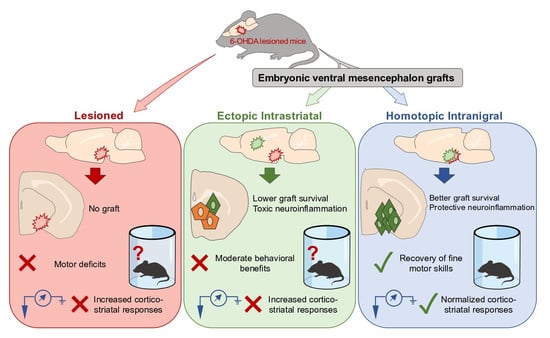
Better Outcomes with Intranigral versus Intrastriatal Cell Transplantation: Relevance for Parkinson’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Surgical Procedures
2.3. Behavioral Tests
2.4. L-DOPA Administration and Behavioral Assessment
2.5. Electrophysiological Measurements
2.6. Tissue Processing and Immunohistochemistry
2.7. Data Acquisition and Quantification
2.8. Statistical Analysis
3. Results
3.1. Both Intranigral and Intrastriatal Transplants Express Dopaminergic Markers
3.2. Dopamine Neurons within the Grafts Express SNpc and VTA Markers
3.3. Astrocyte and Microglia Polarization in the Grafts
3.4. Projection of Grafted Neurons
3.5. Establishment of Reciprocal Synaptic Contacts between Intrastriatal or Intranigral Transplants and Host Neurons
3.6. Functional Recovery of Lesioned Mice with Either Intranigral or Intrastriatal Transplants
3.7. Removal of Intranigral Graft Abolishes Functional Recovery
3.8. Electrophysiological Assessment of Striatal Projection Neurons
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dauer, W.; Przedborski, S. Parkinson’s disease: Mechanisms and models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef] [Green Version]
- Hornykiewicz, O. Dopamine (3-hydroxytyramine) and brain function. Pharmacol. Rev. 1966, 18, 925–964. [Google Scholar] [PubMed]
- Gelb, D.J.; Oliver, E.; Gilman, S. Diagnostic criteria for Parkinson disease. Arch. Neurol. 1999, 56, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Leveque, X.; Nerriere-Daguin, V.; Neveu, I.; Naveilhan, P. Pig neural cells derived from foetal mesencephalon as cell source for intracerebral xenotransplantation. Methods Mol. Biol. 2012, 885, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Wakeman, D.R.; Dodiya, H.B.; Kordower, J.H. Cell transplantation and gene therapy in Parkinson’s disease. Mt. Sinai J. Med. 2011, 78, 126–158. [Google Scholar] [CrossRef]
- Peschanski, M.; Defer, G.; N’Guyen, J.P.; Ricolfi, F.; Monfort, J.C.; Remy, P.; Geny, C.; Samson, Y.; Hantraye, P.; Jeny, R.; et al. Bilateral motor improvement and alteration of L-dopa effect in two patients with Parkinson’s disease following intrastriatal transplantation of foetal ventral mesencephalon. Brain 1994, 117 Pt 3, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Lindvall, O.; Bjorklund, A. Transplantation strategies in the treatment of Parkinson’s disease: Experimental basis and clinical trials. Acta Neurol. Scand. Suppl. 1989, 126, 197–210. [Google Scholar] [CrossRef]
- Bjorklund, A. Dopaminergic transplants in experimental parkinsonism: Cellular mechanisms of graft-induced functional recovery. Curr. Opin. Neurobiol. 1992, 2, 683–689. [Google Scholar] [CrossRef]
- Mendez, I.; Hong, M.; Smith, S.; Dagher, A.; Desrosiers, J. Neural transplantation cannula and microinjector system: Experimental and clinical experience. Technical note. J. Neurosurg. 2000, 92, 493–499. [Google Scholar] [CrossRef]
- Bjorklund, A.; Schmidt, R.H.; Stenevi, U. Functional reinnervation of the neostriatum in the adult rat by use of intraparenchymal grafting of dissociated cell suspensions from the substantia nigra. Cell Tissue Res. 1980, 212, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Freeman, T.B.; Olanow, C.W.; Hauser, R.A.; Nauert, G.M.; Smith, D.A.; Borlongan, C.V.; Sanberg, P.R.; Holt, D.A.; Kordower, J.H.; Vingerhoets, F.J.; et al. Bilateral fetal nigral transplantation into the postcommissural putamen in Parkinson’s disease. Ann. Neurol. 1995, 38, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Lindvall, O.; Rehncrona, S.; Brundin, P.; Gustavii, B.; Astedt, B.; Widner, H.; Lindholm, T.; Bjorklund, A.; Leenders, K.L.; Rothwell, J.C.; et al. Human fetal dopamine neurons grafted into the striatum in two patients with severe Parkinson’s disease. A detailed account of methodology and a 6-month follow-up. Arch. Neurol. 1989, 46, 615–631. [Google Scholar] [CrossRef] [PubMed]
- Mendez, I.; Sanchez-Pernaute, R.; Cooper, O.; Vinuela, A.; Ferrari, D.; Bjorklund, L.; Dagher, A.; Isacson, O. Cell type analysis of functional fetal dopamine cell suspension transplants in the striatum and substantia nigra of patients with Parkinson’s disease. Brain 2005, 128, 1498–1510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piccini, P.; Brooks, D.J.; Bjorklund, A.; Gunn, R.N.; Grasby, P.M.; Rimoldi, O.; Brundin, P.; Hagell, P.; Rehncrona, S.; Widner, H.; et al. Dopamine release from nigral transplants visualized in vivo in a Parkinson’s patient. Nat. Neurosci. 1999, 2, 1137–1140. [Google Scholar] [CrossRef] [PubMed]
- Nishino, H. Intracerebral grafting of catecholamine producing cells and reconstruction of disturbed brain function. Neurosci. Res. 1993, 16, 157–172. [Google Scholar] [CrossRef]
- Schmidt, R.H.; Ingvar, M.; Lindvall, O.; Stenevi, U.; Bjorklund, A. Functional activity of substantia nigra grafts reinnervating the striatum: Neurotransmitter metabolism and [14C]2-deoxy-D-glucose autoradiography. J. Neurochem. 1982, 38, 737–748. [Google Scholar] [CrossRef]
- Triarhou, L.C.; Stotz, E.H.; Low, W.C.; Norton, J.; Ghetti, B.; Landwehrmeyer, B.; Palacios, J.M.; Simon, J.R. Studies on the striatal dopamine uptake system of weaver mutant mice and effects of ventral mesencephalic grafts. Neurochem. Res. 1994, 19, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Fisher, L.J.; Gage, F.H. Grafting in the mammalian central nervous system. Physiol. Rev. 1993, 73, 583–616. [Google Scholar] [CrossRef]
- Bjorklund, A.; Stenevi, U. Regeneration of monoaminergic and cholinergic neurons in the mammalian central nervous system. Physiol. Rev. 1979, 59, 62–100. [Google Scholar] [CrossRef] [PubMed]
- Doucet, G.; Murata, Y.; Brundin, P.; Bosler, O.; Mons, N.; Geffard, M.; Ouimet, C.C.; Bjorklund, A. Host afferents into intrastriatal transplants of fetal ventral mesencephalon. Exp. Neurol. 1989, 106, 1–19. [Google Scholar] [CrossRef]
- Dunnett, S.B. Functional repair of striatal systems by neural transplants: Evidence for circuit reconstruction. Behav. Brain Res. 1995, 66, 133–142. [Google Scholar] [CrossRef]
- Mahalik, T.J.; Finger, T.E.; Stromberg, I.; Olson, L. Substantia nigra transplants into denervated striatum of the rat: Ultrastructure of graft and host interconnections. J. Comp. Neurol. 1985, 240, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Mendez, I.; Elisevich, K.; Flumerfelt, B. Dopaminergic innervation of substance P-containing striatal neurons by fetal nigral grafts: An ultrastructural double-labeling immunocytochemical study. J. Comp. Neurol. 1991, 308, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Nikkhah, G.; Cunningham, M.G.; McKay, R.; Bjorklund, A. Dopaminergic microtransplants into the substantia nigra of neonatal rats with bilateral 6-OHDA lesions. II. Transplant-induced behavioral recovery. J. Neurosci. 1995, 15, 3562–3570. [Google Scholar] [CrossRef]
- Wenning, G.K.; Odin, P.; Morrish, P.; Rehncrona, S.; Widner, H.; Brundin, P.; Rothwell, J.C.; Brown, R.; Gustavii, B.; Hagell, P.; et al. Short- and long-term survival and function of unilateral intrastriatal dopaminergic grafts in Parkinson’s disease. Ann. Neurol. 1997, 42, 95–107. [Google Scholar] [CrossRef]
- Remy, P.; Samson, Y.; Hantraye, P.; Fontaine, A.; Defer, G.; Mangin, J.F.; Fenelon, G.; Geny, C.; Ricolfi, F.; Frouin, V.; et al. Clinical correlates of [18F]fluorodopa uptake in five grafted parkinsonian patients. Ann. Neurol. 1995, 38, 580–588. [Google Scholar] [CrossRef]
- Kordower, J.H.; Freeman, T.B.; Snow, B.J.; Vingerhoets, F.J.; Mufson, E.J.; Sanberg, P.R.; Hauser, R.A.; Smith, D.A.; Nauert, G.M.; Perl, D.P.; et al. Neuropathological evidence of graft survival and striatal reinnervation after the transplantation of fetal mesencephalic tissue in a patient with Parkinson’s disease. N. Engl. J. Med. 1995, 332, 1118–1124. [Google Scholar] [CrossRef]
- Kordower, J.H.; Chu, Y.; Hauser, R.A.; Olanow, C.W.; Freeman, T.B. Transplanted dopaminergic neurons develop PD pathologic changes: A second case report. Mov. Disord. 2008, 23, 2303–2306. [Google Scholar] [CrossRef]
- Li, J.Y.; Christophersen, N.S.; Hall, V.; Soulet, D.; Brundin, P. Critical issues of clinical human embryonic stem cell therapy for brain repair. Trends Neurosci. 2008, 31, 146–153. [Google Scholar] [CrossRef]
- Mendez, I.; Vinuela, A.; Astradsson, A.; Mukhida, K.; Hallett, P.; Robertson, H.; Tierney, T.; Holness, R.; Dagher, A.; Trojanowski, J.Q.; et al. Dopamine neurons implanted into people with Parkinson’s disease survive without pathology for 14 years. Nat. Med. 2008, 14, 507–509. [Google Scholar] [CrossRef]
- Mehta, V.; Spears, J.; Mendez, I. Neural transplantation in Parkinson’s disease. Can. J. Neurol. Sci. 1997, 24, 292–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olanow, C.W.; Kordower, J.H.; Freeman, T.B. Fetal nigral transplantation as a therapy for Parkinson’s disease. Trends Neurosci. 1996, 19, 102–109. [Google Scholar] [CrossRef]
- Nikkhah, G.; Cunningham, M.G.; Cenci, M.A.; McKay, R.D.; Bjorklund, A. Dopaminergic microtransplants into the substantia nigra of neonatal rats with bilateral 6-OHDA lesions. I. Evidence for anatomical reconstruction of the nigrostriatal pathway. J. Neurosci. 1995, 15, 3548–3561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bentlage, C.; Nikkhah, G.; Cunningham, M.G.; Bjorklund, A. Reformation of the nigrostriatal pathway by fetal dopaminergic micrografts into the substantia nigra is critically dependent on the age of the host. Exp. Neurol. 1999, 159, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.A.; Mendez, I. Long distance selective fiber outgrowth of transplanted hNT neurons in white matter tracts of the adult rat brain. J. Comp. Neurol. 2005, 486, 318–330. [Google Scholar] [CrossRef]
- Gaillard, A.; Prestoz, L.; Dumartin, B.; Cantereau, A.; Morel, F.; Roger, M.; Jaber, M. Reestablishment of damaged adult motor pathways by grafted embryonic cortical neurons. Nat. Neurosci. 2007, 10, 1294–1299. [Google Scholar] [CrossRef]
- Gaillard, A.; Decressac, M.; Frappe, I.; Fernagut, P.O.; Prestoz, L.; Besnard, S.; Jaber, M. Anatomical and functional reconstruction of the nigrostriatal pathway by intranigral transplants. Neurobiol. Dis. 2009, 35, 477–488. [Google Scholar] [CrossRef]
- Tabakow, P.; Raisman, G.; Fortuna, W.; Czyz, M.; Huber, J.; Li, D.; Szewczyk, P.; Okurowski, S.; Miedzybrodzki, R.; Czapiga, B.; et al. Functional regeneration of supraspinal connections in a patient with transected spinal cord following transplantation of bulbar olfactory ensheathing cells with peripheral nerve bridging. Cell Transplant. 2014, 23, 1631–1655. [Google Scholar] [CrossRef] [Green Version]
- Thompson, L.H.; Grealish, S.; Kirik, D.; Bjorklund, A. Reconstruction of the nigrostriatal dopamine pathway in the adult mouse brain. Eur. J. Neurosci. 2009, 30, 625–638. [Google Scholar] [CrossRef] [Green Version]
- Dunnett, S.B. Anatomical and behavioral consequences of cholinergic-rich grafts to the neocortex of rats with lesions of the nucleus basalis magnocellularis. Ann. N. Y. Acad. Sci. 1987, 495, 415–430. [Google Scholar] [CrossRef]
- Grealish, S.; Diguet, E.; Kirkeby, A.; Mattsson, B.; Heuer, A.; Bramoulle, Y.; Van Camp, N.; Perrier, A.L.; Hantraye, P.; Bjorklund, A.; et al. Human ESC-derived dopamine neurons show similar preclinical efficacy and potency to fetal neurons when grafted in a rat model of Parkinson’s disease. Cell Stem Cell 2014, 15, 653–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heuer, A.; Vinh, N.N.; Dunnett, S.B. Behavioural recovery on simple and complex tasks by means of cell replacement therapy in unilateral 6-hydroxydopamine-lesioned mice. Eur. J. Neurosci. 2013, 37, 1691–1704. [Google Scholar] [CrossRef] [PubMed]
- Nikkhah, G.; Duan, W.M.; Knappe, U.; Jodicke, A.; Bjorklund, A. Restoration of complex sensorimotor behavior and skilled forelimb use by a modified nigral cell suspension transplantation approach in the rat Parkinson model. Neuroscience 1993, 56, 33–43. [Google Scholar] [CrossRef]
- Dowd, E.; Monville, C.; Torres, E.M.; Dunnett, S.B. The Corridor Task: A simple test of lateralised response selection sensitive to unilateral dopamine deafferentation and graft-derived dopamine replacement in the striatum. Brain Res. Bull. 2005, 68, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Dowd, E.; Dunnett, S.B. Deficits in a lateralized associative learning task in dopamine-depleted rats with functional recovery by dopamine-rich transplants. Eur. J. Neurosci. 2004, 20, 1953–1959. [Google Scholar] [CrossRef]
- Sawamoto, K.; Nakao, N.; Kobayashi, K.; Matsushita, N.; Takahashi, H.; Kakishita, K.; Yamamoto, A.; Yoshizaki, T.; Terashima, T.; Murakami, F.; et al. Visualization, direct isolation, and transplantation of midbrain dopaminergic neurons. Proc. Natl. Acad. Sci. USA 2001, 98, 6423–6428. [Google Scholar] [CrossRef] [Green Version]
- Okabe, M.; Ikawa, M.; Kominami, K.; Nakanishi, T.; Nishimune, Y. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 1997, 407, 313–319. [Google Scholar] [CrossRef] [Green Version]
- Bjorklund, A.; Stenevi, U.; Schmidt, R.H.; Dunnett, S.B.; Gage, F.H. Intracerebral grafting of neuronal cell suspensions. I. Introduction and general methods of preparation. Acta Physiol. Scand. Suppl. 1983, 522, 1–7. [Google Scholar]
- Schallert, T.; Fleming, S.M.; Leasure, J.L.; Tillerson, J.L.; Bland, S.T. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology 2000, 39, 777–787. [Google Scholar] [CrossRef]
- Boix, J.; Padel, T.; Paul, G. A partial lesion model of Parkinson’s disease in mice--characterization of a 6-OHDA-induced medial forebrain bundle lesion. Behav. Brain Res. 2015, 284, 196–206. [Google Scholar] [CrossRef]
- Heuer, A.; Smith, G.A.; Lelos, M.J.; Lane, E.L.; Dunnett, S.B. Unilateral nigrostriatal 6-hydroxydopamine lesions in mice I: Motor impairments identify extent of dopamine depletion at three different lesion sites. Behav. Brain Res. 2012, 228, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Lundblad, M.; Andersson, M.; Winkler, C.; Kirik, D.; Wierup, N.; Cenci, M.A. Pharmacological validation of behavioural measures of akinesia and dyskinesia in a rat model of Parkinson’s disease. Eur. J. Neurosci. 2002, 15, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Francardo, V.; Recchia, A.; Popovic, N.; Andersson, D.; Nissbrandt, H.; Cenci, M.A. Impact of the lesion procedure on the profiles of motor impairment and molecular responsiveness to L-DOPA in the 6-hydroxydopamine mouse model of Parkinson’s disease. Neurobiol. Dis. 2011, 42, 327–340. [Google Scholar] [CrossRef] [Green Version]
- Grealish, S.; Mattsson, B.; Draxler, P.; Bjorklund, A. Characterisation of behavioural and neurodegenerative changes induced by intranigral 6-hydroxydopamine lesions in a mouse model of Parkinson’s disease. Eur. J. Neurosci. 2010, 31, 2266–2278. [Google Scholar] [CrossRef] [PubMed]
- Drucker-Colin, R.; Garcia-Hernandez, F. A new motor test sensitive to aging and dopaminergic function. J. Neurosci. Methods 1991, 39, 153–161. [Google Scholar] [CrossRef]
- Glajch, K.E.; Fleming, S.M.; Surmeier, D.J.; Osten, P. Sensorimotor assessment of the unilateral 6-hydroxydopamine mouse model of Parkinson’s disease. Behav. Brain Res. 2012, 230, 309–316. [Google Scholar] [CrossRef] [Green Version]
- Ungerstedt, U.; Arbuthnott, G.W. Quantitative recording of rotational behavior in rats after 6-hydroxy-dopamine lesions of the nigrostriatal dopamine system. Brain Res. 1970, 24, 485–493. [Google Scholar] [CrossRef]
- Dunnett, S.B.; Torres, E.M. Rotation in the 6-OHDA-Lesioned Rat. In Animal Models of Movement Disorders; Lane, E.L., Dunnett, S.B., Eds.; Humana Press: Totowa, NJ, USA, 2011; pp. 299–315. [Google Scholar]
- Montoya, C.P.; Campbell-Hope, L.J.; Pemberton, K.D.; Dunnett, S.B. The “staircase test”: A measure of independent forelimb reaching and grasping abilities in rats. J. Neurosci. Methods 1991, 36, 219–228. [Google Scholar] [CrossRef]
- Baird, A.L.; Meldrum, A.; Dunnett, S.B. The staircase test of skilled reaching in mice. Brain Res. Bull. 2001, 54, 243–250. [Google Scholar] [CrossRef]
- Whishaw, I.Q.; Pellis, S.M.; Gorny, B.; Kolb, B.; Tetzlaff, W. Proximal and distal impairments in rat forelimb use in reaching follow unilateral pyramidal tract lesions. Behav. Brain Res. 1993, 56, 59–76. [Google Scholar] [CrossRef]
- Whishaw, I.Q.; Coles, B.L. Varieties of paw and digit movement during spontaneous food handling in rats: Postures, bimanual coordination, preferences, and the effect of forelimb cortex lesions. Behav. Brain Res. 1996, 77, 135–148. [Google Scholar] [CrossRef]
- Paxinos, G.; Franklin, K.B.J. The Mouse Brain in Stereotaxic Coordinates, 2nd ed.; Academic Press: San Diego, CA, USA, 2001. [Google Scholar]
- Pinault, D. A novel single-cell staining procedure performed in vivo under electrophysiological control: Morpho-functional features of juxtacellularly labeled thalamic cells and other central neurons with biocytin or Neurobiotin. J. Neurosci. Methods 1996, 65, 113–136. [Google Scholar] [CrossRef]
- Onn, S.P.; Berger, T.W.; Grace, A.A. Identification and characterization of striatal cell subtypes using in vivo intracellular recording in rats: I. Basic physiology and response to corticostriatal fiber stimulation. Synapse 1994, 16, 161–180. [Google Scholar] [CrossRef] [PubMed]
- Mallet, N.; Le Moine, C.; Charpier, S.; Gonon, F. Feedforward inhibition of projection neurons by fast-spiking GABA interneurons in the rat striatum in vivo. J. Neurosci. 2005, 25, 3857–3869. [Google Scholar] [CrossRef] [Green Version]
- Belujon, P.; Lodge, D.J.; Grace, A.A. Aberrant striatal plasticity is specifically associated with dyskinesia following levodopa treatment. Mov. Disord. 2010, 25, 1568–1576. [Google Scholar] [CrossRef] [Green Version]
- Abercrombie, M. Estimation of nuclear population from microtome sections. Anat. Rec. 1946, 94, 239–247. [Google Scholar] [CrossRef]
- Thompson, L.; Barraud, P.; Andersson, E.; Kirik, D.; Bjorklund, A. Identification of dopaminergic neurons of nigral and ventral tegmental area subtypes in grafts of fetal ventral mesencephalon based on cell morphology, protein expression, and efferent projections. J. Neurosci. 2005, 25, 6467–6477. [Google Scholar] [CrossRef] [Green Version]
- Damier, P.; Hirsch, E.C.; Agid, Y.; Graybiel, A.M. The substantia nigra of the human brain. I. Nigrosomes and the nigral matrix, a compartmental organization based on calbindin D(28K) immunohistochemistry. Brain 1999, 122 Pt 8, 1421–1436. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.L.; Sinton, C.M.; Sonsalla, P.K.; German, D.C. Midbrain dopaminergic neurons in the mouse that contain calbindin-D28k exhibit reduced vulnerability to MPTP-induced neurodegeneration. Neurodegeneration 1996, 5, 313–318. [Google Scholar] [CrossRef]
- McRitchie, D.A.; Hardman, C.D.; Halliday, G.M. Cytoarchitectural distribution of calcium binding proteins in midbrain dopaminergic regions of rats and humans. J. Comp. Neurol. 1996, 364, 121–150. [Google Scholar] [CrossRef]
- Rogers, J.H. Immunohistochemical markers in rat brain: Colocalization of calretinin and calbindin-D28k with tyrosine hydroxylase. Brain Res. 1992, 587, 203–210. [Google Scholar] [CrossRef]
- Inanobe, A.; Yoshimoto, Y.; Horio, Y.; Morishige, K.I.; Hibino, H.; Matsumoto, S.; Tokunaga, Y.; Maeda, T.; Hata, Y.; Takai, Y.; et al. Characterization of G-protein-gated K+ channels composed of Kir3.2 subunits in dopaminergic neurons of the substantia nigra. J. Neurosci. 1999, 19, 1006–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schein, J.C.; Hunter, D.D.; Roffler-Tarlov, S. Girk2 expression in the ventral midbrain, cerebellum, and olfactory bulb and its relationship to the murine mutation weaver. Dev. Biol. 1998, 204, 432–450. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.J. Astrocyte Heterogeneity in the Adult Central Nervous System. Front. Cell. Neurosci. 2018, 12, 401. [Google Scholar] [CrossRef] [Green Version]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Munch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Mallet, N.; Ballion, B.; Le Moine, C.; Gonon, F. Cortical inputs and GABA interneurons imbalance projection neurons in the striatum of parkinsonian rats. J. Neurosci. 2006, 26, 3875–3884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, K.Y.; Kasanetz, F.; Kargieman, L.; Riquelme, L.A.; Murer, M.G. Cortical slow oscillatory activity is reflected in the membrane potential and spike trains of striatal neurons in rats with chronic nigrostriatal lesions. J. Neurosci. 2001, 21, 6430–6439. [Google Scholar] [CrossRef] [PubMed]
- Brundin, P.; Li, J.Y.; Holton, J.L.; Lindvall, O.; Revesz, T. Research in motion: The enigma of Parkinson’s disease pathology spread. Nat. Rev. Neurosci. 2008, 9, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, A.; Jaber, M. Rewiring the brain with cell transplantation in Parkinson’s disease. Trends Neurosci. 2011, 34, 124–133. [Google Scholar] [CrossRef]
- Winkler, C.; Kirik, D.; Bjorklund, A.; Dunnett, S.B. Transplantation in the rat model of Parkinson’s disease: Ectopic versus homotopic graft placement. Prog. Brain Res. 2000, 127, 233–265. [Google Scholar]
- Freund, T.F.; Powell, J.F.; Smith, A.D. Tyrosine hydroxylase-immunoreactive boutons in synaptic contact with identified striatonigral neurons, with particular reference to dendritic spines. Neuroscience 1984, 13, 1189–1215. [Google Scholar] [CrossRef]
- Moss, J.; Bolam, J.P. A dopaminergic axon lattice in the striatum and its relationship with cortical and thalamic terminals. J. Neurosci. 2008, 28, 11221–11230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pickel, V.M.; Beckley, S.C.; Joh, T.H.; Reis, D.J. Ultrastructural immunocytochemical localization of tyrosine hydroxylase in the neostriatum. Brain Res. 1981, 225, 373–385. [Google Scholar] [CrossRef]
- Gagnon, D.; Petryszyn, S.; Sanchez, M.G.; Bories, C.; Beaulieu, J.M.; De Koninck, Y.; Parent, A.; Parent, M. Striatal Neurons Expressing D1 and D2 Receptors are Morphologically Distinct and Differently Affected by Dopamine Denervation in Mice. Sci. Rep. 2017, 7, 41432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Descarries, L.; Mechawar, N. Ultrastructural evidence for diffuse transmission by monoamine and acetylcholine neurons of the central nervous system. Prog. Brain Res. 2000, 125, 27–47. [Google Scholar] [CrossRef]
- Adler, A.F.; Cardoso, T.; Nolbrant, S.; Mattsson, B.; Hoban, D.B.; Jarl, U.; Wahlestedt, J.N.; Grealish, S.; Bjorklund, A.; Parmar, M. hESC-Derived Dopaminergic Transplants Integrate into Basal Ganglia Circuitry in a Preclinical Model of Parkinson’s Disease. Cell Rep. 2019, 28, 3462–3473.e5. [Google Scholar] [CrossRef] [Green Version]
- Abdi, A.; Mallet, N.; Mohamed, F.Y.; Sharott, A.; Dodson, P.D.; Nakamura, K.C.; Suri, S.; Avery, S.V.; Larvin, J.T.; Garas, F.N.; et al. Prototypic and arkypallidal neurons in the dopamine-intact external globus pallidus. J. Neurosci. 2015, 35, 6667–6688. [Google Scholar] [CrossRef] [Green Version]
- Glajch, K.E.; Kelver, D.A.; Hegeman, D.J.; Cui, Q.; Xenias, H.S.; Augustine, E.C.; Hernandez, V.M.; Verma, N.; Huang, T.Y.; Luo, M.; et al. Npas1+ Pallidal Neurons Target Striatal Projection Neurons. J. Neurosci. 2016, 36, 5472–5488. [Google Scholar] [CrossRef] [Green Version]
- Bye, C.R.; Thompson, L.H.; Parish, C.L. Birth dating of midbrain dopamine neurons identifies A9 enriched tissue for transplantation into parkinsonian mice. Exp. Neurol. 2012, 236, 58–68. [Google Scholar] [CrossRef]
- Brodacki, B.; Staszewski, J.; Toczylowska, B.; Kozlowska, E.; Drela, N.; Chalimoniuk, M.; Stepien, A. Serum interleukin (IL-2, IL-10, IL-6, IL-4), TNFalpha, and INFgamma concentrations are elevated in patients with atypical and idiopathic parkinsonism. Neurosci. Lett. 2008, 441, 158–162. [Google Scholar] [CrossRef]
- Gelders, G.; Baekelandt, V.; Van der Perren, A. Linking Neuroinflammation and Neurodegeneration in Parkinson’s Disease. J. Immunol. Res. 2018, 2018, 4784268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mogi, M.; Harada, M.; Narabayashi, H.; Inagaki, H.; Minami, M.; Nagatsu, T. Interleukin (IL)-1 beta, IL-2, IL-4, IL-6 and transforming growth factor-alpha levels are elevated in ventricular cerebrospinal fluid in juvenile parkinsonism and Parkinson’s disease. Neurosci. Lett. 1996, 211, 13–16. [Google Scholar] [CrossRef]
- Akiyama, H.; McGeer, P.L. Microglial response to 6-hydroxydopamine-induced substantia nigra lesions. Brain Res. 1989, 489, 247–253. [Google Scholar] [CrossRef]
- He, Y.; Appel, S.; Le, W. Minocycline inhibits microglial activation and protects nigral cells after 6-hydroxydopamine injection into mouse striatum. Brain Res. 2001, 909, 187–193. [Google Scholar] [CrossRef]
- Rodrigues, R.W.; Gomide, V.C.; Chadi, G. Astroglial and microglial reaction after a partial nigrostriatal degeneration induced by the striatal injection of different doses of 6-hydroxydopamine. Int. J. Neurosci. 2001, 109, 91–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Tao, S.; Fang, Y.; Guo, J.; Zhu, L.; Zhang, S. Infiltrating cells from host brain restore the microglial population in grafted cortical tissue. Sci. Rep. 2016, 6, 33080. [Google Scholar] [CrossRef] [Green Version]
- Appel, S.H.; Beers, D.R.; Henkel, J.S. T cell-microglial dialogue in Parkinson’s disease and amyotrophic lateral sclerosis: Are we listening? Trends Immunol. 2010, 31, 7–17. [Google Scholar] [CrossRef] [Green Version]
- Ballout, N.; Rochelle, T.; Brot, S.; Bonnet, M.L.; Francheteau, M.; Prestoz, L.; Zibara, K.; Gaillard, A. Characterization of Inflammation in Delayed Cortical Transplantation. Front. Mol. Neurosci. 2019, 12, 160. [Google Scholar] [CrossRef] [Green Version]
- Damier, P.; Hirsch, E.C.; Zhang, P.; Agid, Y.; Javoy-Agid, F. Glutathione peroxidase, glial cells and Parkinson’s disease. Neuroscience 1993, 52, 1–6. [Google Scholar] [CrossRef]
- Venkateshappa, C.; Harish, G.; Mythri, R.B.; Mahadevan, A.; Bharath, M.M.; Shankar, S.K. Increased oxidative damage and decreased antioxidant function in aging human substantia nigra compared to striatum: Implications for Parkinson’s disease. Neurochem. Res. 2012, 37, 358–369. [Google Scholar] [CrossRef]
- Braak, H.; Sastre, M.; Del Tredici, K. Development of alpha-synuclein immunoreactive astrocytes in the forebrain parallels stages of intraneuronal pathology in sporadic Parkinson’s disease. Acta Neuropathol. 2007, 114, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Nikkhah, G.; Olsson, M.; Eberhard, J.; Bentlage, C.; Cunningham, M.G.; Bjorklund, A. A microtransplantation approach for cell suspension grafting in the rat Parkinson model: A detailed account of the methodology. Neuroscience 1994, 63, 57–72. [Google Scholar] [CrossRef]
- Greenhalgh, A.D.; David, S.; Bennett, F.C. Immune cell regulation of glia during CNS injury and disease. Nat. Rev. Neurosci. 2020, 21, 139–152. [Google Scholar] [CrossRef]
- Goren, B.; Kahveci, N.; Eyigor, O.; Alkan, T.; Korfali, E.; Ozluk, K. Effects of intranigral vs intrastriatal fetal mesencephalic neural grafts on motor behavior disorders in a rat Parkinson model. Surg. Neurol. 2005, 64, S33–S41. [Google Scholar] [CrossRef]
- Nikkhah, G.; Bentlage, C.; Cunningham, M.G.; Bjorklund, A. Intranigral fetal dopamine grafts induce behavioral compensation in the rat Parkinson model. J. Neurosci. 1994, 14, 3449–3461. [Google Scholar] [CrossRef] [PubMed]
- Yurek, D.M. Intranigral transplants of fetal ventral mesencephalic tissue attenuate D1-agonist-induced rotational behavior. Exp. Neurol. 1997, 143, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Winkler, C.; Bentlage, C.; Nikkhah, G.; Samii, M.; Bjorklund, A. Intranigral transplants of GABA-rich striatal tissue induce behavioral recovery in the rat Parkinson model and promote the effects obtained by intrastriatal dopaminergic transplants. Exp. Neurol. 1999, 155, 165–186. [Google Scholar] [CrossRef] [PubMed]
- Mukhida, K.; Baker, K.A.; Sadi, D.; Mendez, I. Enhancement of sensorimotor behavioral recovery in hemiparkinsonian rats with intrastriatal, intranigral, and intrasubthalamic nucleus dopaminergic transplants. J. Neurosci. 2001, 21, 3521–3530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, K.A.; Sadi, D.; Hong, M.; Mendez, I. Simultaneous intrastriatal and intranigral dopaminergic grafts in the parkinsonian rat model: Role of the intranigral graft. J. Comp. Neurol. 2000, 426, 106–116. [Google Scholar] [CrossRef]
- Mendez, I.; Sadi, D.; Hong, M. Reconstruction of the nigrostriatal pathway by simultaneous intrastriatal and intranigral dopaminergic transplants. J. Neurosci. 1996, 16, 7216–7227. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, K.K.; Jiang, W.; Papazoglou, A.; Tenorio, S.B.; Dobrossy, M.; Nikkhah, G. Graft-mediated functional recovery on a skilled forelimb use paradigm in a rodent model of Parkinson’s disease is dependent on reward contingency. Behav. Brain Res. 2010, 212, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Rumpel, R.; Hohmann, M.; Klein, A.; Wesemann, M.; Baumgartner, W.; Ratzka, A.; Grothe, C. Transplantation of fetal ventral mesencephalic progenitor cells overexpressing high molecular weight fibroblast growth factor 2 isoforms in 6-hydroxydopamine lesioned rats. Neuroscience 2015, 286, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Abrous, D.N.; Torres, E.M.; Dunnett, S.B. Dopaminergic grafts implanted into the neonatal or adult striatum: Comparative effects on rotation and paw reaching deficits induced by subsequent unilateral nigrostriatal lesions in adulthood. Neuroscience 1993, 54, 657–668. [Google Scholar] [CrossRef]
- Hargus, G.; Cooper, O.; Deleidi, M.; Levy, A.; Lee, K.; Marlow, E.; Yow, A.; Soldner, F.; Hockemeyer, D.; Hallett, P.J.; et al. Differentiated Parkinson patient-derived induced pluripotent stem cells grow in the adult rodent brain and reduce motor asymmetry in Parkinsonian rats. Proc. Natl. Acad. Sci. USA 2010, 107, 15921–15926. [Google Scholar] [CrossRef] [Green Version]
- Mandel, R.J.; Brundin, P.; Bjorklund, A. The Importance of Graft Placement and Task Complexity for Transplant-Induced Recovery of Simple and Complex Sensorimotor Deficits in Dopamine Denervated Rats. Eur. J. Neurosci. 1990, 2, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Dunnett, S.B. Analysis of skilled forelimb movement in rats: The single pellet reaching test and staircase test. Curr. Protoc. Neurosci. 2012, 58, 8–28. [Google Scholar] [CrossRef]
- Lelos, M.J.; Dowd, E.; Dunnett, S.B. Nigral grafts in animal models of Parkinson’s disease. Is recovery beyond motor function possible? Prog. Brain Res. 2012, 200, 113–142. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.J.; Dunnett, S.B.; Isacson, O.; Sirinathsinghji, D.J.; Bjorklund, A. Striatal grafts in rats with unilateral neostriatal lesions--I. Ultrastructural evidence of afferent synaptic inputs from the host nigrostriatal pathway. Neuroscience 1988, 24, 791–801. [Google Scholar] [CrossRef]
- Freund, T.F.; Bolam, J.P.; Bjorklund, A.; Stenevi, U.; Dunnett, S.B.; Powell, J.F.; Smith, A.D. Efferent synaptic connections of grafted dopaminergic neurons reinnervating the host neostriatum: A tyrosine hydroxylase immunocytochemical study. J. Neurosci. 1985, 5, 603–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strecker, R.E.; Sharp, T.; Brundin, P.; Zetterstrom, T.; Ungerstedt, U.; Bjorklund, A. Autoregulation of dopamine release and metabolism by intrastriatal nigral grafts as revealed by intracerebral dialysis. Neuroscience 1987, 22, 169–178. [Google Scholar] [CrossRef]
- Freed, W.J. Functional brain tissue transplantation: Reversal of lesion-induced rotation by intraventricular substantia nigra and adrenal medulla grafts, with a note on intracranial retinal grafts. Biol. Psychiatry 1983, 18, 1205–1267. [Google Scholar] [PubMed]
- Montoya, C.P.; Astell, S.; Dunnett, S.B. Effects of nigral and striatal grafts on skilled forelimb use in the rat. Prog. Brain Res. 1990, 82, 459–466. [Google Scholar]
- Bouchard, S. Optimum symptomatic control of Parkinson’s disease with dopaminergic therapy. Can. J. Neurol. Sci. 1987, 14, 460–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olanow, C.W.; Gauger, L.L.; Cedarbaum, J.M. Temporal relationships between plasma and cerebrospinal fluid pharmacokinetics of levodopa and clinical effect in Parkinson’s disease. Ann. Neurol. 1991, 29, 556–559. [Google Scholar] [CrossRef] [PubMed]
- Burleigh, A.; Horak, F.; Nutt, J.; Frank, J. Levodopa reduces muscle tone and lower extremity tremor in Parkinson’s disease. Can. J. Neurol. Sci. 1995, 22, 280–285. [Google Scholar] [CrossRef] [Green Version]
- Fabbri, M.; Coelho, M.; Abreu, D.; Guedes, L.C.; Rosa, M.M.; Costa, N.; Antonini, A.; Ferreira, J.J. Do patients with late-stage Parkinson’s disease still respond to levodopa? Parkinsonism. Relat. Disord. 2016, 26, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Sacks, H.S. Cellulitis and agitation: A diagnostic dilemma. Hosp. Pract. Off. Ed. 1982, 17, 179–182; 186. [Google Scholar] [PubMed]
- Castiello, U.; Bennett, K.M.; Bonfiglioli, C.; Peppard, R.F. The reach-to-grasp movement in Parkinson’s disease before and after dopaminergic medication. Neuropsychologia 2000, 38, 46–59. [Google Scholar] [CrossRef]
- Johnels, B.; Ingvarsson, P.E.; Thorselius, M.; Valls, M.; Steg, G. Disability profiles and objective quantitative assessment in Parkinson’s disease. Acta Neurol. Scand. 1989, 79, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Okuma, Y.; Yanagisawa, N. The clinical spectrum of freezing of gait in Parkinson’s disease. Mov. Disord. 2008, 23, S426–S430. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, J.D.; Giladi, N.; Balash, Y.; Bartels, A.L.; Gurevich, T.; Hausdorff, J.M. Gait dynamics in Parkinson’s disease: Relationship to Parkinsonian features, falls and response to levodopa. J. Neurol. Sci. 2003, 212, 47–53. [Google Scholar] [CrossRef]
- Metz, G.A.; Farr, T.; Ballermann, M.; Whishaw, I.Q. Chronic levodopa therapy does not improve skilled reach accuracy or reach range on a pasta matrix reaching task in 6-OHDA dopamine-depleted (hemi-Parkinson analogue) rats. Eur. J. Neurosci. 2001, 14, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.; Nikkhah, G.; Bentlage, C.; Bjorklund, A. Forelimb akinesia in the rat Parkinson model: Differential effects of dopamine agonists and nigral transplants as assessed by a new stepping test. J. Neurosci. 1995, 15, 3863–3875. [Google Scholar] [CrossRef] [PubMed]
- Delaville, C.; McCoy, A.J.; Gerber, C.M.; Cruz, A.V.; Walters, J.R. Subthalamic nucleus activity in the awake hemiparkinsonian rat: Relationships with motor and cognitive networks. J. Neurosci. 2015, 35, 6918–6930. [Google Scholar] [CrossRef] [PubMed]
- Rodter, A.; Winkler, C.; Samii, M.; Nikkhah, G. Complex sensorimotor behavioral changes after terminal striatal 6-OHDA lesion and transplantation of dopaminergic embryonic micrografts. Cell Transplant. 2000, 9, 197–214. [Google Scholar] [CrossRef]
- Navailles, S.; Bioulac, B.; Gross, C.; De Deurwaerdere, P. Serotonergic neurons mediate ectopic release of dopamine induced by L-DOPA in a rat model of Parkinson’s disease. Neurobiol. Dis. 2010, 38, 136–143. [Google Scholar] [CrossRef]
- Robertson, G.S.; Robertson, H.A. Evidence that the substantia nigra is a site of action for L-DOPA. Neurosci. Lett. 1988, 89, 204–208. [Google Scholar] [CrossRef]
- Robertson, G.S.; Robertson, H.A. Evidence that L-dopa-induced rotational behavior is dependent on both striatal and nigral mechanisms. J. Neurosci. 1989, 9, 3326–3331. [Google Scholar] [CrossRef]
- Hefti, F.; Melamed, E.; Wurtman, R.J. The decarboxylation of DOPA in the parkinsonian brain: In vivo studies on an animal model. J. Neural Transm. Suppl. 1980, 16, 95–101. [Google Scholar]
- Mendez, I.; Dagher, A.; Hong, M.; Gaudet, P.; Weerasinghe, S.; McAlister, V.; King, D.; Desrosiers, J.; Darvesh, S.; Acorn, T.; et al. Simultaneous intrastriatal and intranigral fetal dopaminergic grafts in patients with Parkinson disease: A pilot study. Report of three cases. J. Neurosurg. 2002, 96, 589–596. [Google Scholar] [CrossRef]
- Michelsen, K.A.; Acosta-Verdugo, S.; Benoit-Marand, M.; Espuny-Camacho, I.; Gaspard, N.; Saha, B.; Gaillard, A.; Vanderhaeghen, P. Area-specific reestablishment of damaged circuits in the adult cerebral cortex by cortical neurons derived from mouse embryonic stem cells. Neuron 2015, 85, 982–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peron, S.; Droguerre, M.; Debarbieux, F.; Ballout, N.; Benoit-Marand, M.; Francheteau, M.; Brot, S.; Rougon, G.; Jaber, M.; Gaillard, A. A Delay between Motor Cortex Lesions and Neuronal Transplantation Enhances Graft Integration and Improves Repair and Recovery. J. Neurosci. 2017, 37, 1820–1834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, P.; Jones, L.L.; Snyder, E.Y.; Tuszynski, M.H. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp. Neurol. 2003, 181, 115–129. [Google Scholar] [CrossRef]
- Ourednik, J.; Ourednik, V.; Lynch, W.P.; Schachner, M.; Snyder, E.Y. Neural stem cells display an inherent mechanism for rescuing dysfunctional neurons. Nat. Biotechnol. 2002, 20, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Hooshmand, M.J.; Sontag, C.J.; Uchida, N.; Tamaki, S.; Anderson, A.J.; Cummings, B.J. Analysis of host-mediated repair mechanisms after human CNS-stem cell transplantation for spinal cord injury: Correlation of engraftment with recovery. PLoS ONE 2009, 4, e5871. [Google Scholar] [CrossRef]












| Total 118 | 112 Remaining | 22 Intact | |||
| 90 Lesioned | 28 Lesioned | ||||
| 62 Transplanted | 6 β-actin-GFP mouse fetal VM tissue | 3 Intrastriatal | |||
| 3 Intranigral | |||||
| 56 TH-GFP mouse fetal VM tissue | 26 Intrastriatal | ||||
| 30 Intranigral | |||||
| 6 Excluded (Failed to reach the criterion for the staircase test training period) | |||||
| Intrastriatal | Vs | Intranigral | ||
|---|---|---|---|---|
| Anatomical comparison | Transplants | Graft survival | ||
| Decrease in GFP+ neurons within the graft | ||||
| Expression of dopaminergic markers | Same proportion of GFP/Calb+ neurons Same proportion of GFP/Girk2+ neurons | |||
| Differentiation into mature neurons | Increase in GFP/NeuN+ cells | |||
| Astrocytic evaluation | Increase in GFAP+ cells (surrounding and within the graft) | |||
| Level of inflammation | Increase in Iba1+ cells (surrounding and within the graft) | |||
| Inflammatory profile | Increase of Iba1/CD68+ cells | Increase of Iba1/Arg1+ cells | ||
| Increase in GFAP/C3+ cells (surrounding and within the graft) | ||||
| Low percentage of GFAP/CD109 + cells | ||||
| Projection of grafted neurons | Send projections to appropriate dopaminergic targets | |||
| Pathway taken by projections | Covering the entire rostrocaudal extent of the striatum Present in the Acb, FC and PC | Nigrostriatal and mesolimbocortical pathway Extend through the mfb, nigrostriatal pathway, GP exiting the dorsal striatum | ||
| Functional comparison | Synaptic contacts | Establish reciprocal synaptic contacts with the host circuits | ||
| Rotation tests | Decrease apomorphine- or amphetamine-induced rotations | |||
| Simple and complex behavioral tasks | No improvement in all behavioral tests | Improvement in all behavioral tests | ||
| L-DOPA administration | L-DOPA improved motor performance | No potentiation with L-DOPA | ||
| Electrophysiological assessment | Restoration of excitability | |||
| Increase cortical stimulation threshold | Normalize cortico- striatal responses | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Droguerre, M.; Brot, S.; Vitrac, C.; Benoit-Marand, M.; Belnoue, L.; Patrigeon, M.; Lainé, A.; Béré, E.; Jaber, M.; Gaillard, A. Better Outcomes with Intranigral versus Intrastriatal Cell Transplantation: Relevance for Parkinson’s Disease. Cells 2022, 11, 1191. https://doi.org/10.3390/cells11071191
Droguerre M, Brot S, Vitrac C, Benoit-Marand M, Belnoue L, Patrigeon M, Lainé A, Béré E, Jaber M, Gaillard A. Better Outcomes with Intranigral versus Intrastriatal Cell Transplantation: Relevance for Parkinson’s Disease. Cells. 2022; 11(7):1191. https://doi.org/10.3390/cells11071191
Chicago/Turabian StyleDroguerre, Marine, Sébastien Brot, Clément Vitrac, Marianne Benoit-Marand, Laure Belnoue, Maelig Patrigeon, Anaïs Lainé, Emile Béré, Mohamed Jaber, and Afsaneh Gaillard. 2022. "Better Outcomes with Intranigral versus Intrastriatal Cell Transplantation: Relevance for Parkinson’s Disease" Cells 11, no. 7: 1191. https://doi.org/10.3390/cells11071191