Med. 2016, 11, 777–786. [Google Scholar] [CrossRef] [Green Version]
- Peng, S.-P.; Copray, S. Comparison of Human Primary with Human IPS Cell-Derived Dopaminergic Neuron Grafts in the Rat Model for Parkinson’s Disease. Stem Cell Rev. Rep. 2016, 12, 105–120. [Google Scholar] [CrossRef] [Green Version]
- Tiklová, K.; Nolbrant, S.; Fiorenzano, A.; Björklund, Å.K.; Sharma, Y.; Heuer, A.; Gillberg, L.; Hoban, D.B.; Cardoso, T.; Adler, A.F.; et al. Single Cell Transcriptomics Identifies Stem Cell-Derived Graft Composition in a Model of Parkinson’s Disease. Nat. Commun. 2020, 11, 2434. [Google Scholar] [CrossRef]
- Doi, D.; Magotani, H.; Kikuchi, T.; Ikeda, M.; Hiramatsu, S.; Yoshida, K.; Amano, N.; Nomura, M.; Umekage, M.; Morizane, A.; et al. Pre-Clinical Study of Induced Pluripotent Stem Cell-Derived Dopaminergic Progenitor Cells for Parkinson’s Disease. Nat. Commun. 2020, 11, 3369. [Google Scholar] [CrossRef]
- Song, B.; Cha, Y.; Ko, S.; Jeon, J.; Lee, N.; Seo, H.; Park, K.-J.; Lee, I.-H.; Lopes, C.; Feitosa, M.; et al. Human Autologous IPSC-Derived Dopaminergic Progenitors Restore Motor Function in Parkinson’s Disease Models. J. Clin. Invest. 2020, 130, 904–920. [Google Scholar] [CrossRef] [Green Version]
- Hallett, P.J.; Deleidi, M.; Astradsson, A.; Smith, G.A.; Cooper, O.; Osborn, T.M.; Sundberg, M.; Moore, M.A.; Perez-Torres, E.; Brownell, A.-L.; et al. Successful Function of Autologous IPSC-Derived Dopamine Neurons Following Transplantation in a Non-Human Primate Model of Parkinson’s Disease. Cell Stem Cell 2015, 16, 269–274. [Google Scholar] [CrossRef] [Green Version]
- Morizane, A.; Kikuchi, T.; Hayashi, T.; Mizuma, H.; Takara, S.; Doi, H.; Mawatari, A.; Glasser, M.F.; Shiina, T.; Ishigaki, H.; et al. MHC Matching Improves Engraftment of IPSC-Derived Neurons in Non-Human Primates. Nat. Commun. 2017, 8, 385. [Google Scholar] [CrossRef]
- Kikuchi, T.; Morizane, A.; Doi, D.; Magotani, H.; Onoe, H.; Hayashi, T.; Mizuma, H.; Takara, S.; Takahashi, R.; Inoue, H.; et al. Human IPS Cell-Derived Dopaminergic Neurons Function in a Primate Parkinson’s Disease Model. Nature 2017, 548, 592–596. [Google Scholar] [CrossRef]
- Tao, Y.; Vermilyea, S.C.; Zammit, M.; Lu, J.; Olsen, M.; Metzger, J.M.; Yao, L.; Chen, Y.; Phillips, S.; Holden, J.E.; et al. Autologous Transplant Therapy Alleviates Motor and Depressive Behaviors in Parkinsonian Monkeys. Nat. Med. 2021, 27, 632–639. [Google Scholar] [CrossRef]
- Adler, A.F.; Cardoso, T.; Nolbrant, S.; Mattsson, B.; Hoban, D.B.; Jarl, U.; Wahlestedt, J.N.; Grealish, S.; Björklund, A.; Parmar, M. HESC-Derived Dopaminergic Transplants Integrate into Basal Ganglia Circuitry in a Preclinical Model of Parkinson’s Disease. Cell Rep. 2019, 28, 3462–3473. [Google Scholar] [CrossRef] [Green Version]
- Kriks, S.; Shim, J.-W.; Piao, J.; Ganat, Y.M.; Wakeman, D.R.; Xie, Z.; Carrillo-Reid, L.; Auyeung, G.; Antonacci, C.; Buch, A.; et al. Dopamine Neurons Derived from Human ES Cells Efficiently Engraft in Animal Models of Parkinson’s Disease. Nature 2011, 480, 547–551. [Google Scholar] [CrossRef]
- Rhee, Y.-H.; Ko, J.-Y.; Chang, M.-Y.; Yi, S.-H.; Kim, D.; Kim, C.-H.; Shim, J.-W.; Jo, A.-Y.; Kim, B.-W.; Lee, H.; et al. Protein-Based Human IPS Cells Efficiently Generate Functional Dopamine Neurons and Can Treat a Rat Model of Parkinson Disease. J. Clin. Invest. 2011, 121, 2326–2335. [Google Scholar] [CrossRef]
- Hargus, G.; Cooper, O.; Deleidi, M.; Levy, A.; Lee, K.; Marlow, E.; Yow, A.; Soldner, F.; Hockemeyer, D.; Hallett, P.J.; et al. Differentiated Parkinson Patient-Derived Induced Pluripotent Stem Cells Grow in the Adult Rodent Brain and Reduce Motor Asymmetry in Parkinsonian Rats. Proc. Natl. Acad. Sci. USA 2010, 107, 15921–15926. [Google Scholar] [CrossRef] [Green Version]
- de Luzy, I.R.; Niclis, J.C.; Gantner, C.W.; Kauhausen, J.A.; Hunt, C.P.J.; Ermine, C.; Pouton, C.W.; Thompson, L.H.; Parish, C.L. Isolation of LMX1a Ventral Midbrain Progenitors Improves the Safety and Predictability of Human Pluripotent Stem Cell-Derived Neural Transplants in Parkinsonian Disease. J. Neurosci. 2019, 39, 9521–9531. [Google Scholar] [CrossRef]
- Gaillard, A.; Decressac, M.; Frappé, I.; Fernagut, P.O.; Prestoz, L.; Besnard, S.; Jaber, M. Anatomical and Functional Reconstruction of the Nigrostriatal Pathway by Intranigral Transplants. Neurobiol. Dis. 2009, 35, 477–488. [Google Scholar] [CrossRef]
- Kirst, C.; Skriabine, S.; Vieites-Prado, A.; Topilko, T.; Bertin, P.; Gerschenfeld, G.; Verny, F.; Topilko, P.; Michalski, N.; Tessier-Lavigne, M.; et al. Mapping the Fine-Scale Organization and Plasticity of the Brain Vasculature. Cell 2020, 180, 780–795.e25. [Google Scholar] [CrossRef]
- Mouton, P.R.; Gokhale, A.M.; Ward, N.L.; West, M.J. Stereological Length Estimation Using Spherical Probes. J. Microsc. 2002, 206, 54–64. [Google Scholar] [CrossRef]
- Morizane, A.; Takahashi, J. Evading the Immune System: Immune Modulation and Immune Matching in Cell Replacement Therapies for Parkinson’s Disease. J. Parkinsons Dis. 2021, 11, S167–S172. [Google Scholar] [CrossRef]
- Liu, J.; Verma, P.J. Synthetic MRNA Reprogramming of Human Fibroblast Cells. Methods Mol. Biol. 2015, 1330, 17–28. [Google Scholar] [CrossRef]
- Kauhausen, J.A.; Thompson, L.H.; Parish, C.L. Chondroitinase Improves Midbrain Pathway Reconstruction by Transplanted Dopamine Progenitors in Parkinsonian Mice. Mol. Cell. Neurosci. 2015, 69, 22–29. [Google Scholar] [CrossRef]
- Wang, M.; Ling, K.-H.; Tan, J.J.; Lu, C.-B. Development and Differentiation of Midbrain Dopaminergic Neuron: From Bench to Bedside. Cells 2020, 9, 1489. [Google Scholar] [CrossRef]
- Nolbrant, S.; Heuer, A.; Parmar, M.; Kirkeby, A. Generation of High-Purity Human Ventral Midbrain Dopaminergic Progenitors for in Vitro Maturation and Intracerebral Transplantation. Nat. Protoc. 2017, 12, 1962–1979. [Google Scholar] [CrossRef]
- Kirkeby, A.; Nolbrant, S.; Tiklova, K.; Heuer, A.; Kee, N.; Cardoso, T.; Ottosson, D.R.; Lelos, M.J.; Rifes, P.; Dunnett, S.B.; et al. Predictive Markers Guide Differentiation to Improve Graft Outcome in Clinical Translation of HESC-Based Therapy for Parkinson’s Disease. Cell Stem Cell 2017, 20, 135–148. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.W.; Koo, S.Y.; Studer, L. Pluripotent Stem Cell Therapies for Parkinson Disease: Present Challenges and Future Opportunities. Front. Cell Dev. Biol. 2020, 8, 729. [Google Scholar] [CrossRef]
- Politis, M.; Wu, K.; Loane, C.; Quinn, N.P.; Brooks, D.J.; Rehncrona, S.; Bjorklund, A.; Lindvall, O.; Piccini, P. Serotonergic Neurons Mediate Dyskinesia Side Effects in Parkinson’s Patients with Neural Transplants. Sci. Transl. Med. 2010, 2. [Google Scholar] [CrossRef]
- Piao, J.; Zabierowski, S.; Dubose, B.N.; Hill, E.J.; Navare, M.; Claros, N.; Rosen, S.; Ramnarine, K.; Horn, C.; Fredrickson, C.; et al. Preclinical Efficacy and Safety of a Human Embryonic Stem Cell-Derived Midbrain Dopamine Progenitor Product, MSK-DA01. Cell Stem Cell 2021, 28, 217–229. [Google Scholar] [CrossRef]
- Grealish, S.; Diguet, E.; Kirkeby, A.; Mattsson, B.; Heuer, A.; Bramoulle, Y.; Van Camp, N.; Perrier, A.L.; Hantraye, P.; Björklund, A.; et al. Human ESC-Derived Dopamine Neurons Show Similar Preclinical Efficacy and Potency to Fetal Neurons When Grafted in a Rat Model of Parkinson’s Disease. Cell Stem Cell 2014, 15, 653–665. [Google Scholar] [CrossRef] [Green Version]
- Doi, D.; Samata, B.; Katsukawa, M.; Kikuchi, T.; Morizane, A.; Ono, Y.; Sekiguchi, K.; Nakagawa, M.; Parmar, M.; Takahashi, J. Isolation of Human Induced Pluripotent Stem Cell-Derived Dopaminergic Progenitors by Cell Sorting for Successful Transplantation. Stem Cell Rep. 2014, 2, 337–350. [Google Scholar] [CrossRef] [Green Version]
- Gantner, C.W.; de Luzy, I.R.; Kauhausen, J.A.; Moriarty, N.; Niclis, J.C.; Bye, C.R.; Penna, V.; Hunt, C.P.J.; Ermine, C.M.; Pouton, C.W.; et al. Viral Delivery of GDNF Promotes Functional Integration of Human Stem Cell Grafts in Parkinson’s Disease. Cell Stem Cell 2020, 26, 511–526.e5. [Google Scholar] [CrossRef]
- Xi, J.; Liu, Y.; Liu, H.; Chen, H.; Emborg, M.E.; Zhang, S. Specification of Midbrain Dopamine Neurons from Primate Pluripotent Stem Cells. Stem Cells 2012, 30, 1655–1663. [Google Scholar] [CrossRef] [Green Version]
- Alavian, K.N.; Scholz, C.; Simon, H.H. Transcriptional Regulation of Mesencephalic Dopaminergic Neurons: The Full Circle of Life and Death. Mov. Disord. 2008, 23, 319–328. [Google Scholar] [CrossRef]
- Betarbet, R.; Sherer, T.B.; MacKenzie, G.; Garcia-Osuna, M.; Panov, A.V.; Greenamyre, J.T. Chronic Systemic Pesticide Exposure Reproduces Features of Parkinson’s Disease. Nat. Neurosci. 2000, 3, 1301–1306. [Google Scholar] [CrossRef]
- Giguère, N.; Burke Nanni, S.; Trudeau, L.-E. On Cell Loss and Selective Vulnerability of Neuronal Populations in Parkinson’s Disease. Front. Neurol. 2018, 9, 455. [Google Scholar] [CrossRef]
- Surmeier, D.J.; Obeso, J.A.; Halliday, G.M. Selective Neuronal Vulnerability in Parkinson Disease. Nat. Rev. Neurosci. 2017, 18, 101–113. [Google Scholar] [CrossRef]
- Sortwell, C.E.; Pitzer, M.R.; Collier, T.J. Time Course of Apoptotic Cell Death within Mesencephalic Cell Suspension Grafts: Implications for Improving Grafted Dopamine Neuron Survival. Exp. Neurol. 2000, 165, 268–277. [Google Scholar] [CrossRef]
- Isacson, O.; Deacon, T.W. Specific Axon Guidance Factors Persist in the Adult Brain as Demonstrated by Pig Neuroblasts Transplanted to the Rat. Neuroscience 1996, 75, 827–837. [Google Scholar] [CrossRef]
- Michelsen, K.A.; Acosta-Verdugo, S.; Benoit-Marand, M.; Espuny-Camacho, I.; Gaspard, N.; Saha, B.; Gaillard, A.; Vanderhaeghen, P. Area-Specific Reestablishment of Damaged Circuits in the Adult Cerebral Cortex by Cortical Neurons Derived from Mouse Embryonic Stem Cells. Neuron 2015, 85, 982–997. [Google Scholar] [CrossRef] [Green Version]
- Niclis, J.C.; Turner, C.; Durnall, J.; McDougal, S.; Kauhausen, J.A.; Leaw, B.; Dottori, M.; Parish, C.L.; Thompson, L.H. Long-Distance Axonal Growth and Protracted Functional Maturation of Neurons Derived from Human Induced Pluripotent Stem Cells After Intracerebral Transplantation. Stem Cells Transl. Med. 2017, 6, 1547–1556. [Google Scholar] [CrossRef] [Green Version]
- Thompson, L.H.; Grealish, S.; Kirik, D.; Björklund, A. Reconstruction of the Nigrostriatal Dopamine Pathway in the Adult Mouse Brain. Eur J. Neurosci. 2009, 30, 625–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrán, E.; Ruiz-Ortega, J.Á.; Aristieta, A.; Igartua, M.; Requejo, C.; Lafuente, J.V.; Ugedo, L.; Pedraz, J.L.; Hernández, R.M. In Vivo Administration of VEGF- and GDNF-Releasing Biodegradable Polymeric Microspheres in a Severe Lesion Model of Parkinson’s Disease. Eur. J. Pharm. Biopharm. 2013, 85, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, T.; Adler, A.F.; Mattsson, B.; Hoban, D.B.; Nolbrant, S.; Wahlestedt, J.N.; Kirkeby, A.; Grealish, S.; Björklund, A.; Parmar, M. Target-Specific Forebrain Projections and Appropriate Synaptic Inputs of HESC-Derived Dopamine Neurons Grafted to the Midbrain of Parkinsonian Rats. J. Comp. Neurol. 2018, 526, 2133–2146. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.P.; Burrell, J.C.; Struzyna, L.A.; Chen, H.I.; Serruya, M.D.; Wolf, J.A.; Duda, J.E.; Cullen, D.K. Emerging Regenerative Medicine and Tissue Engineering Strategies for Parkinson’s Disease. npj Parkinsons Dis. 2020, 6, 4. [Google Scholar] [CrossRef] [Green Version]
- Kordower, J.H.; Freeman, T.B.; Chen, E.-Y.; Mufson, E.J.; Sanberg, P.R.; Hauser, R.A.; Snow, B.; Warren Olanow, C. Fetal Nigral Grafts Survive and Mediate Clinical Benefit in a Patient with Parkinson’s Disease: FETAL NIGRAL GRAFTS IN PD. Mov. Disord. 1998, 13, 383–393. [Google Scholar] [CrossRef]
- Piccini, P.; Brooks, D.J.; Björklund, A.; Gunn, R.N.; Grasby, P.M.; Rimoldi, O.; Brundin, P.; Hagell, P.; Rehncrona, S.; Widner, H.; et al. Dopamine Release from Nigral Transplants Visualized in Vivo in a Parkinson’s Patient. Nat. Neurosci. 1999, 2, 1137–1140. [Google Scholar] [CrossRef]
- Tiklová, K.; Nolbrant, S.; Fiorenzano, A.; Björklund, Å.K.; Sharma, Y.; Heuer, A.; Gillberg, L.; Hoban, D.B.; Cardoso, T.; Adler, A.F.; et al. Single Cell Gene Expression Analysis Reveals Human Stem Cell-Derived Graft Composition in a Cell Therapy Model. of Parkinson’s Disease. Neuroscience 2019. [Google Scholar]
- Kim, T.W.; Piao, J.; Koo, S.Y.; Kriks, S.; Chung, S.Y.; Betel, D.; Socci, N.D.; Choi, S.J.; Zabierowski, S.; Dubose, B.N.; et al. Biphasic Activation of WNT Signaling Facilitates the Derivation of Midbrain Dopamine Neurons from HESCs for Translational Use. Cell Stem Cell 2021, 28, 343–355.e5. [Google Scholar] [CrossRef]
- Hiramatsu, S.; Morizane, A.; Kikuchi, T.; Doi, D.; Yoshida, K.; Takahashi, J. Cryopreservation of Induced Pluripotent Stem Cell-Derived Dopaminergic Neurospheres for Clinical Application. J. Parkinsons Dis. 2021. [Google Scholar] [CrossRef]
- Kim, J.; Jeon, J.; Song, B.; Lee, N.; Ko, S.; Cha, Y.; Leblanc, P.; Seo, H.; Kim, K.-S. Spotting-Based Differentiation of Functional Dopaminergic Progenitors from Human Pluripotent Stem Cells. Nat. Protoc. 2022, 17, 890–909. [Google Scholar] [CrossRef]
- Ganat, Y.M.; Calder, E.L.; Kriks, S.; Nelander, J.; Tu, E.Y.; Jia, F.; Battista, D.; Harrison, N.; Parmar, M.; Tomishima, M.J.; et al. Identification of Embryonic Stem Cell–Derived Midbrain Dopaminergic Neurons for Engraftment. J. Clin. Invest. 2012, 122, 2928–2939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinbeck, J.A.; Choi, S.J.; Mrejeru, A.; Ganat, Y.; Deisseroth, K.; Sulzer, D.; Mosharov, E.V.; Studer, L. Optogenetics Enables Functional Analysis of Human Embryonic Stem Cell-Derived Grafts in a Parkinson’s Disease Model. Nat. Biotechnol. 2015, 33, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Liao, M.-C.; Chen, A.K.; Wei, S.; Xie, S.; Reuveny, S.; Zhou, Z.D.; Hunziker, W.; Tan, E.K.; Oh, S.K.W.; et al. Immature Midbrain Dopaminergic Neurons Derived from Floor-Plate Method Improve Cell Transplantation Therapy Efficacy for Parkinson’s Disease. Stem Cells Transl Med. 2017, 6, 1803–1814. [Google Scholar] [CrossRef]
- Kirkeby, A.; Grealish, S.; Wolf, D.A.; Nelander, J.; Wood, J.; Lundblad, M.; Lindvall, O.; Parmar, M. Generation of Regionally Specified Neural Progenitors and Functional Neurons from Human Embryonic Stem Cells under Defined Conditions. Cell Rep. 2012, 1, 703–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grealish, S.; Heuer, A.; Cardoso, T.; Kirkeby, A.; Jönsson, M.; Johansson, J.; Björklund, A.; Jakobsson, J.; Parmar, M. Monosynaptic Tracing Using Modified Rabies Virus Reveals Early and Extensive Circuit Integration of Human Embryonic Stem Cell-Derived Neurons. Stem Cell Rep. 2015, 4, 975–983. [Google Scholar] [CrossRef] [Green Version]
- Droguerre, M.; Brot, S.; Vitrac, C.; Benoit-Marand, M.; Belnoue, L.; Patrigeon, M.; Lainé, A.; Béré, E.; Jaber, M.; Gaillard, A. Better Outcomes with Intranigral versus Intrastriatal Cell Transplantation: Relevance for Parkinson’s Disease. Cells 2022, 11, 1191. [Google Scholar] [CrossRef]
- Xiong, M.; Tao, Y.; Gao, Q.; Feng, B.; Yan, W.; Zhou, Y.; Kotsonis, T.A.; Yuan, T.; You, Z.; Wu, Z.; et al. Human Stem Cell-Derived Neurons Repair Circuits and Restore Neural Function. Cell Stem Cell 2021, 28, 112–126.e6. [Google Scholar] [CrossRef]
- Schweitzer, J.S.; Song, B.; Herrington, T.M.; Park, T.-Y.; Lee, N.; Ko, S.; Jeon, J.; Cha, Y.; Kim, K.; Li, Q.; et al. Personalized IPSC-Derived Dopamine Progenitor Cells for Parkinson’s Disease. N. Engl J. Med. 2020, 382, 1926–1932. [Google Scholar] [CrossRef]
- Takahashi, J. IPS Cell-Based Therapy for Parkinson’s Disease: A Kyoto Trial. Regen. Ther. 2020, 13, 18–22. [Google Scholar] [CrossRef]
- Gordián-Vélez, W.J.; Chouhan, D.; España, R.A.; Chen, H.I.; Burdick, J.A.; Duda, J.E.; Cullen, D.K. Restoring Lost Nigrostriatal Fibers in Parkinson’s Disease Based on Clinically-Inspired Design Criteria. Brain Res. Bull. 2021, 175, 168–185. [Google Scholar] [CrossRef]
- Moriarty, N.; Gantner, C.W.; Hunt, C.P.J.; Ermine, C.M.; Frausin, S.; Viventi, S.; Ovchinnikov, D.A.; Kirik, D.; Parish, C.L.; Thompson, L.H. A Combined Cell and Gene Therapy Approach for Homotopic Reconstruction of Midbrain Dopamine Pathways Using Human Pluripotent Stem Cells. Cell Stem Cell 2022, 29, 434–448.e5. [Google Scholar] [CrossRef] [PubMed]
- Sauer, H.; Rosenblad, C.; Björklund, A. Glial Cell Line-Derived Neurotrophic Factor but Not Transforming Growth Factor Beta 3 Prevents Delayed Degeneration of Nigral Dopaminergic Neurons Following Striatal 6-Hydroxydopamine Lesion. Proc. Natl. Acad. Sci. USA 1995, 92, 8935–8939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomac, A.; Lindqvist, E.; Lin, L.F.; Ogren, S.O.; Young, D.; Hoffer, B.J.; Olson, L. Protection and Repair of the Nigrostriatal Dopaminergic System by GDNF in Vivo. Nature 1995, 373, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Gillmore, J.D.; Gane, E.; Taubel, J.; Kao, J.; Fontana, M.; Maitland, M.L.; Seitzer, J.; O’Connell, D.; Walsh, K.R.; Wood, K.; et al. CRISPR-Cas9 In Vivo Gene Editing for Transthyretin Amyloidosis. N. Engl. J. Med. 2021, 385, 493–502. [Google Scholar] [CrossRef]












Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brot, S.; Thamrin, N.P.; Bonnet, M.-L.; Francheteau, M.; Patrigeon, M.; Belnoue, L.; Gaillard, A. Long-Term Evaluation of Intranigral Transplantation of Human iPSC-Derived Dopamine Neurons in a Parkinson’s Disease Mouse Model. Cells 2022, 11, 1596. https://doi.org/10.3390/cells11101596
Brot S, Thamrin NP, Bonnet M-L, Francheteau M, Patrigeon M, Belnoue L, Gaillard A. Long-Term Evaluation of Intranigral Transplantation of Human iPSC-Derived Dopamine Neurons in a Parkinson’s Disease Mouse Model. Cells. 2022; 11(10):1596. https://doi.org/10.3390/cells11101596
Chicago/Turabian StyleBrot, Sébastien, Nabila Pyrenina Thamrin, Marie-Laure Bonnet, Maureen Francheteau, Maëlig Patrigeon, Laure Belnoue, and Afsaneh Gaillard. 2022. "Long-Term Evaluation of Intranigral Transplantation of Human iPSC-Derived Dopamine Neurons in a Parkinson’s Disease Mouse Model" Cells 11, no. 10: 1596. https://doi.org/10.3390/cells11101596