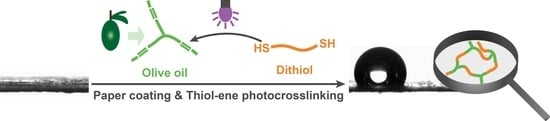
A Solvent-Free Approach to Crosslinked Hydrophobic Polymeric Coatings on Paper Using Vegetable Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Glass Wafer Hydrophobization
2.2.2. Paper Making
2.2.3. Coating Preparation and Crosslinking
2.2.4. 1H-NMR Spectroscopy
2.2.5. FTIR Spectroscopy
2.2.6. DSC Measurement
2.2.7. Contact Angle Measurement
2.2.8. Surface Roughness Measurements
2.2.9. Optical Microscopy
2.2.10. SEM
3. Results and Discussion
4. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dankovich, T.A.; Hsieh, Y.L. Surface modification of cellulose with plant triglycerides for hydrophobicity. Cellulose 2007, 14, 469–480. [Google Scholar] [CrossRef]
- Saha, P.; Manna, S.; Sen, R.; Roy, D.; Adhikari, B. Durability of lignocellulosic fibers treated with vegetable oil–phenolic resin. Carbohydr. Polym. 2012, 87, 1628–1636. [Google Scholar] [CrossRef]
- Crépy, L.; Chaveriat, L.; Banoub, J.; Martin, P.; Joly, N. Synthesis of cellulose fatty esters as plastics-influence of the degree of substitution and the fatty chain length on mechanical properties. ChemSusChem 2009, 2, 165–170. [Google Scholar] [CrossRef]
- Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P.; Belgacem, M.N.; Gandini, A. Controlled heterogeneous modification of cellulose fibers with fatty acids: Effect of reaction conditions on the extent of esterification and fiber properties. J. Appl. Polym. Sci. 2006, 100, 1093–1102. [Google Scholar] [CrossRef]
- Geissler, A.; Bonaccurso, E.; Heim, L.O.; Heinze, T.; Zhang, K. Temperature-responsive thin films from cellulose stearoyl triester. J. Phys. Chem. C 2014, 118, 2408–2417. [Google Scholar] [CrossRef]
- Huang, X.; Wang, A.; Xu, X.; Liu, H.; Shang, S. Enhancement of hydrophobic properties of cellulose fibers via grafting with polymeric epoxidized soybean oil. ACS Sustain. Chem. Eng. 2017, 5, 1619–1627. [Google Scholar] [CrossRef]
- Onwukamike, K.N.; Grelier, S.; Grau, E.; Cramail, H.; Meier, M.A.R. Sustainable transesterification of cellulose with high oleic sunflower oil in a DBU-CO2 switchable solvent. ACS Sustain. Chem. Eng. 2018, 6, 8826–8835. [Google Scholar] [CrossRef]
- Samyn, P.; Schoukens, G.; Stanssens, D.; Vonck, L.; van den Abbeele, H. Hydrophobic waterborne coating for cellulose containing hybrid organic nanoparticle pigments with vegetable oils. Cellulose 2013, 20, 2625–2646. [Google Scholar] [CrossRef]
- Cabrales, L.; Abidi, N. Microwave plasma induced grafting of oleic acid on cotton fabric surfaces. Appl. Surf. Sci. 2012, 258, 4636–4641. [Google Scholar] [CrossRef]
- Sharmin, E.; Zafar, F.; Akram, D.; Alam, M.; Ahmad, S. Recent advances in vegetable oils based environment friendly coatings: A review. Ind. Crops Prod. 2015, 76, 215–229. [Google Scholar] [CrossRef]
- Montero de Espinosa, L.; Meier, M.A.R. Plant oils: The perfect renewable resource for polymer science?! Eur. Pol. J. 2011, 47, 837–852. [Google Scholar] [CrossRef] [Green Version]
- Miao, S.; Wang, P.; Su, Z.; Zhang, S. Vegetable-oil-based polymers as future polymeric biomaterials. Acta Biomater. 2014, 10, 1692–1704. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Garrison, T.F.; Madbouly, S.A.; Kessler, M.R. Recent advances in vegetable oil-based polymers and their composites. Prog. Polym. Sci. 2017, 71, 91–143. [Google Scholar] [CrossRef]
- Hong, J.; Shah, B.K.; Petrović, Z.S. Vegetable oil cast resins via click chemistry: Effects of cross-linkers. Eur. J. Lipid Sci. Technol. 2013, 115, 55–60. [Google Scholar] [CrossRef]
- Kreye, O.; Tóth, T.; Meier, M.A.R. Copolymers derived from rapeseed derivatives via ADMET and thiol-ene addition. Eur. Pol. J. 2011, 47, 1804–1816. [Google Scholar] [CrossRef]
- Lomège, J.; Lapinte, V.; Negrell, C.; Robin, J.J.; Caillol, S. Fatty acid-based radically polymerizable monomers: From novel poly(meth)acrylates to cutting-edge properties. Biomacromolecules 2019, 20, 4–26. [Google Scholar] [CrossRef] [PubMed]
- Altuna, F.I.; Pettarin, V.; Williams, R.J.J. Self-healable polymer networks based on the cross-linking of epoxidised soybean oil by an aqueous citric acid solution. Green Chem. 2013, 15, 3360. [Google Scholar] [CrossRef]
- Wuzella, G.; Mahendran, A.R.; Müller, U.; Kandelbauer, A.; Teischinger, A. Photocrosslinking of an acrylated epoxidized linseed oil: Kinetics and its application for optimized wood coatings. J. Polym. Environ. 2012, 20, 1063–1074. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, G.; Cui, Y.; Tian, J.; He, M.; Yang, J.W. Castor oil based biothiol as a highly stable and self-initiated oligomer for photoinitiator-free UV coatings. ACS Sustain. Chem. Eng. 2017, 5, 376–381. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Bowman, C.N. Thiol-ene click chemistry. Angew. Chem. Int. Ed. Engl. 2010, 49, 1540–1573. [Google Scholar] [CrossRef]
- Roper, T.M.; Guymon, C.A.; Jönsson, E.S.; Hoyle, C.E. Influence of the alkene structure on the mechanism and kinetics of thiol-alkene photopolymerizations with real-time infrared spectroscopy. J. Polym. Sci. A Polym. Chem. 2004, 42, 6283–6298. [Google Scholar] [CrossRef]
- Claudino, M.; Johansson, M.; Jonsson, M. Thiol–ene coupling of 1,2-disubstituted alkene monomers: The kinetic effect of cis/trans-isomer structures. Eur. Pol. J. 2010, 46, 2321–2332. [Google Scholar] [CrossRef]
- Desroches, M.; Caillol, S.; Lapinte, V.; Auvergne, R.; Boutevin, B. Synthesis of biobased polyols by thiol−ene coupling from vegetable oils. Macromolecules 2011, 44, 2489–2500. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.H.; Vuluga, D.; Lecamp, L.; Burel, F. Photoinitiated thiol–epoxy addition for the preparation of photoinduced self-healing fatty coatings. RSC Adv. 2016, 6, 32098–32105. [Google Scholar] [CrossRef] [Green Version]
- Shang, Q.; Chen, J.; Liu, C.; Hu, Y.; Hu, L.; Yang, X.; Zhou, Y. Facile fabrication of environmentally friendly bio-based superhydrophobic surfaces via UV-polymerization for self-cleaning and high efficient oil/water separation. Prog. Org. Coat. 2019, 137, 105346. [Google Scholar] [CrossRef]
- Shang, Q.; Liu, C.; Chen, J.; Yang, X.; Hu, Y.; Hu, L.; Zhou, Y.; Ren, X. Sustainable and robust superhydrophobic cotton fabrics coated with castor oil-based nanocomposites for effective oil–water separation. ACS Sustain. Chem. Eng. 2020, 8, 7423–7435. [Google Scholar] [CrossRef]
- Wang, T.; Li, L.; Cao, Y.; Wang, Q.; Guo, C. Preparation and flame retardancy of castor oil based UV-cured flame retardant coating containing P/Si/S on wood surface. Ind. Crops Prod. 2019, 130, 562–570. [Google Scholar] [CrossRef]
- Meng, L.; Qiu, H.; Wang, D.; Feng, B.; Di, M.; Shi, J.; Wei, S. Castor-oil-based waterborne acrylate/SiO2 hybrid coatings prepared via sol–gel and thiol-ene reactions. Prog. Org. Coat. 2020, 140, 105492. [Google Scholar] [CrossRef]
- Black, M.; Rawlins, J.W. Thiol–ene UV-curable coatings using vegetable oil macromonomers. Eur. Polym. J. 2009, 45, 1433–1441. [Google Scholar] [CrossRef]
- Türünç, O.; Meier, M.A.R. The thiol-ene (click) reaction for the synthesis of plant oil derived polymers. Eur. J. Lipid Sci. Technol. 2013, 115, 41–54. [Google Scholar] [CrossRef]
- Resetco, C.; Hendriks, B.; Badi, N.; Du Prez, F. Thiol–ene chemistry for polymer coatings and surface modification—Building in sustainability and performance. Mater. Horiz. 2017, 4, 1041–1053. [Google Scholar] [CrossRef]
- Moser, B.R.; Doll, K.M.; Peterson, S.C. Renewable poly(thioether-ester)s from fatty acid derivatives via thiol-ene photopolymerization. J. Am. Oil Chem. Soc. 2019, 96, 825–837. [Google Scholar] [CrossRef]
- Samuelsson, J.; Jonsson, M.; Brinck, T.; Johansson, M. Thiol-ene coupling reaction of fatty acid monomers. J. Polym. Sci. A Polym. Chem. 2004, 42, 6346–6352. [Google Scholar] [CrossRef]
- Bouaziz, K.; Ayadi, M.; Allouche, N.; Chemtob, A. Renewable photopolymer films derived from low-grade lampante and pomace olive oils. Eur. J. Lipid Sci. Technol. 2017, 119, 1700003. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.H.; Vuluga, D.; Lecamp, L.; Burel, F. A rapid, eco- and environmental friendly alternative to oil oxidation for the preparation of fatty coatings using photoinitiated thiol-ene chemistry. Prog. Org. Coat. 2016, 101, 216–224. [Google Scholar] [CrossRef]
- Xiao, Y.; Ritcey, A.M. Phase Transitions in Spread Monolayers of Cellulose Ethers. Langmuir 2000, 16, 4252–4258. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, C.; Sun, Z.; Hu, X.; Shen, Q.; Wu, J. Authentication of edible vegetable oils adulterated with used frying oil by fourier transform infrared spectroscopy. Food Chem. 2012, 132, 1607–1613. [Google Scholar] [CrossRef]
- Şeker, H.; Çakmakçi, E. Fully bio-based thiol-ene photocured thermosets from isosorbide and tung oil. J. Polym. Sci. 2020, 58, 1105–1114. [Google Scholar] [CrossRef]






| Ra (μm) | Rq (μm) | Rz (μm) | Sa (μm) | Sq (μm) | Sz (μm) | |
|---|---|---|---|---|---|---|
| Cotton linters paper, uncoated | 6.3 ± 0.7 | 8.0 ± 0.9 | 39.8 ± 4.8 | 7.6 ± 0.6 | 10.0 ± 0.7 | 124.2 ± 12.0 |
| Cotton linters paper, olive oil coating | 6.1 ± 0.6 | 7.8 ± 0.8 | 40.8 ± 5.5 | 6.8 ± 0.6 | 9.3 ± 1.0 | 126.3 ± 10.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loesch-Zhang, A.; Cordt, C.; Geissler, A.; Biesalski, M. A Solvent-Free Approach to Crosslinked Hydrophobic Polymeric Coatings on Paper Using Vegetable Oil. Polymers 2022, 14, 1773. https://doi.org/10.3390/polym14091773
Loesch-Zhang A, Cordt C, Geissler A, Biesalski M. A Solvent-Free Approach to Crosslinked Hydrophobic Polymeric Coatings on Paper Using Vegetable Oil. Polymers. 2022; 14(9):1773. https://doi.org/10.3390/polym14091773
Chicago/Turabian StyleLoesch-Zhang, Amelia, Cynthia Cordt, Andreas Geissler, and Markus Biesalski. 2022. "A Solvent-Free Approach to Crosslinked Hydrophobic Polymeric Coatings on Paper Using Vegetable Oil" Polymers 14, no. 9: 1773. https://doi.org/10.3390/polym14091773