MAPK Signaling Pathway Is Essential for Female Reproductive Regulation in the Cabbage Beetle, Colaphellus bowringi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Gene Cloning and Sequence Analysis
2.3. RNA Extraction and Quantitative Real Time-PCR (qRT-PCR)
2.4. RNAi Experiments
2.5. Statistical Analysis
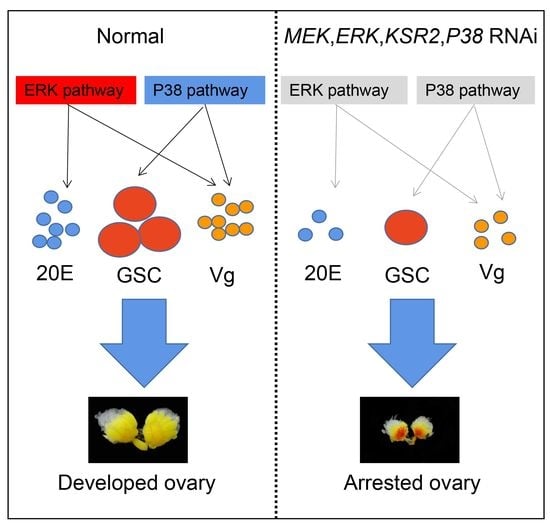
3. Results
3.1. RNAi Knockdown of Candidate Genes in MAPK Signaling Pathway in C. bowringi Females
3.2. Effects of Knocking down Key Genes in Ovarian Development
3.3. Survivorship and Fecundity of Silencing Key Genes in Females
3.4. Expression of JH, 20E and GSC Relative Genes after Knocking down Key Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Gibson, T.B.; Robinson, F.; Silvestro, L.; Pearson, G.; Xu, B.; Wright, A.; Vanderbilt, C.; Cobb, M.H. MAP kinases. Chem. Rev. 2001, 101, 2449–2476. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, M.V. RTK/Ras/MAPK signaling. WormBook 2006, 11, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Garrington, T.P.; Johnson, G.L. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr. Opin. Cell Biol. 1999, 11, 211–218. [Google Scholar] [CrossRef]
- Chang, L.; Karin, M. Mammalian MAP kinase signalling cascades. Nature 2001, 410, 37–40. [Google Scholar] [CrossRef]
- Keshet, Y.; Seger, R. The MAP kinase signaling cascades: A system of hundreds of components regulates a diverse array of physiological functions. Methods Mol. Biol. 2010, 661, 3–38. [Google Scholar]
- Lavoie, H.; Gagnon, J.; Therrien, M. ERK signalling: A master regulator of cell behaviour, life and fate. Nat. Rev. Mol. Cell Biol. 2020, 21, 607–632. [Google Scholar] [CrossRef]
- Rios-Barrera, L.D.; Riesgo-Escovar, J.R. Regulating cell morphogenesis: The Drosophila Jun N-terminal kinase pathway. Genesis 2013, 51, 147–162. [Google Scholar] [CrossRef]
- Hammouda, M.B.; Ford, A.E.; Liu, Y.; Zhang, J.Y. The JNK signaling pathway in inflammatory skin disorders and cancer. Cells 2020, 9, 857. [Google Scholar] [CrossRef] [Green Version]
- Zarubin, T.; Han, J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005, 15, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Harper, S.J.; LoGrasso, P. Signalling for survival and death in neurones: The role of stress-activated kinases, JNK and p38. Cell. Signal. 2001, 13, 299–310. [Google Scholar] [CrossRef]
- Yamashita, M. Molecular mechanisms of meiotic maturation and arrest in fish and amphibian oocytes. Semin. Cell Dev. Biol. 1998, 9, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.Y.; Sun, Q.Y. Involvement of mitogen-activated protein kinase cascade during oocyte maturation and fertilization in mammals. Biol. Reprod. 2004, 70, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.Y.; Liu, Z.; Paquet, M.; Wang, J.; Lydon, J.P.; DeMayo, F.J.; Richards, J.S. Cell type-specific targeted mutations of Kras and Pten document proliferation arrest in granulosa cells versus oncogenic insult to ovarian surface epithelial cells. Cancer Res. 2009, 69, 6463–6472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oktem, O.; Buyuk, E.; Oktay, K. Preantral follicle growth is regulated by c-Jun-N-terminal kinase (JNK) pathway. Reprod. Sci. 2011, 18, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.P.; Wang, H.W.; Liu, J.; Tan, P.P.; Lin, L.; Zhou, B.H. JNK/STAT signalling pathway is involved in fluoride-induced follicular developmental dysplasia in female mice. Chemosphere 2018, 209, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Tanel, A.; Averill-Bates, D.A. P38 and ERK mitogen-activated protein kinases mediate acrolein-induced apoptosis in Chinese hamster ovary cells. Cell. Signal. 2007, 19, 968–977. [Google Scholar] [CrossRef]
- Brand, A.H.; Perrimon, N. Raf acts downstream of the EGF receptor to determine dorsoventral polarity during Drosophila oogenesis. Genes Dev. 1994, 8, 629–639. [Google Scholar] [CrossRef] [Green Version]
- Castanieto, A.; Johnston, M.J.; Nystul, T.G. EGFR signaling promotes self-renewal through the establishment of cell polarity in Drosophila follicle stem cells. eLife 2014, 3, e04437. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Chi, B.; Chen, P.; Liu, Y. 20E and MAPK signal pathway involved in the effect of reproduction caused by cyantraniliprole in Bactrocera dorsalis Hendel (Diptera: Tephritidae). Pest Manag. Sci. 2022, 78, 63–72. [Google Scholar] [CrossRef]
- Bastock, R.; St Johnston, D. Drosophila oogenesis. Curr. Biol. 2008, 18, R1082–R1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLaughlin, J.M.; Bratu, D.P. Drosophila melanogaster oogenesis: An overview. In Drosophila Oogenesis; Methods in Molecular Biology; Bratu, D., McNeil, G., Eds.; Humana Press: New York, NY, USA, 2015; Volume 1328, pp. 1–20. [Google Scholar]
- Dansereau, D.A.; Lasko, P. The development of germline stem cells in Drosophila. Methods Mol. Biol. 2008, 450, 3–26. [Google Scholar] [PubMed] [Green Version]
- Nakao, H.; Takasu, Y. Complexities in Bombyx germ cell formation process revealed by Bm-nosO (a Bombyx homolog of nanos) knockout. Dev. Biol. 2019, 445, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Saha, T.T.; Zou, Z.; Raikhel, A.S. Regulatory pathways controlling female insect reproduction. Annu. Rev. Entomol. 2018, 63, 489–511. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, L.; He, Q.; Zhou, S. Regulatory mechanisms of vitellogenesis in insects. Front. Cell Dev. Biol. 2020, 8, 593613. [Google Scholar] [CrossRef]
- Santos, C.G.; Humann, F.C.; Hartfelder, K. Juvenile hormone signaling in insect oogenesis. Curr. Opin. Insect Sci. 2019, 31, 43–48. [Google Scholar] [CrossRef]
- Belles, X.; Piulachs, M.D. Ecdysone signalling and ovarian development in insects: From stem cells to ovarian follicle formation. Biochim. Biophys. Acta 2015, 1849, 181–186. [Google Scholar] [CrossRef]
- Khalid, M.Z.; Ahmad, S.; Ngegba, P.M.; Zhong, G. Role of endocrine system in the regulation of female insect reproduction. Biology 2021, 10, 614. [Google Scholar] [CrossRef]
- Lin, X.; Smagghe, G. Roles of the insulin signaling pathway in insect development and organ growth. Peptides 2019, 122, 169923. [Google Scholar] [CrossRef]
- Lin, K.Y.; Hsu, H.J. Regulation of adult female germline stem cells by nutrient-responsive signaling. Curr. Opin. Insect Sci. 2020, 37, 16–22. [Google Scholar] [CrossRef]
- Gu, S.H.; Li, G.; Hsieh, H.Y.; Lin, P.L.; Li, S. Stimulation of JNK phosphorylation by the PTTH in prothoracic glands of the silkworm, Bombyx mori. Front. Physiol. 2018, 9, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Z.; Kang, S.; Sun, D.; Gong, L.; Zhou, J.; Qin, J.; Guo, L.; Zhu, L.; Bai, Y.; Ye, F.; et al. MAPK-dependent hormonal signaling plasticity contributes to overcoming Bacillus thuringiensis toxin action in an insect host. Nat. Commun. 2020, 11, 3003. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Zhang, T.; Feng, Y.; Liu, X.; Zhang, L.; Chen, H.; Zeng, F.; Wang, M.; Liu, C.; Li, Y.; et al. Two insulin receptors coordinate oogenesis and oviposition via two pathways in the green lacewing, Chrysopa pallens. J. Insect Physiol. 2020, 123, 104049. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B. Occurrence and control of common pests in rape (In Chinese). J. Agric. Catastrophol. 2020, 10, 15–16. [Google Scholar]
- Xue, F.S.; Li, A.Q.; Zhu, X.F.; Gui, A.L.; Jiang, P.L.; Liu, X.F. Diversity in life history of the leaf beetle, Colaphellus bowringi Baly. Acta Entomol. Sin. 2002, 45, 494–498. [Google Scholar]
- Tian, Z.; Guo, S.; Li, J.X.; Zhu, F.; Liu, W.; Wang, X.P. Juvenile hormone biosynthetic genes are critical for regulating reproductive diapause in the cabbage beetle. Insect Biochem. Mol. Biol. 2021, 139, 103654. [Google Scholar] [CrossRef]
- Guo, S.; Wu, Q.W.; Tian, Z.; Zhu, L.; King-Jones, K.; Zhu, F.; Wang, X.P.; Liu, W. Krüppel homolog 1 regulates photoperiodic reproductive plasticity in the cabbage beetle Colaphellus bowringi. Insect Biochem. Mol. Biol. 2021, 134, 103582. [Google Scholar] [CrossRef]
- Guo, S.; Tian, Z.; Wu, Q.W.; King-Jones, K.; Liu, W.; Zhu, F.; Wang, X.P. Steroid hormone ecdysone deficiency stimulates preparation for photoperiodic reproductive diapause. PLoS Genet. 2021, 17, e1009352. [Google Scholar] [CrossRef]
- Xue, F.; Spieth, H.R.; Aiqing, L.; Ai, H. The role of photoperiod and temperature in determination of summer and winter diapause in the cabbage beetle, Colaphellus bowringi (Coleoptera: Chrysomelidae). J. Insect Physiol. 2002, 48, 279–286. [Google Scholar] [CrossRef]
- Gallo, K.A.; Johnson, G.L. Mixed-lineage kinase control of JNK and p38 MAPK pathways. Nat. Rev. Mol. Cell Biol. 2002, 3, 663–672. [Google Scholar] [CrossRef]
- Shilo, B.Z. The regulation and functions of MAPK pathways in Drosophila. Methods 2014, 68, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.Q.; Zhu, L.; Li, Y.; Liu, W.; Ma, W.H.; Lei, C.L.; Wang, X.P. A de novo transcriptome and valid reference genes for quantitative real-time PCR in Colaphellus bowringi. PLoS ONE 2015, 10, e0118693. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, Y.; Zhu, L.; Zhu, F.; Lei, C.L.; Wang, X.P. Juvenile hormone facilitates the antagonism between adult reproduction and diapause through the methoprene-tolerant gene in the female Colaphellus bowringi. Insect Biochem. Mol. Biol. 2016, 74, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Sun, D.; Tian, Z.; Liu, W.; Zhu, F.; Wang, X.P. The limited regulatory roles of juvenile hormone degradation pathways in reproductive diapause preparation of the cabbage beetle, Colaphellus bowringi. J. Insect Physiol. 2019, 119, 103967. [Google Scholar] [CrossRef]
- Jindra, M.; Palli, S.R.; Riddiford, L.M. The juvenile hormone signaling pathway in insect development. Annu. Rev. Entomol. 2013, 58, 181–204. [Google Scholar] [CrossRef]
- Lagueux, M.; Hirn, M.; Hoffmann, J.A. Ecdysone during ovarian development in Locusta migratoria. J. Insect Physiol. 1977, 23, 109–119. [Google Scholar] [CrossRef]
- Fan, H.Y.; Liu, Z.; Mullany, L.K.; Richards, J.S. Consequences of RAS and MAPK activation in the ovary: The good, the bad and the ugly. Mol. Cell. Endocrinol. 2012, 356, 74–79. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Jiang, J.C.; Dai, X.X.; Fan, H.Y. Function and molecular mechanism of mitogen-activated protein kinase (MAPK) in regulating oocyte meiotic maturation and ovulation. Shengli Xuebao 2020, 72, 48–62. [Google Scholar]
- Das, D.; Arur, S. Conserved insulin signaling in the regulation of oocyte growth, development, and maturation. Mol. Reprod. Dev. 2017, 84, 444–459. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Ren, Z.; Tang, L.; Yao, H.; Li, X.; Wang, C.; Mu, C.; Shi, C.; Wang, H. JNK signaling pathway regulates the development of ovaries and synthesis of vitellogenin (Vg) in the swimming crab Portunus trituberculatus. Cell Stress Chaperones 2020, 25, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, D.S.; Tachibana, K.; Sumitani, M.; Lee, J.M.; Hatakeyama, M. Involvement of Mos-MEK-MAPK pathway in cytostatic factor (CSF) arrest in eggs of the parthenogenetic insect, Athalia rosae. Mech. Dev. 2008, 125, 996–1008. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, D.S.; Hatakeyama, M.; Matsuoka, H. Artificial activation of mature unfertilized eggs in the malaria vector mosquito, Anopheles stephensi (Diptera, Culicidae). J. Exp. Biol. 2013, 216, 2960–2966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brennan, D.F.; Dar, A.C.; Hertz, N.T.; Chao, W.C.; Burlingame, A.L.; Shokat, K.M.; Barford, D. A Raf-induced allosteric transition of KSR stimulates phosphorylation of MEK. Nature 2011, 472, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Raabe, T.; Rapp, U.R. KSR—A regulator and scaffold protein of the MAPK pathway. Sci. STKE 2002, 2002, pe28. [Google Scholar] [CrossRef]
- Frodyma, D.; Neilsen, B.; Costanzo-Garvey, D.; Fisher, K.; Lewis, R. Coordinating ERK signaling via the molecular scaffold Kinase Suppressor of Ras. F1000Res. 2017, 6, 1621. [Google Scholar] [CrossRef] [PubMed]
- Suzanne, M.; Irie, K.; Glise, B.; Agnes, F.; Mori, E.; Matsumoto, K.; Noselli, S. The Drosophila p38 MAPK pathway is required during oogenesis for egg asymmetric development. Genes Dev. 1999, 13, 1464–1474. [Google Scholar] [CrossRef] [Green Version]
- Zarse, K.; Schmeisser, S.; Groth, M.; Priebe, S.; Beuster, G.; Kuhlow, D.; Guthke, R.; Platzer, M.; Kahn, C.R.; Ristow, M. Impaired insulin/IGF1 signaling extends life span by promoting mitochondrial L-proline catabolism to induce a transient ROS signal. Cell Metab. 2012, 15, 451–465. [Google Scholar] [CrossRef] [Green Version]
- Hou, W.; Pei, J.; Wang, Y.; Zhang, J.; Zheng, H.; Cui, R. Anti-ageing effects of red ginseng on female Drosophila melanogaster. J. Cell. Mol. Med. 2020, 24, 3751–3755. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, L.I. Halloween genes encode P450 enzymes that mediate steroid hormone biosynthesis in Drosophila melanogaster. Mol. Cell. Endocrinol. 2004, 215, 1–10. [Google Scholar] [CrossRef]
- Cruz, J.; Martin, D.; Franch-Marro, X. Egfr signaling is a major regulator of ecdysone biosynthesis in the Drosophila prothoracic gland. Curr. Biol. 2020, 30, 1547–1554.e4. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.L.; Gu, S.H. In vitro and in vivo stimulation of extracellular signal-regulated kinase (ERK) by the prothoracicotropic hormone in prothoracic gland cells and its developmental regulation in the silkworm, Bombyx mori. J. Insect Physiol. 2007, 53, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.T.; Meng, Q.W.; Guo, W.C.; Li, G.Q. RNA interference suppression of the receptor tyrosine kinase Torso gene impaired pupation and adult emergence in Leptinotarsa decemlineata. J. Insect Physiol. 2015, 83, 53–64. [Google Scholar] [CrossRef]
- Kidokoro, K.; Iwata, K.; Fujiwara, Y.; Takeda, M. Effects of juvenile hormone analogs and 20-hydroxyecdysone on diapause termination in eggs of Locusta migratoria and Oxya yezoensis. J. Insect Physiol. 2006, 52, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Saffman, E.E.; Lasko, P. Germline development in vertebrates and invertebrates. Cell. Mol. Life Sci. 1999, 55, 1141–1163. [Google Scholar] [CrossRef]
- Dehghani, M.; Lasko, P. Multiple functions of the DEAD-Box helicase vasa in Drosophila oogenesis. Results Probl. Cell Differ. 2017, 63, 127–147. [Google Scholar] [PubMed]
- Martin-Blanco, E. P38 MAPK signalling cascades: Ancient roles and new functions. Bioessays 2000, 22, 637–645. [Google Scholar] [CrossRef]
- Adachi-Yamada, T.; Nakamura, M.; Irie, K.; Tomoyasu, Y.; Sano, Y.; Mori, E.; Goto, S.; Ueno, N.; Nishida, Y.; Matsumoto, K. P38 mitogen-activated protein kinase can be involved in transforming growth factor beta superfamily signal transduction in Drosophila wing morphogenesis. Mol. Cell. Biol. 1999, 19, 2322–2329. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z.; Tian, Z.; Zhao, Y.; Zhu, F.; Liu, W.; Wang, X. MAPK Signaling Pathway Is Essential for Female Reproductive Regulation in the Cabbage Beetle, Colaphellus bowringi. Cells 2022, 11, 1602. https://doi.org/10.3390/cells11101602
Huang Z, Tian Z, Zhao Y, Zhu F, Liu W, Wang X. MAPK Signaling Pathway Is Essential for Female Reproductive Regulation in the Cabbage Beetle, Colaphellus bowringi. Cells. 2022; 11(10):1602. https://doi.org/10.3390/cells11101602
Chicago/Turabian StyleHuang, Zijie, Zhong Tian, Yulian Zhao, Fen Zhu, Wen Liu, and Xiaoping Wang. 2022. "MAPK Signaling Pathway Is Essential for Female Reproductive Regulation in the Cabbage Beetle, Colaphellus bowringi" Cells 11, no. 10: 1602. https://doi.org/10.3390/cells11101602