Biosorbents Based on Biopolymers from Natural Sources and Food Waste to Retain the Methylene Blue Dye from the Aqueous Medium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Batch Biosorption Methodology
- the biosorption capacity (q, mg of dye/g of biosorbent):
- the percentage of dye removal (R),
2.2.2. Physicochemical Characterization of Biosorbents
2.2.3. The Biosorption Equilibrium Data Analysis
- ➢
- Freundlich—takes the heterogeneity of the surface and the exponential distribution of the active sites of the biosorbent into account. The general equation is:
- ➢
- Langmuir—starts from the idea that the maximum biosorption corresponds to a monolayer of solute molecules on the biosorbent surface, which contain a finite number of energetically equivalent sites. The general equation is:
- ➢
- Dubinin–Radushkevich—allows the appreciation of the nature of the biosorption process (physical or chemical) depending on the value of the adsorption energy, E. Thus E < 8 kJ/moll characterizes a physical biosorption mechanism, and values between 8 and 16 kJ/mol suggest an ion exchange mechanism. The general equation is Equation (8).
3. Results
3.1. Preparation of Microbial Biosorbent and Their Physical–Chemical Characterization
3.2. The pHPZC Value
3.3. Evaluation of the Biosorbent Potential of the Obtained Microbial Biosorbents
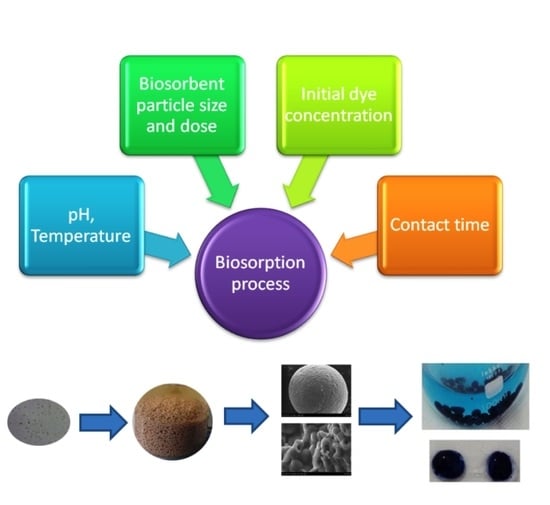
Effect of the Main Physical–Chemical Operating Parameters on Biosorption of Methylene Blue Dye onto Microbial Biosorbent
- According to the values of the parameter pHPZC, the retention of the cationic dye MB occurs in the environment with a pH > 5, and the optimum is reached around the pH value = 9. The degree of recovery (R%) follows the same evolution curve as the maximum biosorption capacity (q) (Figure 4a,b). It is also observed that although the curves q = f (pH) and R (%) = f (pH) have the same allure in both types of biomass immobilization, the maximum values for q and R are different, respectively higher (104.45 mg/g, 29.09%) in the case of immobilization by microencapsulation with the help of the Buchi device compared to those obtained by a simple dropping technique (76.89 mg/g, 24.83%).
- Figure 4c,d show a decrease in the amount of dye retained per unit mass of biosorbent from 15.5 mg/g to 6.1 mg/g (Figure 4c) and respectively from 50.803 mg/g to 6.537 mg/g (Figure 4d) as the amount of biosorbent increases from 0.264 g/L to 3.08 g/L. There is also an increase in the percentage of dye recovered with the increase of biosorbent, which can be explained by the increase in the number of active positions favorable to biosorption as the amount of biosorbent increases. By analyzing the variations of the two parameters (q and R), it was established that a dose of 0.264 g d.w./L (respectively, 5.28 g/L biosorbent) in the case of the biosorbent obtained by a simple dripping technique, and 0.15 g d.w./L (respectively, 3 g/L biosorbent) in the case of biosorbent obtained by encapsulation using a Buchi microencapsulator can be considered optimal for the removal of MB dye from aqueous solutions.
- Figure 4e shows an increase in biosorption capacity with contact time, which increases faster in the first 100 min regardless of the diameter of the biosorbent particles, followed by a slower increase until equilibrium is reached.
- Figure 4g,f show an increase in biosorption capacity as the initial concentration of the dye in the aqueous solution increases until the saturation value of the biosorbent is reached. It also can be observed the positive influence of temperature increase on the biosorption capacity; in both cases, the biosorption process proving to be endothermic.
3.4. Evaluation the Equilibrium of the Biosorption Process of Cationic Dye Methylene Blue onto Biosorbent by Immobilization of Microbial Residual Biomass onto Alginate Matrix
- ➢
- The values of the calculated quantitative parameters are a function of temperature and have higher values in the case of smaller diameter granules (obtained by encapsulated using a Buchi microencapsulator) because in this case, a larger specific contract area is ensured, which facilitates the biosorption process.
- ➢
- Temperature dependence suggested an endothermic biosorption process favored by relative high temperature.
- ➢
- Taking as appreciation criterion the values of the regression coefficient R2 and the values of the maximum biosorption capacities (q0, mg/g), among the two linearized forms of the Langmuir isotherm, the form I is best suited to model the data in the case of biosorbent obtained by a simple dropping technique, and the form II in the case of biosorbent obtained by encapsulation using a Buchi microencapsulator.
- ➢
- The mean free biosorption energy, E, calculated by DR equation, can be useful to estimate the nature of the biosorption process (physical or chemical) [33]. In this case, there are two distinct situations: in the case of biosorbent obtained by a simple dripping technique the energy values, E, are between 13.87–15.4 kJ/mol suggesting for the process of MB dye biosorption an ion exchange mechanism (the sorption energy is within 8–16 kJ/mol). In the case of the biosorbent obtained by encapsulation using a Buchi microencapsulator, the energy values, E, are lower, in the range 7.4–7.9 kJ/mol for temperature between 5–17 °C and 10 kJ/mol for 45 °C, which suggests a process of biosorption of the same MB dye by physical mechanism as a result of the electrostatic interaction bonds, for the lower temperatures and ion exchange for the higher temperature. This behavior of biosorbents differs not only from what we found in the case of our studies about biosorption the reactive dyes [14,16] but also between these two types of biosorbents and can be explained by the structure and size of the dye molecule. Methylene blue dye has a much smaller molecule than the previously studied dye (Brilliant Red HE-3B reactive dye with MW 1463), which implies a higher probability to penetrate the internal structure of the biosorbent. Thus, its retention can be explained both on the basis of the formation of chemical and physical bonds (hydrogen bonds, van der Waals, dipole–dipole, etc.) made with the functional groups existing in the biosorbent structure and on its surface. On the other hand, in the case of smaller diameter biosorbent beads, the bisorption process is determined by the physical binding of the dye molecules only on the biosorbent surface due to a larger granule–dye contact surface and the more compact structure of the granules. This behavior could also explain why the values of the maximum biosorption capacities, q0 (represents the total specific meso- and macropore volume of the biosorbent, mg/g), calculated with the DR model have values closer to those resulting from the Langmuir model in the case of biosorbent obtained by a simple dropping technique with granules diameter size φ 1 = 4 mm.
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pham, V.H.T.; Kim, J.; Chang, S.; Chung, W. Bacterial biosorbents, an efficient heavy metals green clean-up strategy: Prospects, challenges, and opportunities. Microorganisms 2022, 10, 610. [Google Scholar] [CrossRef] [PubMed]
- Abidli, A.; Huang, Y.; Ben Rejeb, Z.; Zaoui, A.; Park, C.B. Sustainable and efficient technologies for removal and recovery of toxic and valuable metals from wastewater: Recent progress, challenges, and future perspectives. Chemosphere 2021, 292, 133102. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, V.; Singh Dhanjal, D.; Datta, S.; Singh, S.; Singh, J. Biosorbents for heavy metal removal from industrial effluents. In Bioremediation for Environmental Sustainability; Kumar, V., Saxena, G., Shah, M.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 219–233. [Google Scholar] [CrossRef]
- Fathollahi, A.; Khasteganan, N.; Coupe, S.J.; Newman, A.P. A meta-analysis of metal biosorption by suspended bacteria from three phyla. Chemosphere 2021, 268, 129290. [Google Scholar] [CrossRef]
- Rizvi, A.; Ahmed, B.; Zaidi, A.; Khan, M.S. Biosorption of heavy metals by dry biomass of metal tolerant bacterial biosorbents: An efficient metal clean-up strategy. Environ. Monit. Assess. 2020, 192, 12. [Google Scholar] [CrossRef]
- Roy, U.; Manna, S.; Sengupta, S.; Das, P.; Datta, S.; Mukhopadhyay, A.; Bhowal, A. Dye removal using microbial biosorbents. In Green Adsorbents for Pollutant Removal. Environmental Chemistry for a Sustainable World; Crini, G., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2018; Volume 19. [Google Scholar] [CrossRef]
- Rismondo, J.; Gillis, A.; Gründling, A. Modifications of cell wall polymers in Gram-positive bacteria by multi-component transmembrane glycosylation systems. Curr. Opin. Microbiol. 2021, 60, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rubio, R.; de Oliveira, H.C.; Rivera, J.; Trevijano-Contador, N. The fungal cell wall: Candida, Cryptococcus, and Aspergillus Species. Front. Microbiol. 2020, 10, 2993. [Google Scholar] [CrossRef] [PubMed]
- Fomina, M.; Gadd, G.M. Biosorption: Current perspectives on concept, definition and application. Bioresour. Technol. 2014, 160, 3–14. [Google Scholar] [CrossRef]
- Song, W.; Huang, T.; Zuo, T.; Deng, D.; Tang, C. Application of microbial immobilization on chitosan composite membrane for manganese removal in water treatment. Polymer 2022, 243, 124531. [Google Scholar] [CrossRef]
- Blaga, A.C.; Zaharia, C.; Suteu, D. Polysaccharides as support for microbial biomass-based adsorbents with applications in removal of heavy metals and dyes. Polymers 2021, 13, 2893. [Google Scholar] [CrossRef]
- Benettayeb, A.; Ghosh, S.; Usman, M.; Seihoub, F.Z.; Sohoo, I.; Chia, C.H.; Sillanpää, M. Some Well-Known Alginate and chitosan modifications used in adsorption: A Review. Water 2022, 14, 1353. [Google Scholar] [CrossRef]
- Maurya, R.; Ghosh, T.; Paliwal, C.; Shrivastav, A.; Chokshi, K.; Pancha, I.; Ghosh, A.; Mishra, S. Biosorption of methylene blue by de-oiled algal biomass: Equilibrium, kinetics and artificial neural network modelling. PLoS ONE 2014, 9, e109545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suteu, D.; Blaga, A.C.; Zaharia, C.; Cimpoesu, R.; Puițel, A.C.; Tataru-Farmus, R.-E.; Tanasă, A.M. Polysaccharides used in biosorbents preparation for organic dyes retaining from aqueous media. Polymers 2022, 14, 588. [Google Scholar] [CrossRef] [PubMed]
- Rusu, L.; Grigoraș, C.-G.; Simion, A.-I.; Suceveanu, E.-M.; Blaga, A.-C.; Harja, M. Encapsulation of Saccharomyces pastorianus residual biomass in calcium alginate matrix with insights in ethacridine lactate biosorption. Polymers 2022, 14, 170. [Google Scholar] [CrossRef] [PubMed]
- Suteu, D.; Blaga, A.C.; Cimpoesu, R.; Puiţel, A.C.; Tataru-Farmus, R.-E. Composites based on natural polymers and microbial biomass for biosorption of Brilliant Red HE-3B reactive dye from aqueous solutions. Polymers 2021, 13, 4314. [Google Scholar] [CrossRef]
- Velkova, Z.; Kirova, G.; Stoytcheva, M.; Kostadinova, S.; Todorova, K.; Gochev, V. Immobilized microbial biosorbents for heavy metals removal. Eng. Life Sci. 2018, 18, 871–881. [Google Scholar] [CrossRef] [Green Version]
- Stewart, G.G. Saccharomyces species in the production of beer. Beverages 2016, 2, 34. [Google Scholar] [CrossRef]
- Isik, B.; Ugraskan, V.; Cankurtaran, O. Effective biosorption of methylene blue dye from aqueous solution using wild macrofungus (Lactarius piperatus). Sep. Sci. Technol. 2022, 57, 854–871. [Google Scholar] [CrossRef]
- Seoane, R.; Santaeufemia, S.; Abalde, J.; Torres, E. Efficient removal of methylene blue using living biomass of the microalga Chlamydomonas moewusii: Kinetics and equilibrium studies. Int. J. Environ. Res. Public Health 2022, 19, 2653. [Google Scholar] [CrossRef]
- De Morais Sobral, D.; Montero-Rodríguez, D.; Silva, A.F.S.; de Souza, A.F.; dos Santos, V.A.; Campos-Takaki, G.M. Removal of methylene blue dye from aqueous solution using biomass of Cunninghamella echinulata Ucp 1297 as superbiosorbent. Braz. J. Dev. 2022, 8, 17341–17350. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on methylene blue: Its properties, uses, toxicity and photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Xiong, Z.M.; O’Donovan, M.; Sun, L.; Choi, J.Y.; Ren, M.; Cao, K. Anti-aging potentials of methylene blue for human skin longevity. Sci. Rep. 2017, 7, 2475. [Google Scholar] [CrossRef] [PubMed]
- Bouras, H.D.; Isik, Z.; Arikan, E.B.; Yeddou, A.R.; Bouras, N.; Chergui, A.; Favier, L.; Amrane, A.; Dizge, N. Biosorption characteristics of methylene blue dye by two fungal biomasses. Int. J. Environ. Stud. 2021, 78, 365–381. [Google Scholar] [CrossRef]
- Wazir, A.H.; Waseem, I.; Qureshi, I.; Manan, A. Saccharum Arundinaceum leaves as a versatile biosorbent for removal of methylene blue dye from wastewater. Environ. Eng. Sci. 2020, 37, 737–745. [Google Scholar] [CrossRef]
- Silva, F.; Nascimento, L.; Brito, M.; da Silva, K.; Paschoal, W.; Fujiyama, R. Biosorption of methylene blue dye using natural biosorbents made from weeds. Materials 2019, 12, 2486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouras, H.D.; Yeddou, A.R.; Bouras, N.; Chergui, A.; Favier, L.; Amrane, A.; Dizge, N. Biosorption of cationic and anionic dyes using the biomass of Aspergillus parasiticus CBS 100926T. Water Sci. Technol. 2021, 83, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Putro, J.N.; Ju, Y.H.; Soetaredjo, F.E.; Santoso, S.P.; Ismadji, S. Biosorption of Dyes, Green Chemistry and Water Remediation: Research and Applications, in Advances in Green and Sustainable Chemistry; Sharma, S.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 99–133. [Google Scholar] [CrossRef]
- Tabaraki, R.; Sadeghinejad, N. Biosorption of six basic and acidic dyes on brown alga Sargassum ilicifolium: Optimization, kinetic and isotherm studies. Water Sci. Technol. 2017, 75, 2631–2638. [Google Scholar] [CrossRef]
- De Araujo, T.P.; Tavares, F.O.; Vareschini, D.T.; Barros, M.A.S.D. Biosorption mechanisms of cationic and anionic dyes in a low-cost residue from brewer’s spent grain. Environ. Technol. 2021, 42, 2925–2940. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Xia, J.; Long, J. Biosorption of methylene blue by nonliving biomass of the brown macroalga Sargassum hemiphyllum. Water Sci. Technol. 2017, 76, 1574–1583. [Google Scholar] [CrossRef]
- Ge, Q.; Liu, H. Tunable amine-functionalized silsesquioxane-based hybrid networks for efficient removal of heavy metal ions and selective adsorption of anionic dyes. Chem. Eng. J. 2022, 428, 131370. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insight into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Li, M.; Elder, T.; Buschle-Diller, G. Alginate-based polysaccharide beads for cationic contaminant sorption from water. Polym. Bull. 2017, 74, 1267–1281. [Google Scholar] [CrossRef]
- Saeed, A.; Iqbal, M.; Zafar, S.I. Immobilization of Trichoderma viride for enhanced methylene blue biosorption: Batch and column studies. J. Hazard. Mater. 2009, 168, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Upendar, G.; Dutta, S.; Chakraborty, J.; Bhattacharyya, P. Removal of methylene blue dye using immobilized Bacillus subtilis in batch & column reactor. Mater. Today Proc. 2016, 3, 3467–3472. [Google Scholar] [CrossRef]






| Parameters | Studied Limits of Variation |
|---|---|
| pH | 2–11 |
| T, °C | 5, 20, 60 |
| t, min | 10 min–24 h |
| Biosorbent dose, g/L | 2.4–50.4 |
| Initial dye concentration in solution, mg/L | 14.32–229.2 |
Granule size:
| φ = 4 mm φ = 1.5 mm |
| Isotherm | φ 1 = 4 mm | φ 2 = 1.5 mm | ||||
|---|---|---|---|---|---|---|
| 278 K | 290 K | 313 K | 278 K | 290 K | 313 K | |
| Freundlich | ||||||
| KF ((mg/g)(L/mg)1/n) | 15.59 | 19.87 | 8.79 | 0.51 | 19.40 | 19.59 |
| n | 4.52 | 4.64 | 2.89 | 3.29 | 2.01 | 1.50 |
| R2 | 0.91 | 0.93 | 0.94 | 0.99 | 0.95 | 0.90 |
| Langmuir | ||||||
| Langmuir I | ||||||
| q0 (mg/g) | 49.75 | 40.81 | 88.49 | 204.08 | 188.67 | 434.78 |
| KL (L/g) | 0.22 | 0.18 | 0.11 | 0.01 | 0.04 | 0.02 |
| R2 | 0.99 | 0.99 | 0.99 | 0.96 | 0.97 | 0.97 |
| Langmuir II | ||||||
| q0 (mg/g) | 47.61 | 40.00 | 87.71 | 163.93 | 200.00 | 227.27 |
| KL (L/g) | 0.22 | 0.22 | 0.12 | 0.02 | 0.04 | 0.01 |
| R2 | 0.99 | 0.99 | 0.99 | 0.95 | 0.95 | 0.71 |
| Dubinin-Radushkevich (DR) | ||||||
| q0 (mg/g) | 108.37 | 90.18 | 301.74 | 2364.63 | 8.413 × 10−3 | 3527.96 |
| β (mol2/kJ2) | 0.002 | 0.002 | 0.002 | 0.008 | 0.0089 | 0.005 |
| E (kJ/mol) | 15.43 | 15.81 | 13.867 | 7.906 | 7.495 | 10.00 |
| R2 | 0.9400 | 0.9300 | 0.9598 | 0.9305 | 0.9991 | 0.9159 |
| Biosorbent | Conditions | Maximum Adsorption Capacity, mg/g | References |
|---|---|---|---|
| Sodium alginates beads | pH = 9 | 0.25 | [34] |
| Lactarius piperatus | T = 25 °C; pH = 7 | 384.6 ± 3.4 | [18] |
| Microspora sp | pH = 7, 150 rpm, 24 h | 139.11 | [19] |
| Chlamydomonas moewusii | pH 10, 7 h | 212.41 | [20] |
| Sargassum ilicifolium | 0.6 h | 99.7 | [29] |
| Brewer’s spent grain | 7 h | 298.35 | [30] |
| Sargassum hemiphyllum | 2 h, pH = 5 | 729.93 | [31] |
| Trichoderma viride, entrapped in loofa sponge | 90 min, pH = 10 | 201.52 | [35] |
| Bacillus subtilis immobilized in calcium alginate | 30 °C, 20 g/L biosorbent, shaking speed 900 rpm | 90% removal | [36] |
| Residual biomass of Saccharomyces pastorianus immobilized in sodium alginate by a simple dropping technique | pH = 3; t = 24 h; amount of biosorbent = 0.26 g/L (with 5% d.w); φ = 4 mm; T = 5–40 °C | 47.62–87.72 | This study |
| Residual biomass of Saccharomyces pastorianus immobilized in sodium alginate by encapsulation using a microencapsulator | pH = 3; t = 24 h; amount of biosorbent = 0.15 g/L (with 5% d.w); φ = 1.5 mm; T = 5–40 °C | 188.68–434.78 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blaga, A.C.; Tanasă, A.M.; Cimpoesu, R.; Tataru-Farmus, R.-E.; Suteu, D. Biosorbents Based on Biopolymers from Natural Sources and Food Waste to Retain the Methylene Blue Dye from the Aqueous Medium. Polymers 2022, 14, 2728. https://doi.org/10.3390/polym14132728
Blaga AC, Tanasă AM, Cimpoesu R, Tataru-Farmus R-E, Suteu D. Biosorbents Based on Biopolymers from Natural Sources and Food Waste to Retain the Methylene Blue Dye from the Aqueous Medium. Polymers. 2022; 14(13):2728. https://doi.org/10.3390/polym14132728
Chicago/Turabian StyleBlaga, Alexandra Cristina, Alexandra Maria Tanasă, Ramona Cimpoesu, Ramona-Elena Tataru-Farmus, and Daniela Suteu. 2022. "Biosorbents Based on Biopolymers from Natural Sources and Food Waste to Retain the Methylene Blue Dye from the Aqueous Medium" Polymers 14, no. 13: 2728. https://doi.org/10.3390/polym14132728