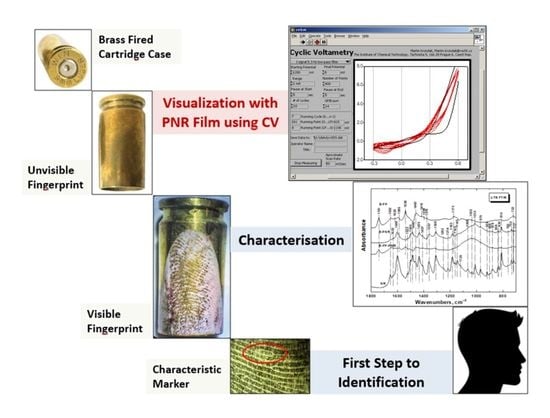
Optimization of Electrochemical Visualization of Latent Fingerprints with Poly(Neutral Red) on Brass Surfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Instrumentation
2.3. Experimental Procedures
2.3.1. Electrochemical Cell Cleaning
2.3.2. Treatment of Plates and Cartridges Prior to the Application of Fingerprints
2.3.3. Fingerprint Visualization
2.3.4. Characterization of Properties of Brass Surfaces
2.3.5. Optimization of the Fingerprint Visualization Process
3. Results and Discussion
3.1. Electrochemical Visualization of Fingerprints by Deposition of Poly(Neutral Red) Film onto Brass Substrates
3.2. Optimization of Electrochemical Visualization of Fingerprints
3.3. Microscopic Visualization
3.4. Comparison of the Properties of a Brass Substrate Prior to and after Applying PNR to a Fingerprint
3.4.1. Spectroscopic Characterization
3.4.2. Electrochemical Characterization
3.5. Fingerprint Visualization on Fired Cartridges (from the Shooting Range to the Laboratory)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Straus, J.; Porada, V.; Fürbach, M.; Nožička, V.; Rudáš, Z.; Suchánek, J.; Vavera, F.; Veselá, J. Kriminalistická Daktyloskopie; Policejní Akademie ČR: Praha, Czech Republic, 2005; p. 288. ISBN 80-7251-192-0. [Google Scholar]
- Konrád, Z.; Porada, V.; Straus, J.; Suchánek, J. Kriminalistika: Teorie, Metodologie a Metody Kriminalistické Techniky; Aleš Čeněk s.r.o.: Plzeň, Czech Republic, 2014; p. 464. ISBN 978-80-7380-490-9. [Google Scholar]
- Francese, S.; Bradshaw, R.; Ferguson, L.S.; Wolstenholme, R.; Clench, M.R.; Bleay, S. Beyond the ridge pattern: Multi-informative analysis of latent fingermarks by MALDI mass spectrometry. Analyst 2013, 138, 4215–4228. [Google Scholar] [CrossRef]
- Brown, R.M.; Hillman, A.R. Electrochromic enhancement of latent fingerprints by poly(3,4-ethylenedioxythiophene). Phys. Chem. Chem. Phys. 2012, 14, 8653–8661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langenburg, G.; Hall, C. Friction Ridge Skin: Comparison and Identification. In Wiley Encyclopedia of Forensic Science; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2013. [Google Scholar] [CrossRef]
- Sapstead, R.M.; Corden, N.; Hillman, A.R. Latent fingerprint enhancement via conducting electrochromic copolymer films of pyrrole and 3,4-ethylenedioxythiophene on stainless steel. Electrochim. Acta 2015, 162, 119–128. [Google Scholar] [CrossRef]
- Rak, R.; Matyáš, V.; Říha, Z. Biometrie a Identita Člověka ve Forenzních a Komerčních Aplikacích; Grada Publishing: Praha, Czech Republic, 2008; p. 664. ISBN 978-80-247-2365-5. [Google Scholar]
- Najdoski, M.; Oklevski, S.; Stojkovic, G. A Simple Chemical Method for Visualization of Sebaceous Fingerprints on Unfi red Cartridge Cases by Prussian Blue Deposition. Russ. J. Appl. Chem. 2015, 88, 1896–1901. [Google Scholar] [CrossRef]
- Christofidis, G.; Morrissey, J.; Birkett, J.W. Detection of fingermarks—Applicability to metallic surfaces: A literature review. J. Forensic Sci. 2018, 63, 1616–1627. [Google Scholar] [CrossRef] [Green Version]
- Beresford, A.L.; Hillman, A.R. Electrochromic enhancement of latent fingerprints on stainless steel surfaces. Anal. Chem. 2010, 82, 483–486. [Google Scholar] [CrossRef]
- Challinger, S.E.; Baikie, I.D.; Flannigan, G.; Halls, S.; Laing, K.; Daly, L.; Daeid, N.N. Comparison of scanning Kelvin probe with SEM/EPMA techniques for fingermark recovery from metallic surfaces. Forensic Sci. Int. 2018, 291, 44–52. [Google Scholar] [CrossRef] [Green Version]
- Beresford, A.L.; Brown, R.M.; Hillman, A.R.; Bond, J.W. Comparative study of electrochromic enhancement of latent fingerprints with existing development techniques. J. Forensic Sci. 2012, 57, 93–102. [Google Scholar] [CrossRef] [PubMed]
- James, R.M.; Altamimi, M.J. The enhancement of friction ridge detail on brass ammunition casings using cold patination fluid. Forensic Sci. Int. 2015, 257, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Jasuja, O.P.; Singh, G.; Almog, J. Development of latent fingermarks by aqueous electrolytes. Forensic Sci. Int. 2011, 207, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Bond, J.W.; Phil, D. Visualization of Latent Fingerprint Corrosion of Metallic Surfaces. J. Forensic Sci. 2008, 53, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Slaninová, T.; Broncová, G.; Straus, J.; Shishkanova, T.V. The Visualization of Fingerprints using Conducting Polymer Layers. Chem. Listy 2019, 113, 530–539. [Google Scholar]
- Broncova, G.; Shishkanova, T.V.; Matejka, P.; Volf, R.; Král, V. Citrate selectivity of poly(neutral red) electropolymerized films. Anal. Chim. Acta 2004, 511, 197–205. [Google Scholar] [CrossRef]
- Broncová, G.; Slaninová, T.; Dendisová, M. Poly(neutral red) modified metal substrates for fingerprint visualization. Chem. Pap. 2021, 1–4. [Google Scholar] [CrossRef]
- Karyakin, A.A.; Bobrova, O.A.; Karyakina, E.E. Electroreduction of NAD+ to enzymatically active NADH at poly(neutral red) modified electrodes. J. Electroanal. Chem. 1995, 399, 179–184. [Google Scholar] [CrossRef]
- Chen, S.M.; Lin, K.C. The electrocatalytic properties of polymerized neutral red film modified electrodes. Electroanal. Chem. 2001, 511, 101–104. [Google Scholar] [CrossRef]
- Bauldreay, J.M.; Archer, M.D. Dye-modified electrodes for photogalvanic cells. Electrochim. Acta 1983, 28, 1515–1552. [Google Scholar] [CrossRef]
- Champod, C.; Lennard, C.J.; Margot, P.; Stoilovic, M. Fingerprints and Other Ridge Skin Impressions, 1st ed.; CRC Press LLC: Boca Raton, FL, USA, 2004; p. 304. [Google Scholar] [CrossRef]
- Beresford, A.L. The Electrochromic Enhancement of Latent Fingerprints on Metal Surfaces. Ph.D. Dissertation, Department of Chemistry University of Leicester, Leicester, UK, 2013. Available online: https://leicester.figshare.com/articles/thesis/The_Electrochromic_Enhancement_of_Latent_Fingerprints_on_Metal_Surfaces/1012657 (accessed on 20 August 2021).
- Bersellini, C.; Garofano, L.; Giannetto, M.; Lusardi, F.; Mori, G. Development of latent fingerprints on metallic surfaces using electropolymerization processes. J. Forensic Sci. 2001, 46, 871–877. [Google Scholar] [CrossRef]
- Kabasakalog, M.; Kıyak, T.; Sendil, O.; Asan, A. Electrochemical behavior of brass in 0.1 M NaCl. Appl. Surf. Sci. 2002, 193, 167–174. [Google Scholar] [CrossRef]
- Scruton, B.; Robins, B.W.; Blott, B.H. The Deposition of Fingerprint Films. J. Phys. D Appl. Phys. 1975, 8, 714–723. [Google Scholar] [CrossRef]
- Crane, N.J.; Bartick, E.G.; Perlman, R.S.; Huffman, S. Infrared Spectroscopic Imaging for Noninvasive Detection of Latent Fingerprints. J. Forensic Sci. 2007, 52, 48–53. [Google Scholar] [CrossRef]
- Bailey, M.J.; Bright, N.J.; Croxton, R.S.; Francese, S.; Ferguson, L.S.; Hinder, S.; Jickells, S.; Jones, B.J.; Jones, B.N.; Kazarian, S.G.; et al. Chemical Characterization of Latent Fingerprints by Matrix-Assisted Laser Desorption Ionization, Time-of-Flight Secondary Ion Mass Spectrometry, Mega Electron Volt Secondary Mass Spectrometry, Gas Chromatography/Mass Spectrometry, X-ray Photoelectron Spectroscopy, and Attenuated Total Reflection Fourier Transform Infrared Spectroscopic Imaging: An Intercomparison. Anal. Chem. 2012, 84, 8514–8523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorakumbura, B.N.; Boseley, R.E.; Becker, T.; Martin, D.E.; Richter, A.; Tobin, M.J.; van Bronswjik, W.; Vongsvivut, J.; Hackett, M.J.; Lewis, S.W. Revealing the spatial distribution of chemical species within latent fingermarks using vibrational spectroscopy. Analyst 2018, 143, 3961–4208. [Google Scholar] [CrossRef] [Green Version]
- Cadd, S.; Islam, M.; Manson, P.; Bleay, S. Fingerprint composition and aging: A literature review. Sci. Justice 2015, 55, 219–238. [Google Scholar] [CrossRef]
- Frick, A.A.; Fritz, P.; Lewis, S.W. Chemical methods for the detection of latent fingermarks. In Forensic Chemistry: Fundamentals and Applications; Siegal, J.A., Ed.; John Wiley & Sons, Ltd.: New York, NY, USA, 2016; pp. 354–399. ISBN 1118897722. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; Wiley: New York, NY, USA, 2001; p. 364. ISBN 978-0-470-09307-8. [Google Scholar]
- Silverstein, R.M.; Bassler, G.C.; Morrill, T.C. Spectrometric Identification of Organic Compounds, 5th ed.; Wiley: New York, NY, USA, 1991; p. 430. ISBN 0471-63404-2. [Google Scholar]
- Ozkan, S.Z.; Karpacheva, G.P.; Bondarenko, G.N.; Kolyagin, Y.G. Polymers Based on 3-amino-7-dimethylamino-2-methylphenazine hydrochloride: Synthesis, structure, and properties. Polym. Sci. Ser. B 2015, 57, 106–115. [Google Scholar] [CrossRef]
- Jeništová, A.; Dendisová, M.; Matějka, P. Study of plasmonic nanoparticles interactions with skin layers by vibrational spectroscopy. Eur. J. Pharm. Biopharm. 2017, 116, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Hendel, S.J.; Young, E.R. Introduction to Electrochemistry and the Use of Electrochemistry to Synthesize and Evaluate Catalysts for Water Oxidation and Reduction. J. Chem. Educ. 2016, 93, 1951–1956. [Google Scholar] [CrossRef]
- Broaddus, E.; Brubaker, J.; Gold, S.A. Electrochemical Characterization of Platinum Nanotubules Made via Template Wetting Nanofabrication. Int. J. Electrochem. 2013, 7, 960513. [Google Scholar] [CrossRef] [Green Version]
- Girelli, C.M.A.; Lobo, B.J.M.; Cunha, A.G.; Freitas, J.C.C.; Emmerich, F.G. Comparison of practical techniques to develop latent fingermarks on fired and unfired cartridge cases. Forensic Sci. Int. 2015, 250, 17–26. [Google Scholar] [CrossRef] [PubMed]







| Common Bands for Powdered NR and Brass-PNR (cm−1) | Assignments of Observed FTIR Bands |
|---|---|
| 3325 | N–H stretching vibrations in phenyleneamine fragments C–NH–C |
| 3220 | N–H stretching vibrations in C=NH groups |
| 2919, 2858/2850, 2807/2803 a | C–H stretching vibrations in–N(CH3)2 |
| below 2800 b, c mainly 2735, 2658 | amine hydrochloride |
| 1600 b–d with two side peaks at 1652 and 1632 | N–H deformation vibration of amines and/or –C=N stretching vibrations in heteroaromatic rings or further Ar–C=N– (in-plane) vibrations |
| 1485 b, c | Ring C–C stretching modes (vibrations of the quinonoid ring) |
| 1435 b | Aromatic in-plane stretching mode and bending vibrations of CH3 groups |
| 1357, 1308, 1252 c, d | Coupled C–N stretching vibrations |
| 1173, 1162, 1145 b | C–H in-plane bending vibrations of the aromatic rings |
| 1035, 1010 c | C–H bending vibrations of the aromatic rings |
| 920, 878, 830, 810, 733, 717 b–d | 1,2,4-trisubstituted benzenes and 1,2,4,5-tetrasubstituted benzenes C–H out of plane (Oop) deformation and ring Oop deformation vibrations |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Broncová, G.; Slaninová, T.; Trchová, M.; Prokopec, V.; Matějka, P.; Shishkanova, T.V. Optimization of Electrochemical Visualization of Latent Fingerprints with Poly(Neutral Red) on Brass Surfaces. Polymers 2021, 13, 3220. https://doi.org/10.3390/polym13193220
Broncová G, Slaninová T, Trchová M, Prokopec V, Matějka P, Shishkanova TV. Optimization of Electrochemical Visualization of Latent Fingerprints with Poly(Neutral Red) on Brass Surfaces. Polymers. 2021; 13(19):3220. https://doi.org/10.3390/polym13193220
Chicago/Turabian StyleBroncová, Gabriela, Tereza Slaninová, Miroslava Trchová, Vadim Prokopec, Pavel Matějka, and Tatiana V. Shishkanova. 2021. "Optimization of Electrochemical Visualization of Latent Fingerprints with Poly(Neutral Red) on Brass Surfaces" Polymers 13, no. 19: 3220. https://doi.org/10.3390/polym13193220