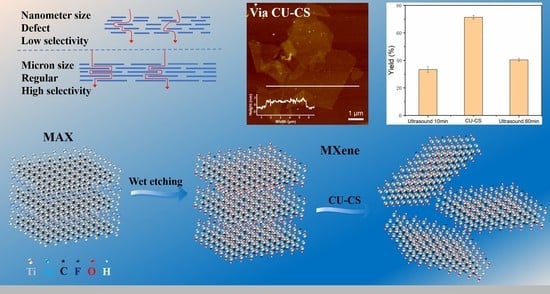
Enhanced Yield of Large-Sized Ti3C2Tx MXene Polymers Nanosheets via Cyclic Ultrasonic-Centrifugal Separation
Abstract
:1. Introduction
2. Experiments and Methods
2.1. Materials
2.2. Preparation of Ti3C2Tx MXene Polymers Nanosheets
2.3. Characterization
2.4. Yield Calculation
2.5. Membrane Water Flux Test
3. Results and Discussion
3.1. Composition and Structure Characterization
3.2. Effect of the Ultrasonic Method on the Size and Yield of the Nanosheets
3.3. Application of Ti3C2Tx MXene Nanosheets
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhatnagar, A.; Sillanpaa, M.; Witek-Krowiak, A. Agricultural waste peels as versatile biomass for water purification—A review. Chem. Eng. J. 2015, 270, 244–271. [Google Scholar] [CrossRef]
- Ma, N.L.; Zhang, N.; Yong, W.T.L.; Misbah, S.; Hashim, F.; Soon, C.F.; Sonne, C. Use, exposure and omics characterisation of potential hazard in nanomaterials. Mater. Today Adv. 2023, 17, 100341. [Google Scholar] [CrossRef]
- Joseph, L.; Boateng, L.K.; Flora, J.R.V.; Park, Y.-G.; Son, A.; Badawy, M.; Yoon, Y. Removal of bisphenol A and 17 alpha-ethinyl estradiol by combined coagulation and adsorption using carbon nanomaterials and powdered activated carbon. Sep. Purif. Technol. 2013, 107, 37–47. [Google Scholar] [CrossRef]
- Al-Hamadani, Y.A.J.; Chu, K.H.; Flora, J.R.V.; Kim, D.-H.; Jang, M.; Sohn, J.; Yoon, Y. Sonocatalytical degradation enhancement for ibuprofen and sulfamethoxazole in the presence of glass beads and single-walled carbon nanotubes. Ultrason. Sonochemistry 2016, 32, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-X.; Chen, C.-B.; Wang, Y.-J.; Fu, X.-Z.; Cui, S.; Lu, J.-Y.; Lau, T.-C. Insights on the pH-dependent roles of peroxymonosulfate and chlorine ions in phenol oxidative transformation. Chem. Eng. J. 2019, 362, 570–575. [Google Scholar] [CrossRef]
- Liu, S.S.; Chai, Y.C.; Guan, N.J.; Li, L.D. Small Molecule Adsorption and Separation on Zeolites. Chem. J. Chin. Univ. Chin. 2021, 42, 268–288. [Google Scholar] [CrossRef]
- Mansas, C.; Mendret, J.; Brosillon, S.; Ayral, A. Coupling catalytic ozonation and membrane separation: A review. Sep. Purif. Technol. 2020, 236, 116221. [Google Scholar] [CrossRef]
- Gu, L.P.; Tang, X.; Sun, Y.; Kou, H.J. Bioavailability of dissolved organic matter in biogas slurry enhanced by catalytic ozonation combined with membrane separation. Ecotoxicol. Environ. Saf. 2020, 196, 110547. [Google Scholar] [CrossRef]
- Paixao, R.M.; da Silva, L.; Reck, I.M.; Vieira, M.F.; Bergamasco, R.; Vieira, A.M.S. Deposition of graphene nanoparticles associated with tannic acid in microfiltration membrane for removal of food colouring. Environ. Technol. 2021, 42, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Ke, Q.R.; Ulbricht, M. In situ reactive coating of porous filtration membranes with functional polymer layers to integrate boron adsorber property. J. Membr. Sci. 2022, 660, 120851. [Google Scholar] [CrossRef]
- Yuan, S.S.; Li, X.; Zhu, J.Y.; Zhang, G.; Van Puyvelde, P.; Van der Bruggen, B. Covalent organic frameworks for membrane separation. Chem. Soc. Rev. 2019, 48, 2665–2681. [Google Scholar] [CrossRef]
- Saeid, P.; Zeinolabedini, M.; Khamforoush, M. Simulation of a crossflow ultrafiltration polysulfone/polyvinylpyrrolidone membrane separation using finite element analysis to separate oil/water emulsion. Iran. Polym. J. 2022, 40, 1–9. [Google Scholar] [CrossRef]
- Lu, Y.Q.; Zhu, Y.Y.; Ma, H.; Chen, F.Y.; Gao, C.J.; Xue, L.X. Wetting-induced superlyophobic polyacrylonitrile membranes: From reversible wettability to switchable on-demand emulsion separation. Sep. Purif. Technol. 2022, 297, 121438. [Google Scholar] [CrossRef]
- Wang, J.T.; Liu, T.; Lu, C.L.; Gong, C.J.; Miao, M.Y.; Wei, Z.L.; Wang, Y. Efficient oil-in-water emulsion separation in the low-cost bauxite ceramic membranes with hierarchically oriented straight pores. Sep. Purif. Technol. 2022, 303, 122244. [Google Scholar] [CrossRef]
- Xu, R.X.; Feng, J.Y.; Zhang, L.X.; Li, S.Q. Low viscosity of spinning liquid to prepare organic-inorganic hybrid ultrafine nanofiber membrane for high-efficiency filtration application. Sep. Purif. Technol. 2022, 303, 122224. [Google Scholar] [CrossRef]
- Ju, X.H.; Lu, J.P.; Zhao, L.L.; Lu, T.D.; Cao, X.L.; Jia, T.Z.; Sun, S.P. Electrospun transition layer that enhances the structure and performance of thin-film nanofibrous composite membranes. J. Membr. Sci. 2021, 620, 118927. [Google Scholar] [CrossRef]
- Fahrina, A.; Arahman, N.; Aprilia, S.; Bilad, M.R.; Silmina, S.; Sari, W.P.; Rajabzadeh, S. Functionalization of PEG-AgNPs Hybrid Material to Alleviate Biofouling Tendency of Polyethersulfone Membrane. Polymers 2022, 14, 1908. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.M.; Chen, J.C.; Liu, S.H.; Yang, H.; Ji, S.F.; Chen, H.Y. A double anti-fouling mechanism established by self-assembly of TiO2 on F127 chains for improving the hydrophilicity of PES membrane based on RTIPS method. Sep. Purif. Technol. 2021, 255, 117742. [Google Scholar] [CrossRef]
- Huang, T.F.; Zhang, L.; Chen, H.L.; Gao, C.J. Sol-gel fabrication of a non-laminated graphene oxide membrane for oil/water separation. J. Mater. Chem. A 2015, 3, 19517–19524. [Google Scholar] [CrossRef]
- Su, P.C.; Wang, F.; Li, Z.J.; Tang, C.Y.; Li, W.B. Graphene oxide membranes: Controlling their transport pathways. J. Mater. Chem. A 2020, 8, 15319–15340. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, Y.S.; Gao, X.L.; Ma, Z.; Wang, X.J.; Gao, C.J. Multilayered graphene oxide membranes for water treatment: A review. Carbon 2018, 139, 964–981. [Google Scholar] [CrossRef]
- Bai, Z.Y.; Liu, Q.; Zhang, H.S.; Yu, J.; Chen, R.R.; Liu, J.Y.; Wang, J. Anti-Biofouling and Water-Stable Balanced Charged Metal Organic Framework-Based Polyelectrolyte Hydrogels for Extracting Uranium from Seawater. Acs. Appl. Mater. Interfaces 2020, 12, 18012–18022. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, K.; Luo, B.; Hu, H.; Sun, D.; Wang, S.C.; Wang, L.Z. Polyethylenimine Expanded Graphite Oxide Enables High Sulfur Loading and Long-Term Stability of Lithium-Sulfur Batteries. Small 2019, 15, 1804578. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Zhang, H.Q.; Li, Y.F.; Wang, J.T.; Shi, B.B.; Mao, H.; Liu, J.D. Graphene oxide-embedded nanocomposite membrane for solvent resistant nanofiltration with enhanced rejection ability. Chem. Eng. Sci. 2015, 138, 227–238. [Google Scholar] [CrossRef]
- Weng, Q.; Wang, X.; Wang, X.; Bando, Y.; Golberg, D. Functionalized hexagonal boron nitride nanomaterials: Emerging properties and applications. Chem. Soc. Rev. 2016, 45, 3989–4012. [Google Scholar] [CrossRef] [Green Version]
- Fan, G.; Li, F.; Evans, D.G.; Duan, X. Catalytic applications of layered double hydroxides: Recent advances and perspectives. Chem. Soc. Rev. 2014, 43, 7040–7066. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Yang, Z.; Chen, K.; Wang, C.; Xiong, Y. 2D Layered Double Hydroxides for Oxygen Evolution Reaction: From Fundamental Design to Application. Adv. Energy Mater. 2019, 9, 1803358. [Google Scholar] [CrossRef]
- Choi, W.; Choudhary, N.; Han, G.H.; Park, J.; Akinwande, D.; Lee, Y.H. Recent development of two-dimensional transition metal dichalcogenides and their applications. Mater. Today 2017, 20, 116–130. [Google Scholar] [CrossRef]
- Liu, G.Z.; Shen, J.; Ji, Y.F.; Liu, Q.; Liu, G.P.; Yang, J.; Jin, W.Q. Two-dimensional Ti2CTx MXene membranes with integrated and ordered nanochannels for efficient solvent dehydration. J. Mater. Chem. A 2019, 7, 12095–12104. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.J.; Heon, M.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [Green Version]
- Sokol, M.; Natu, V.; Kota, S.; Barsoum, M.W. On the Chemical Diversity of the MAX Phases. Trends Chem. 2019, 1, 210–223. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Mathis, T.S.; Sarycheva, A.; Hatter, C.B.; Uzun, S.; Gogotsi, Y. Selective Etching of Silicon from Ti3SiC2 (MAX) To Obtain 2D Titanium Carbide (MXene). Angew. Chem. Int. Ed. 2018, 57, 5444–5448. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Zhao, G.Q.; Hu, J.; Zheng, Y.J.; Zhang, J.Y.; Zuo, Y.; Jiao, F.P. Cracked-earth-like titanium carbide MXene membranes with abundant hydroxyl groups for oil-in-water emulsion separation. J. Colloid Interface Sci. 2022, 607, 378–388. [Google Scholar] [CrossRef]
- Lv, K.; Zhang, J.Z.; Zhao, X.; Kong, N.; Tao, J.L.; Zhou, J. Understanding the Effect of Pore Size on Electrochemical Capacitive Performance of MXene Foams. Small 2022, 18, 2202203. [Google Scholar] [CrossRef]
- Tang, Q.; Zhou, Z.; Shen, P. Are MXenes Promising Anode Materials for Li Ion Batteries? Computational Studies on Electronic Properties and Li Storage Capability of Ti3C2 and Ti3C2X2 (X = F, OH) Monolayer. J. Am. Chem. Soc. 2012, 134, 16909–16916. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, L.B.; Liu, Z.B.; Chen, L.; Guo, J.K.; Kang, N.; Ren, W.C. Large-area high-quality 2D ultrathin Mo2C superconducting crystals. Nat. Mater. 2015, 14, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Zhang, C.F.; Kremer, M.P.; Seral-Ascaso, A.; Park, S.H.; McEvoy, N.; Anasori, B.; Nicolosi, V. Stamping of Flexible, Coplanar Micro-Supercapacitors Using MXene Inks. Adv. Funct. Mater. 2018, 28, 1705506. [Google Scholar] [CrossRef]
- Lakhe, P.; Prehn, E.M.; Habib, T.; Lutkenhaus, J.L.; Radovic, M.; Mannan, M.S.; Green, M.J. Process Safety Analysis for Ti3C2Tx MXene Synthesis and Processing. Ind. Eng. Chem. Res. 2019, 58, 1570–1579. [Google Scholar] [CrossRef]
- Ding, L.; Wei, Y.Y.; Wang, Y.J.; Chen, H.B.; Caro, J.; Wang, H.H. A Two-Dimensional Lamellar Membrane: MXene Nanosheet Stacks. Angew. Chem. Int. Ed. 2017, 56, 1825–1829. [Google Scholar] [CrossRef]
- Xuan, J.N.; Wang, Z.Q.; Chen, Y.Y.; Liang, D.J.; Cheng, L.; Yang, X.J.; Geng, F.X. Organic-Base-Driven Intercalation and Delamination for the Production of Functionalized Titanium Carbide Nanosheets with Superior Photothermal Therapeutic Performance. Angew. Chem. Int. Ed. 2016, 55, 14569–14574. [Google Scholar] [CrossRef]
- Qu, D.Y.; Jian, Y.Y.; Guo, L.H.; Su, C.; Tang, N.; Zhang, X.M.; Wu, W.W. An Organic Solvent-Assisted Intercalation and Collection (OAIC) for Ti3C2Tx MXene with Controllable Sizes and Improved Yield. Nano Micro Lett. 2021, 13, 188. [Google Scholar] [CrossRef]
- Huang, X.W.; Wu, P.Y. A Facile, High-Yield, and Freeze-and-Thaw-Assisted Approach to Fabricate MXene with Plentiful Wrinkles and Its Application in On-Chip Micro-Supercapacitors. Adv. Funct. Mater. 2020, 30, 1910048. [Google Scholar] [CrossRef]
- Han, F.; Luo, S.J.; Xie, L.Y.; Zhu, J.J.; Wei, W.; Chen, X.; Huang, Y. Boosting the Yield of MXene 2D Sheets via a Facile Hydrothermal-Assisted Intercalation. Acs. Appl. Mater. Interfaces 2019, 11, 8443–8452. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.G.; Zhu, J.L.; Gong, C.; Guan, Z.X.; Yang, D.; Shen, Z.H.; Wu, H.J. Achieving high yield of Ti3C2Tx MXene few-layer flakes with enhanced pseudocapacior performance by decreasing precursor size. Chin. Chem. Lett. 2020, 31, 1039–1043. [Google Scholar] [CrossRef]
- Naguib, M.; Unocic, R.R.; Armstrong, B.L.; Nanda, J. Large-scale delamination of multi-layers transition metal carbides and carbonitrides “MXenes”. Dalton Trans. 2015, 44, 9353–9358. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Liao, S.Y.; Qi, F.Y.; Liu, R.T.; Xiao, T.H.; Hu, J.Q.; Min, Y.G. Direct deposition of two-dimensional MXene nanosheets on commercially available filter for fast and efficient dye removal. J. Hazard. Mater. 2020, 384, 121367. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.K.; Liu, Y.C.; Li, L.B.; Wei, Y.Y.; Caro, J.; Wang, H.H. Ultra-thin titanium carbide (MXene) sheet membranes for high-efficient oil/water emulsions separation. J. Membr. Sci. 2019, 592, 117361. [Google Scholar] [CrossRef]
- Wu, X.Y.; Han, B.Y.; Zhang, H.B.; Xie, X.; Tu, T.X.; Zhang, Y.; Yu, Z.Z. Compressible, durable and conductive polydimethylsiloxane-coated MXene foams for high-performance electromagnetic interference shielding. Chem. Eng. J. 2020, 381, 122622. [Google Scholar] [CrossRef]
- Yang, G.; Lei, W.; Chen, C.; Qin, S.; Zhang, L.; Su, Y.; Liu, D. Ultrathin Ti3C2Tx (MXene) membrane for pressure-driven electrokinetic power generation. Nano Energy 2020, 75, 104954. [Google Scholar] [CrossRef]
- Liu, G.Z.; Shen, J.; Liu, Q.; Liu, G.P.; Xiong, J.; Yang, J.; Jin, W.Q. Ultrathin two-dimensional MXene membrane for pervaporation desalination. J. Membr. Sci. 2018, 548, 548–558. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, K.; Yang, Y.; Zhou, H.; Chen, X.; Ge, S. Enhanced Yield of Large-Sized Ti3C2Tx MXene Polymers Nanosheets via Cyclic Ultrasonic-Centrifugal Separation. Polymers 2023, 15, 1330. https://doi.org/10.3390/polym15061330
Hou K, Yang Y, Zhou H, Chen X, Ge S. Enhanced Yield of Large-Sized Ti3C2Tx MXene Polymers Nanosheets via Cyclic Ultrasonic-Centrifugal Separation. Polymers. 2023; 15(6):1330. https://doi.org/10.3390/polym15061330
Chicago/Turabian StyleHou, Kun, Yafeng Yang, Hu Zhou, Xiangmeng Chen, and Shengbo Ge. 2023. "Enhanced Yield of Large-Sized Ti3C2Tx MXene Polymers Nanosheets via Cyclic Ultrasonic-Centrifugal Separation" Polymers 15, no. 6: 1330. https://doi.org/10.3390/polym15061330