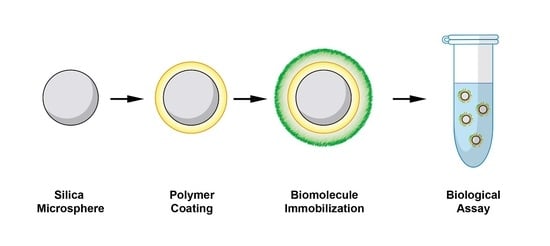
Polymeric Coating of Silica Microspheres for Biological Applications: Suppression of Non-Specific Binding and Functionalization with Biomolecules
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of MCP-6
2.3. Coating of Silica Microspheres Using MCP-6
2.4. Zeta Potential Measurement
2.5. Antifouling Properties Evaluation
2.6. Immobilization of Oligonucleotides on MCP-6 Coated Silica Microspheres
2.6.1. Immobilization of Oligonucleotides
2.6.2. Hybridization with Complementary DNA
2.7. Immobilization of Streptavidin on MCP-6 Coated Silica Microspheres
2.7.1. Synthesis of DBCO-Modified Streptavidin
2.7.2. Streptavidin Immobilization
2.7.3. Capture of Biotinylated Oligonucleotides
2.8. Immobilization of Antibodies on MCP-6 Coated Silica Microspheres
2.8.1. Synthesis of DBCO-Modified Rabbit IgG
2.8.2. Rabbit IgG Immobilization
2.8.3. Interaction with Secondary Antibody
2.9. Transmission Electron Microscopy
3. Results & Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campos, E.; Branquinho, J.; Carreira, A.S.; Carvalho, A.; Coimbra, P.; Ferreira, P.; Gil, M.H. Designing polymeric microparticles for biomedical and industrial applications. Eur. Polym. J. 2013, 49, 2005–2021. [Google Scholar] [CrossRef]
- Daly, A.C.; Riley, L.; Segura, T.; Burdick, J.A. Hydrogel microparticles for biomedical applications. Nat. Rev. Mater. 2020, 5, 20–43. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, L.; Ge, X.; Xu, B.; Zhang, W.; Qu, L.; Choi, C.H.; Xu, J.; Zhang, A.; Lee, H.; et al. Microfluidic fabrication of microparticles for biomedical applications. Chem. Soc. Rev. 2018, 47, 5646–5683. [Google Scholar] [CrossRef] [PubMed]
- Audonnet, V.; Malaquin, L.; Viovy, J.L. Polymeric coatings on micro- and nanometric particles for bioapplications. Bioanal. Rev. 2011, 3, 41–66. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, T.; Chu, E.; Zhang, A.; Du, J.; Sha, M.Y. A high-throughput microsphere-based immunoassay of anti-SARS-CoV-2 IgM testing for COVID-19 diagnostics. PLoS ONE 2021, 16, e0248444. [Google Scholar] [CrossRef]
- Camilla, C.; Ly, L.M.; Magnan, A.; Casano, B.; Prato, S.; Debono, S.; Montero, F.; Defoort, J.-P.; Martin, M.; Fert, V. Flow Cytometric Microsphere-Based Immunoassay: Analysis of Secreted Cytokines in Whole-Blood Samples from Asthmatics. Clin. Diagn. Lab. Immunol. 2001, 8, 776–784. [Google Scholar] [CrossRef] [Green Version]
- Meza, M. Application of Magnetic Particles in Immunoassays. In Scientific and Clinical Applications of Magnetic Carriers; Springer: Boston, MA, USA, 1997; pp. 303–309. [Google Scholar] [CrossRef]
- Khalife, J.; Sanchez, J.F.; Pichiorri, F. Extracellular Vesicles in Hematological Malignancies: From Biomarkers to Therapeutic Tools. Diagnostics 2020, 10, 1065. [Google Scholar] [CrossRef]
- Sharma, P.; Ludwig, S.; Muller, L.; Hong, C.S.; Kirkwood, J.M.; Ferrone, S.; Whiteside, T.L. Immunoaffinity-based isolation of melanoma cell-derived exosomes from plasma of patients with melanoma. J. Extracell. Vesicles 2018, 7, 1435138. [Google Scholar] [CrossRef]
- Akhavan, B.; Jarvis, K.; Majewski, P. Tuning the hydrophobicity of plasma polymer coated silica particles. Powder Technol. 2013, 249, 403–411. [Google Scholar] [CrossRef]
- Grewal, M.S.; Abe, H.; Matsuo, Y.; Yabu, H. Aqueous dispersion and tuning surface charges of polytetrafluoroethylene particles by bioinspired polydopamine–polyethyleneimine coating via one-step method. R. Soc. Open Sci. 2021, 8, 210582. [Google Scholar] [CrossRef]
- Reza, R.T.; Pérez, C.A.M.; González, C.A.R.; Romero, H.M.; Casillas, P.E.G. Effect of the polymeric coating over Fe3O4 particles used for magnetic separation. Cent. Eur. J. Chem. 2010, 8, 1041–1046. [Google Scholar] [CrossRef]
- Miller, P.J.; Shantz, D.F. Covalently functionalized uniform amino-silica nanoparticles. Synthesis and validation of amine group accessibility and stability. Nanoscale Adv. 2020, 2, 860–868. [Google Scholar] [CrossRef] [Green Version]
- Pirri, G.; Damin, F.; Chiari, M.; Bontempi, E.; Depero, L.E. Characterization of A Polymeric Adsorbed Coating for DNA Microarray Glass Slides. Anal. Chem. 2004, 5, 1352–1358. [Google Scholar] [CrossRef] [PubMed]
- Starling, J.; Da Silva, C.M.; Silva Dantas, M.S.; De Fátima, Â.; Oréfice, R.L. N-acryloxysuccinimide: Synthesis, characterization, and incorporation in dental adhesives. Int. J. Adhes. Adhes. 2011, 31, 767–774. [Google Scholar] [CrossRef]
- Marcelo, G.; Martinho, J.M.G.; Farinha, J.P.S. Polymer-coated nanoparticles by adsorption of hydrophobically modified poly(N,N-dimethylacrylamide). J. Phys. Chem. B 2013, 117, 3416–3427. [Google Scholar] [CrossRef] [PubMed]
- Cretich, M.; Pirri, G.; Damin, F.; Solinas, I.; Chiari, M. A new polymeric coating for protein microarrays. Anal. Biochem. 2004, 332, 67–74. [Google Scholar] [CrossRef]
- Brambilla, D.; Sola, L.; Chiari, M. Advantageous antibody microarray fabrication through DNA-directed immobilization: A step toward use of extracellular vesicles in diagnostics. Talanta 2021, 222, 121542. [Google Scholar] [CrossRef]
- Gori, A.; Romanato, A.; Greta, B.; Strada, A.; Gagni, P.; Frigerio, R.; Brambilla, D.; Vago, R.; Galbiati, S.; Picciolini, S.; et al. Membrane-binding peptides for extracellular vesicles on-chip analysis. J. Extracell. Vesicles 2020, 9, 1751428. [Google Scholar] [CrossRef]
- Sola, L.; Damin, F.; Gagni, P.; Consonni, R.; Chiari, M. Synthesis of clickable coating polymers by postpolymerization modification: Applications in microarray technology. Langmuir 2016, 32, 10284–10295. [Google Scholar] [CrossRef]
- Carl, P.; Ramos, I.I.; Segundo, M.A.; Schneider, R.J. Antibody conjugation to carboxyl-modified microspheres through N-hydroxysuccinimide chemistry for automated immunoassay applications: A general procedure. PLoS ONE 2019, 14, e0218686. [Google Scholar] [CrossRef]
- Lim, C.Y.; Owens, N.A.; Wampler, R.D.; Ying, Y.; Granger, J.H.; Porter, M.D.; Takahashi, M.; Shimazu, K. Succinimidyl Ester Surface Chemistry: Implications of the Competition between Aminolysis and Hydrolysis on Covalent Protein Immobilization. Langmuir 2014, 30, 12868–12878. [Google Scholar] [CrossRef] [PubMed]
- Poonthiyil, V.; Lindhorst, T.K.; Golovko, V.B.; Fairbanks, A.J. Recent applications of click chemistry for the functionalization of gold nanoparticles and their conversion to glyco-gold nanoparticles. Beilstein J. Org. Chem. 2018, 14, 11–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyde, A.M.; Zultanski, S.L.; Waldman, J.H.; Zhong, Y.L.; Shevlin, M.; Peng, F. General Principles and Strategies for Salting-Out Informed by the Hofmeister Series. Org. Process Res. Dev. 2017, 21, 1355–1370. [Google Scholar] [CrossRef] [Green Version]
- Zilio, C.; Sola, L.; Damin, F.; Faggioni, L.; Chiari, M. Universal hydrophilic coating of thermoplastic polymers currently used in microfluidics. Biomed. Microdevices 2014, 16, 107–114. [Google Scholar] [CrossRef] [Green Version]
- Sola, L.; Chiari, M. Tuning capillary surface properties by charged polymeric coatings. J. Chromatogr. A 2015, 1414, 173–181. [Google Scholar] [CrossRef]
- Medda, L.; Monduzzi, M.; Salis, A. The molecular motion of bovine serum albumin under physiological conditions is ion specific. Chem. Commun. 2015, 51, 6663–6666. [Google Scholar] [CrossRef] [Green Version]
- Abeyrathne, E.D.N.S.; Lee, H.Y.; Ahn, D.U. Sequential separation of lysozyme, ovomucin, ovotransferrin, and ovalbumin from egg white. Poult. Sci. 2014, 93, 1001–1009. [Google Scholar] [CrossRef]
- Meissner, J.; Prause, A.; Bharti, B.; Findenegg, G.H. Characterization of protein adsorption onto silica nanoparticles: Influence of pH and ionic strength. Colloid Polym. Sci. 2015, 293, 3381–3391. [Google Scholar] [CrossRef] [Green Version]
- Parida, S.K.; Dash, S.; Patel, S.; Mishra, B.K. Adsorption of organic molecules on silica surface. Adv. Colloid Interface Sci. 2006, 121, 77–110. [Google Scholar] [CrossRef]






| Concentration * (µM) | 1 | 2 | 5 | 10 | 20 |
| Density ** (ng/mm2) | 0.11 | 0.18 | 0.61 | 1.04 | 1.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brambilla, D.; Mussida, A.; Ferretti, A.M.; Sola, L.; Damin, F.; Chiari, M. Polymeric Coating of Silica Microspheres for Biological Applications: Suppression of Non-Specific Binding and Functionalization with Biomolecules. Polymers 2022, 14, 730. https://doi.org/10.3390/polym14040730
Brambilla D, Mussida A, Ferretti AM, Sola L, Damin F, Chiari M. Polymeric Coating of Silica Microspheres for Biological Applications: Suppression of Non-Specific Binding and Functionalization with Biomolecules. Polymers. 2022; 14(4):730. https://doi.org/10.3390/polym14040730
Chicago/Turabian StyleBrambilla, Dario, Alessandro Mussida, Anna M. Ferretti, Laura Sola, Francesco Damin, and Marcella Chiari. 2022. "Polymeric Coating of Silica Microspheres for Biological Applications: Suppression of Non-Specific Binding and Functionalization with Biomolecules" Polymers 14, no. 4: 730. https://doi.org/10.3390/polym14040730