Status and Dietary Intake of Phytoene and Phytofluene in Spanish Adults and the Effect of a Four-Week Dietary Intervention with Lutein-Rich Fruits or Vegetables
Abstract
:1. Introduction
2. Material and Methods
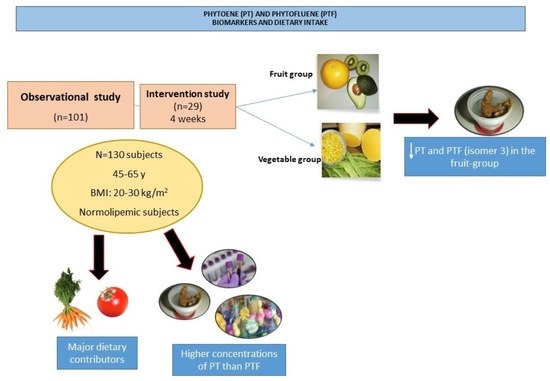
2.1. Subjects
2.2. Carotenoid Extraction and Analysis in Blood, Faeces and Food Sample
2.3. HPLC-DAD Carotenoid Analysis
2.4. Phytoene and Phytofluene Dietary Intake Assessment
2.5. Statistics
3. Results
3.1. Identification of PT and PTF by HPLC
3.2. PT Dietary Intake
3.3. PT and PTF Serum Concentrations
3.4. PT and PTF Concentrations in Faeces
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Engelmann, N.J.; Clinton, S.K.; Erdman, J.W., Jr. Nutritional aspects of phytoene and phytofluene, carotenoid precursors to lycopene. Adv. Nutr. 2011, 2, 51–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olmedilla-Alonso, B.; Benítez-González, A.M.; Estévez-Santiago, R.; Mapelli-Brahm, P.; Stinco, C.M.; Meléndez-Martínez, A.J. Assessment of Food Sources and the Intake of the Colourless Carotenoids Phytoene and Phytofluene in Spain. Nutrients 2021, 13, 4436. [Google Scholar] [CrossRef] [PubMed]
- Biehler, E.; Alkerwi, A.; Hoffmann, L.; Krause, E.; Guillaume, M.; Lair, M.; Bohn, T. Contribution of violaxanthin, neoxanthin, phytoene and phytofluene to total carotenoid intake: Assessment in Luxembourg. J. Food Compos. Anal. 2012, 25, 56–65. [Google Scholar] [CrossRef]
- Böhm, V.; Lietz, G.; Olmedilla-Alonso, B.; Phelan, D.; Reboul, E.; Bánati, D.; Borel, P.; Corte-Real, J.; de Lera, A.R.; Desmarchelier, C.; et al. From carotenoid intake to carotenoid blood and tissue concentrations—implications for dietary intake recommendations. Nutr. Rev. 2021, 79, 544–573. [Google Scholar] [CrossRef] [PubMed]
- Porrini, M.; Riso, P.; Brusamolino, A.; Berti, C.; Guarnieri, S.; Visioli, F. Daily intake of a formulated tomato drink affects carotenoid plasma and lymphocyte concentrations and improves cellular antioxidant protection. Br. J. Nutr. 2005, 93, 93–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, J.K.; Engelmann, N.J.; Lila, M.A.; Erdman, J.W., Jr. Phytoene, phytofluene, and lycopene from tomato powder differentially accumulate in tissues of male Fisher 344 rats. Nutr. Res. 2007, 27, 794. [Google Scholar] [CrossRef] [Green Version]
- Meléndez-Martínez, A.J.; Mapelli-Brahm, P.; Benítez-González, A.; Stinco, C.M. A comprehensive review on the colorless carotenoids phytoene and phytofluene. Arch. Biochem. Biophys. 2015, 572, 188–200, Erratum in Arch. Biochem. Biophys. 2017, 631, 30. [Google Scholar] [CrossRef] [Green Version]
- Richelle, M.; Bortlik, K.; Liardet, S.; Hager, C.; Lambelet, P.; Baur, M.; Applegate, L.A.; Offord, E.A. A food-based formulation provides lycopene with the same bioavailability to humans as that from tomato paste. J. Nutr. 2002, 132, 404–408. [Google Scholar] [CrossRef]
- Edwards, A.J.; Vinyard, B.T.; Wiley, E.R.; Brown, E.D.; Collins, J.K.; Perkins-Veazie, P.; Baker, R.A.; Clevidence, B.A. Consumption of watermelon juice increases plasma concentrations of lycopene and beta-carotene in humans. J. Nutr. 2003, 133, 1043–1050. [Google Scholar] [CrossRef]
- Müller, H.; Bub, A.; Watzl, B.; Rechkemmer, G. Plasma concentrations of carotenoids in healthy volunteers after intervention with carotenoid-rich foods. Eur. J. Nutr. 1999, 38, 35–44. [Google Scholar] [CrossRef]
- Fuller, B.; Smith, D.; Howerton, A.; Kern, D. Anti-inflammatory effects of CoQ10 and colorless carotenoids. J. Cosmet. Dermatol. 2006, 5, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Nara, E.; Hayashi, H.; Kotake, M.; Miyashita, K.; Nagao, A. Acyclic carotenoids and their oxidation mixtures inhibit the growth of HL-60 human promyelocytic leukemia cells. Nutr. Cancer 2001, 39, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, K.; Atzmon, A.; Danilenko, M.; Levy, J.; Sharoni, Y. Lycopene and other carotenoids inhibit estrogenic activity of 17beta-estradiol and genistein in cancer cells. Breast Cancer Res. Treat. 2007, 104, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Ben-Dor, A.; Steiner, M.; Gheber, L.; Danilenko, M.; Dubi, N.; Linnewiel, K.; Zick, A.; Sharoni, Y.; Levy, J. Carotenoids activate the antioxidant response element transcription system. Mol. Cancer Ther. 2005, 4, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Mapelli-Brahm, P.; Corte-Real, J.; Meléndez-Martínez, A.J.; Bohn, T. Bioaccessibility of phytoene and phytofluene is superior to other carotenoids from selected fruit and vegetable juices. Food Chem. 2017, 229, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Martínez, A.J.; Mandić, A.I.; Bantis, F.; Böhm, V.; Borge, G.I.A.; Brnčić, M.; Bysted, A.; Cano, M.P.; Dias, M.G.; Elgersma, A.; et al. A comprehensive review on carotenoids in foods and feeds: Status quo, applications, patents, and research needs. Crit. Rev. Food Sci. Nutr. 2022, 62, 1999–2049. [Google Scholar] [CrossRef] [PubMed]
- Hsu, B.Y.; Pu, Y.S.; Inbaraj, B.S.; Chen, B.H. An improved high performance liquid chromatography-diode array detection-mass spectrometry method for determination of carotenoids and their precursors phytoene and phytofluene in human serum. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2012, 899, 36–45. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, E.; Beltrán-de-Miguel, B.; Samaniego-Aguilar, K.X.; Sánchez-Prieto, M.; Estévez-Santiago, R.; Olmedilla-Alonso, B. Extraction and Analysis by HPLC-DAD of Carotenoids in Human Faeces from Spanish Adults. Antioxid. (Basel) 2020, 9, 484. [Google Scholar] [CrossRef]
- Olmedilla-Alonso, B.; Rodríguez-Rodríguez, E.; Beltrán-De-Miguel, B.; Estévez-Santiago, R.; Sánchez-Prieto, M. Predictors of macular pigment and contrast threshold in normolipemic subjects aged 45–65. PLoS ONE 2021, 16, e0251324. [Google Scholar] [CrossRef]
- Olmedilla-Alonso, B.; Rodríguez-Rodríguez, E.; Beltrán-de-Miguel, B.; Sánchez-Prieto, M.; Estévez-Santiago, R. Changes in Lutein Status Markers (Serum and Faecal Concentrations, Macular Pigment) in Response to a Lutein-Rich Fruit or Vegetable (Three Pieces/Day) Dietary Intervention in Normolipemic Subjects. Nutrients 2021, 13, 3614. [Google Scholar] [CrossRef]
- Dias, M.G.; Olmedilla-Alonso, B.; Hornero-Méndez, D.; Mercadante, A.Z.; Osorio, C.; Vargas-Murga, L.; Meléndez-Martínez, A.J. Comprehensive database of carotenoid contents in ibero-american foods. A valuable tool in the context of functional foods and the establishment of recommended intakes of bioactives. J. Agric. Food Chem. 2018, 66, 5055–5107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Rodríguez, E.; Sánchez-Prieto, M.; Olmedilla-Alonso, B. Assessment of carotenoid concentrations in red peppers (Capsicum annuum) under domestic refrigeration for three weeks as determined by HPLC-DAD. Food Chem. X 2020, 6, 100092. [Google Scholar] [CrossRef] [PubMed]
- de Rosso, V.V.; Mercadante, A.Z. Identification and quantification of carotenoids, by HPLC-PDA-MS/MS, from Amazonian fruits. J. Agric. Food Chem. 2007, 55, 5062–5072. [Google Scholar] [CrossRef]
- Estévez-Santiago, R.; Beltrán-de-Miguel, B.; Cuadrado-Vives, C.; Olmedilla-Alonso, B. Software application for the calculation of dietary intake of individual carotenoids and of its contribution to vitamin A intake. Nutr. Hosp. 2013, 28, 823–829. [Google Scholar] [PubMed]
- Meléndez-Martínez, A.J.; Mapelli-Brahm, P.; Stinco, C.M. The colourless carotenoids phytoene and phytofluene: From dietary sources to their usefulness for the functional foods and nutricosmetics industries. J. Food Compos. Anal. 2018, 67, 91–103. [Google Scholar] [CrossRef]
- Maiani, G.; Castón, M.J.; Catasta, G.; Toti, E.; Cambrodón, I.G.; Bysted, A.; Granado-Lorencio, F.; Olmedilla-Alonso, B.; Knuthsen, P.; Valoti, M.; et al. Carotenoids: Actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol. Nutr. Food Res. 2009, 53 (Suppl S2), S194–S218. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Martínez, A.J.; Fraser, P.D.; Bramley, P.M. Accumulation of health promoting phytochemicals in wild relatives of tomato and their contribution to in vitro antioxidant activity. Phytochemistry 2012, 71, 1104–1114. [Google Scholar] [CrossRef]
- Olmedilla-Alonso, B.; Beltrán-de-Miguel, B.; Estévez-Santiago, R.; Cuadrado-Vives, C. Markers of lutein and zeaxanthin status in two age groups of men and women: Dietary intake, serum concentrations, lipid profile and macular pigment optical density. Nutr. J. 2014, 13, 52. [Google Scholar] [CrossRef] [Green Version]
- Beltrán-de-Miguel, B.; Estévez-Santiago, R.; Olmedilla-Alonso, B. Assessment of dietary vitamin A intake (retinol, α-carotene, β-carotene, β-cryptoxanthin) and its sources in the National Survey of Dietary Intake in Spain (2009–2010). Int. J. Food Sci. Nutr. 2015, 66, 706–712. [Google Scholar] [CrossRef] [Green Version]
- Estévez-Santiago, R.; Beltrán-de-Miguel, B.; Olmedilla-Alonso, B. Assessment of dietary lutein, zeaxanthin and lycopene intakes and sources in the Spanish survey of dietary intake (2009–2010). Int. J. Food Sci. Nutr. 2016, 67, 305–313. [Google Scholar] [CrossRef]
- Than, A.; Bramley, P.M.; Davies, B.H.; Rees, A.F. Stereochemistry of phytoene. Phytochemistry 1972, 11, 3187–3192. [Google Scholar] [CrossRef]
- Mapelli-Brahm, P.; Desmarchelier, C.; Margier, M.; Reboul, E.; Meléndez Martínez, A.J.; Borel, P. Phytoene and Phytofluene Isolated from a Tomato Extract are Readily Incorporated in Mixed Micelles and Absorbed by Caco-2 Cells, as Compared to Lycopene, and SR-BI is Involved in their Cellular Uptake. Mol. Nutr. Food Res. 2018, 62, e1800703. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Gleize, B.; Zhang, L.; Caris-Veyrat, C.; Renard, C.M.G.C. Impact of onions in tomato-based sauces on isomerization and bioaccessibility of colorless carotenes: Phytoene and phytofluene. Food Funct. 2020, 11, 5122–5132. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Martínez, A.J.; Paulino, M.; Stinco, C.M.; Mapelli-Brahm, P.; Wang, X.D. Study of the time-course of cis/trans (Z/E) isomerization of lycopene, phytoene, and phytofluene from tomato. J. Agric. Food Chem. 2014, 62, 12399–12406. [Google Scholar] [CrossRef]
- de Rosso, V.V.; Mercadante, A.Z. HPLC-PDA-MS/MS of anthocyanins and carotenoids from dovyalis and tamarillo fruits. J. Agric. Food Chem. 2007, 55, 9135–9141. [Google Scholar] [CrossRef]
- Melendez-Martinez, A.J.; Stinco, C.M.; Liu, C.; Wang, X.D. A simple HPLC method for the comprehensive analysis of cis/trans (Z/E) geometrical isomers of carotenoids for nutritional studies. Food Chem. 2013, 138, 1341–1350. [Google Scholar] [CrossRef]
- Stinco, C.M.; Benítez-González, A.M.; Meléndez-Martínez, A.J.; Hernanz, D.; Vicario, I.M. Simultaneous determination of dietary isoprenoids (carotenoids, chlorophylls and tocopherols) in human faeces by Rapid Resolution Liquid Chromatography. J. Chromatogr. A 2019, 1583, 63–72. [Google Scholar] [CrossRef]
- Khachik, F.; Spangler, C.J.; Smith, J.C., Jr.; Canfield, L.M.; Steck, A.; Pfander, H. Identification, quantification, and relative concentrations of carotenoids and their metabolites in human milk and serum. Anal. Chem. 1997, 69, 1873–1881. [Google Scholar] [CrossRef]
- Paetau, I.; Khachik, F.; Brown, E.D.; Beecher, G.R.; Kramer, T.R.; Chittams, J.; Clevidence, B.A. Chronic ingestion of lycopene-rich tomato juice or lycopene supplements significantly increases plasma concentrations of lycopene and related tomato carotenoids in humans. Am. J. Clin. Nutr. 1998, 68, 1187–1195. [Google Scholar] [CrossRef]
- Aust, O.; Stahl, W.; Sies, H.; Tronnier, H.; Heinrich, U. Supplementation with tomato-based products increases lycopene, phytofluene, and phytoene levels in human serum and protects against UV-light-induced erythema. Int. J. Vitam. Nutr. Res. 2005, 75, 54–60. [Google Scholar] [CrossRef]



| Serum Concentration (µmol/L) | |
| Phytoene | 0.166 ± 0.067 (0.154) |
| Phytofluene | 0.058 ± 0.039 (0.044) |
| Faecal Concentration (μg/g d.w.) 1 | |
| Phytoene | 16.43 ± 22.04 (11.4) |
| Phytofluene | 5.53 ± 8.11 (3.0) |
| Phytoene Dietary Intake From (µg/day) 2 | |
| Apricot | 126.1 ± 481.8 (0.0) |
| Carrot | 1424.6 ± 2069.9 (697.3) |
| Orange | 31.5 ± 62.8 (0.0) |
| Orange juice | 26.0 ± 57.1 (0.0) |
| Tomato | 1135.3 ± 1195.7 (894.3) |
| Tomato fried | 0.0 ± 0.0 (0.0) |
| Tomato pure | 21.0 ± 65.6 (0.0) |
| Tomato juice | 26.3 ± 263.9 (0.0) |
| Watermelon | 35.0 ± 143.2 (0.0) |
| Phytofluene Dietary Intake From (µg/day) 1 | |
| Apricot | 27.6 ± 105.3 (0.0) |
| Carrot | 333.6 ± 484.7 (163.3) |
| Orange | 14.4 ± 28.7 (0.0) |
| Orange juice | 8.5 ± 18.7 (0.0) |
| Tomato | 220.8 ± 232.5 (173.9) |
| Tomato fried | 76.0 ± 149.4 (0.0) |
| Tomato pure | 11.2 ± 34.9 (0.0) |
| Tomato juice | 11.6 ± 117.0 (0.0) |
| Watermelon | 13.4 ± 54.7 (0.0) |
| Fruit Group (n = 14) | Vegetable Group (n = 15) | |||
|---|---|---|---|---|
| Basal | Final | Basal | Final | |
| Serum Concentration | ||||
| Phytoene | 0.119 ± 0.035 (0.107) | 0.110 ± 0.025 (0.109) | 0.143 ± 0.082 (0.116) | 0.138 ± 0.074 (0.110) |
| Phytofluene | 0.036 ± 0.027 (0.026) | 0.034 ± 0.018 (0.027) | 0.043 ± 0.030 (0.038) | 0.049 ± 0.031 (0.038) |
| Faecal Concentration | ||||
| Phytoene | 23.27 ± 12.57 (24.47) a | 14.48 ± 8.69 (10.91) a,* | 19.97 ± 11.29 (19.13) | 23.37 ± 11.56 (19.49) * |
| Total phytofluene | 10.98 ± 6.96 (13.34) | 7.39 ± 4.20 (5.83) | 8.39 ± 5.82 (7.53) | 10.35 ± 5.44 (8.87) |
| Isomer 1 | 6.61 ± 4.73 (7.27) | 4.23 ± 2.96 (3.41) | 4.41 ± 3.52 (4.26) | 5.49 ± 3.49 (4.33) |
| Isomer 2 | 3.63 ± 2.19 (4.03) | 2.70 ± 1.19 (2.55) | 3.07 ± 2.18 (2.95) | 3.79 ± 1.76 (3.30) |
| Isomer 3 | 0.73 ± 0.50 (0.94) a | 0.47 ± 0.27 (0.47) a* | 0.92 ± 0.66 (0.97) | 1.07 ± 0.75 (1.01) * |
| Dietary Intake | ||||
| Phytoene | 2229.8 ± 2212.8 (1657.7) | 1856.7 ± 1505.9 (1675.1) | 3037.2 ± 1784.8 (2837.8) | 2281.7± 1542.3 (2043.2) |
| Phytofluene | 547.4 ± 491.1 (437.7) | 459.5 ± 305.5 (440.4) | 797.4 ± 438.7 (640.0) | 518.9 ± 344.8 (412.5) |
| PT Dietary Intake From | ||||
| Apricot | 0.0 ± 0.0 (0.0) | 0.0 ± 0.0 (0.0) | 28.7 ± 111.3(0.0) | 488.5 ± 1078.5 (0.0) |
| Carrot | 1172.1 ± 2157.9 (526.7) a | 479.4 ± 806.1 (0.0) a | 1475.1 ± 2068.9 (632.0) | 621.8 ± 1106.6 (0.0) |
| Orange | 93.0 ± 80.0 (89.7) | 126.3 ± 52.2 (120.5) | 32.4 ± 60.7 (0.0) | 20.5 ± 38.0 (0.0) |
| Orange juice | 0.0 ± 0.0 (0.0) * | 5.8 ± 21.8 (0.0) * | 29.9 ± 58.6 (0.0) a,* | 0.0 ± 0.0 (0.0) a,* |
| Tomato | 935.3 ± 953.2 (531.2) | 1212.3 ± 985.4 (1095.4) | 1406.1 ± 1313.2 (1169.2) | 970.1 ± 759.1 (797.6) |
| Tomato fried | 0.0 ± 0.0 (0.0) | 0.0 ± 0.0 (0.0) | 0.0 ± 0.0 (0.0) | 0.0 ± 0.0 (0.0) |
| Tomato pure | 20.5 ± 55.0 (0.0) | 20.1 ± 75.3 (0.0) | 12.5 ± 33.0 (0.0) | 12.5 ± 48.5 (0.0) |
| Tomato juice | 0.0 ± 0.0 (0.0) | 0.0 ± 0.0 (0.0) | 0.0 ± 0.0 (0.0) | 0.0 ± 0.0 (0.0) |
| Watermelon | 0.0 ± 0.0 (0.0) | 2.9 ± 10.8 (0.0) | 40.4 ± 91.4 (0.0) | 88.2 ± 146.9 (0.0) |
| PTF Dietary Intake From | ||||
| Apricot | 0.0 ± 0.0 (0.0) | 0.0 ± 0.0 (0.0) | 6.3 ± 24.3 (0.0) | 106.8 ± 235.8 (0.0) |
| Carrot | 274.5 ± 505.3 (123.4) a | 112.3 ± 188.8 (0.0) a | 345.4 ± 484.5 (148.0) | 145.6 ± 259.1 (0.0) |
| Orange | 42.4 ± 36.5 (40.9.) | 57.6 ± 23.8 (55.0) * | 14.8 ± 27.7 (0.0) | 9.3 ± 17.4 (0.0) * |
| Orange juice | 0.0 ± 0.0 (0.0) * | 1.9 ± 7.1 (0.0) | 9.8 ± 19.2 (0.0) a,* | 0.0 ± 0.0 (0.0) a |
| Tomato | 181.9 ± 185.4 (103.3) | 235.7 ± 191.6 (213.0) | 273.4 ± 255.4 (227.4) | 188.7 ± 147.6 (155.1) |
| Tomato fried | 28.7 ± 49.8 (0.0) | 36.1 ± 52.2 (0.0) * | 112.3 ± 178.2 (0.0) a | 8.8 ± 34.0 (0.0) a,* |
| Tomato pure | 10.9 ± 29.2 (0.0) | 10.7 ± 40.1 (0.0) | 6.7 ± 17.6 (0.0) | 6.7 ± 25.8 (0.0) |
| Tomato juice | 0.0 ± 0.0 (0.0) | 0.0 ± 0.0 (0.0) | 0.0 ± 0.0 (0.0) | 0.0 ± 0.0 (0.0) |
| Watermelon | 0.0 ± 0.0 (0.0) | 1.1 ± 4.1 (0.0) | 15.4 ± 34.9 (0.0) | 33.7 ± 56.1 (0.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Rodríguez, E.; Estévez-Santiago, R.; Sánchez-Prieto, M.; Olmedilla-Alonso, B. Status and Dietary Intake of Phytoene and Phytofluene in Spanish Adults and the Effect of a Four-Week Dietary Intervention with Lutein-Rich Fruits or Vegetables. Nutrients 2022, 14, 2922. https://doi.org/10.3390/nu14142922
Rodríguez-Rodríguez E, Estévez-Santiago R, Sánchez-Prieto M, Olmedilla-Alonso B. Status and Dietary Intake of Phytoene and Phytofluene in Spanish Adults and the Effect of a Four-Week Dietary Intervention with Lutein-Rich Fruits or Vegetables. Nutrients. 2022; 14(14):2922. https://doi.org/10.3390/nu14142922
Chicago/Turabian StyleRodríguez-Rodríguez, Elena, Rocío Estévez-Santiago, Milagros Sánchez-Prieto, and Begoña Olmedilla-Alonso. 2022. "Status and Dietary Intake of Phytoene and Phytofluene in Spanish Adults and the Effect of a Four-Week Dietary Intervention with Lutein-Rich Fruits or Vegetables" Nutrients 14, no. 14: 2922. https://doi.org/10.3390/nu14142922