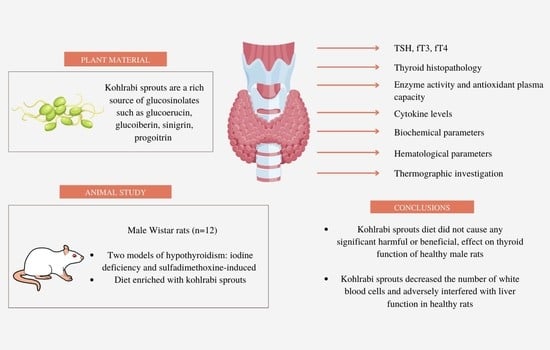
The Impact of Kohlrabi Sprouts on Various Thyroid Parameters in Iodine Deficiency- and Sulfadimethoxine-Induced Hypothyroid Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Animal Study
2.3. TSH, fT3, fT4 Analysis
2.4. Thyroid Histopathology Analysis
2.5. Enzyme Activity and Antioxidant Plasma Capacity Analysis
2.6. Measurement of Cytokine Levels
2.7. Biochemical Analysis
2.8. Haematological Evaluation
2.9. Thermographic Investigation
2.10. Statistical Analysis and Chemometric Approach
3. Results and Discussion
3.1. Hormones Level and Histopathology Analysis
3.2. Antioxidant Effect
3.3. Immunological Parameters
3.4. Biochemical Parameters
3.5. Haematological Parameters
3.6. Body Temperature Determination
3.7. Chemometric Analysis
3.8. The Importance of the Breed of the Rats Used in the Experiment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Dalmazi, G.; Giuliani, C. Plant constituents and thyroid: A revision of the main phytochemicals that interfere with thyroid function. Food Chem. Toxicol. 2021, 152, 112158. [Google Scholar] [CrossRef] [PubMed]
- Montané, X.; Kowalczyk, O.; Reig-Vano, B.; Bajek, A.; Roszkowski, K.; Tomczyk, R.; Pawliszak, W.; Giamberini, M.; Mocek-Płóciniak, A.; Tylkowski, B. Current perspectives of the applications of polyphenols and flavonoids in cancer therapy. Molecules 2020, 25, 3342. [Google Scholar] [CrossRef] [PubMed]
- Benvenga, S.; Ferrari, S.M.; Elia, G.; Ragusa, F.; Patrizio, A.; Paparo, S.R.; Camastra, S.; Bonofiglio, D.; Antonelli, A.; Fallahi, P. Nutraceuticals in thyroidology: A review of in vitro, and in vivo animal studies. Nutrients 2020, 12, 1337. [Google Scholar] [CrossRef] [PubMed]
- Paśko, P.; Krośniak, M.; Prochownik, E.; Tyszka-Czochara, M.; Fołta, M.; Francik, R.; Sikora, J.; Malinowski, M.; Zagrodzki, P. Effect of broccoli sprouts on thyroid function, haematological, biochemical, and immunological parameters in rats with thyroid imbalance. Biomed. Pharmacother. 2018, 97, 82–90. [Google Scholar] [CrossRef]
- Felker, P.; Bunch, R.; Leung, A.M. Concentrations of thiocyanate and goitrin in human plasma, their precursor concentrations in brassica vegetables, and associated potential risk for hypothyroidism. Nutr. Rev. 2016, 74, 248–258. [Google Scholar] [CrossRef] [Green Version]
- Langer, P.; Michajlovskij, N.; Sedlak, J.; Kutka, M. Studies on the antithyroid activity of naturally occurring L-5-vinyl-2-thiooxazolidone in man. Endokrinologie 1971, 57, 225–229. [Google Scholar]
- Paśko, P.; Galanty, A.; Tyszka-Czochara, M.; Żmudzki, P.; Zagrodzki, P.; Gdula-Argasińska, J.; Prochownik, E.; Gorinstein, S. Health Promoting vs. Anti-nutritive Aspects of Kohlrabi Sprouts, a Promising Candidate for Novel Functional Food. Plant Foods Hum. Nutr. 2021, 76, 76–82. [Google Scholar] [CrossRef]
- Chartoumpekis, D.V.; Ziros, P.G.; Chen, J.G.; Groopman, J.D.; Kensler, T.W.; Sykiotis, G.P. Broccoli sprout beverage is safe for thyroid hormonal and autoimmune status: Results of a 12-week randomized trial. Food Chem. Toxicol. 2019, 126, 1–6. [Google Scholar] [CrossRef]
- Babiker, A.; Alawi, A.; Al Atawi, M.; Al Alwan, I. The role of micronutrients in thyroid dysfunction. Sudan. J. Paediatr. 2020, 20, 13. [Google Scholar] [CrossRef]
- Chu, M.; Seltzer, T.F. Myxedema coma induced by ingestion of raw bok choy. N. Engl. J. Med. 2010, 362, 1945–1946. [Google Scholar] [CrossRef]
- Ikeda, T.; Nishikawa, A.; Imazawa, T.; Kimura, S.; Hirose, M. Dramatic synergism between excess soybean intake and iodine deficiency on the development of rat thyroid hyperplasia. Carcinogenesis 2000, 21, 707–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, H.Y.; Nishikawa, A.; Ikeda, T.; Imazawa, T.; Kimura, S.; Hirose, M. Lack of Effect of Soy Isoflavone on Thyroid Hyperplasia in Rats Receiving an Iodine-deficient Diet. Jpn. J. Cancer Res. 2001, 92, 103–108. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, T.; Nishikawa, A.; Son, H.Y.; Nakamura, H.; Miyauchi, M.; Imazawa, T.; Kimura, S.; Hirose, M. Synergistic Effects of High-dose Soybean Intake with Iodine Deficiency, but Not Sulfadimethoxine or Phenobarbital, on Rat Thyroid Proliferation. Jpn. J. Cancer Res. 2001, 92, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolska-Iwanek, J.; Zagrodzki, P.; Prochownik, E.; Jarkiewicz, A.; Paśko, P. Influence of brassica sprouts on short chain fatty acids concentration in stools of rats with thyroid dysfunction. Acta Pol. Pharm. 2019, 76, 1005–1014. [Google Scholar] [CrossRef]
- Paśko, P.; Okoń, K.; Krośniak, M.; Prochownik, E.; Żmudzki, P.; Kryczyk-Kozioł, J.; Zagrodzki, P. Interaction between iodine and glucosinolates in rutabaga sprouts and selected biomarkers of thyroid function in male rats. J. Trace Elem. Med. Biol. 2018, 46, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Morris, V.C.; Levander, O.A. Rapid determination of glutathione peroxidase and thioredoxin reductase activities using a 96-well microplate format: Comparison to standard cuvette-based assays. Int. J. Vitam. Nutr. Res. 2001, 71, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Paśko, P.; Prochownik, E.; Krośniak, M.; Tyszka-Czochara, M.; Francik, R.; Marcinkowska, M.; Sikora, J.; Malinowski, M.; Zagrodzki, P. Animals in iodine deficiency or sulfadimethoxine models of thyroid damage are differently affected by the consumption of Brassica sprouts. Biol. Trace Elem. Res. 2020, 193, 204–213. [Google Scholar] [CrossRef] [Green Version]
- Zagrodzki, P.; Krzyczkowska-Sendrakowska, M.; Nicol, F.; Wietecha-Posłuszny, R.; Milewicz, T.; Kryczyk-Kozioł, J.; Chaykivska, Z.; Jach, R. Selenium status parameters in patients with polycystic ovary syndrome. J. Trace Elem. Med. Biol. 2017, 44, 241–246. [Google Scholar] [CrossRef] [PubMed]
- McMillan, M.; Spinks, E.A.; Fenwick, G.R. Preliminary observations on the effect of dietary brussels sprouts on thyroid function. Hum. Toxicol. 1986, 5, 15–19. [Google Scholar] [CrossRef]
- Schone, F.; Jahreis, G.; Lange, R.; Seffner, W.; Groppel, B.; Hennig, A.; Ludke, H. Effect of varying glucosinolate and iodine intake via rapeseed meal diets on serum thyroid hormone level and total iodine in the thyroid in growing pigs. Endocrinol. Exp. 1990, 24, 415–427. [Google Scholar]
- Chandra, A.K.; Mukhopadhyay, S.; Ghosh, D.; Tripathy, S. Effect of radish (Raphanus sativus Linn.) on thyroid status under conditions of varying iodine intake in rats. Indian J. Exp. Biol. 2006, 44, 653–661. [Google Scholar] [PubMed]
- Schöne, F.; Rudolph, B.; Kirchheim, U.; Knapp, G. Counteracting the negative effects of rapeseed and rapeseed press cake in pig diets. Br. J. Nutr. 1997, 78, 947–962. [Google Scholar] [CrossRef]
- McKinnon, P.J.; Bowland, J.P. Effects of feeding low and high glucosinolate rapeseed meals and soybean meal on thyroid function of young pigs. Can. J. Anim. Sci. 1979, 59, 589–596. [Google Scholar] [CrossRef]
- Rajab, N.M.A.; Ukropina, M.; Cakic-Milosevic, M. Histological and ultrastructural alterations of rat thyroid gland after short-term treatment with high doses of thyroid hormones. Saudi J. Biol. Sci. 2017, 24, 1117–1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, J.M.; Benjamin, B.R.; Giovannetti, P.M. Histopathology of thyroids and livers of rats and mice fed diets containing Brassica glucosinolates. Can. J. Anim. Sci. 1972, 52, 395–406. [Google Scholar] [CrossRef] [Green Version]
- Leoni, S.G.; Kimura, E.T.; Santisteban, P.; De la Vieja, A. Regulation of thyroid oxidative state by thioredoxin reductase has a crucial role in thyroid responses to iodide excess. Mol. Endocrinol. 2011, 25, 1924–1935. [Google Scholar] [CrossRef] [Green Version]
- Clarke, J.D.; Dashwood, R.H.; Ho, E. Multi-targeted prevention of cancer by sulforaphane. Cancer Lett. 2008, 269, 291–304. [Google Scholar] [CrossRef] [Green Version]
- Abbas, A.M.; Sakr, H.F. Effect of magnesium sulfate and thyroxine on inflammatory markers in a rat model of hypothyroidism. Can. J. Physiol. Pharmacol. 2016, 94, 426–432. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, D.Y.; Baek, D.R.; Jeong, R.H.; Lee, D.S.; Kim, Y.C.; Kim, G.S.; Baek, N.I.; Lee, Y.H. Phenylpropanoids from red kohlrabi sprouts inhibits nitric oxide production in RAW 264.7 macrophage cells. Food Sci. Biotechnol. 2004, 23, 965–969. [Google Scholar] [CrossRef]
- Jung, H.A.; Karki, S.; Ehom, N.Y.; Yoon, M.H.; Kim, E.J.; Choi, J.S. Anti-diabetic and anti-inflammatory effects of green and red kohlrabi cultivars (Brassica oleracea var. gongylodes). Prev. Nutr. Food Sci. 2014, 19, 281. [Google Scholar] [CrossRef] [Green Version]
- Contreras-Jurado, C.; Alonso-Merino, E.; Saiz-Ladera, C.; Valiño, A.J.; Regadera, J.; Alemany, S.; Aranda, A. The thyroid hormone receptors inhibit hepatic interleukin-6 signaling during endotoxemia. Sci. Rep. 2016, 6, 30990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, P.H.; Black, E.G.; Sheppard, M.C.; Franklyn, J.A. Relation between serum interleukin-6 and thyroid hormone concentrations in 270 hospital in-patients with non-thyroidal illness. Clin. Endocrinol. 1996, 44, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Quispe, Á.; Li, X.M.; Yi, H. Comparison and relationship of thyroid hormones, IL-6, IL-10 and albumin as mortality predictors in case-mix critically ill patients. Cytokine. 2016, 81, 94–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messarah, M.; Boumendjel, A.; Chouabia, A.; Klibet, F.; Abdennour, C.; Boulakoud, M.S.; El Feki, A. Influence of thyroid dysfunction on liver lipid peroxidation and antioxidant status in experimental rats. Exp. Toxicol. Pathol. 2010, 62, 301–310. [Google Scholar] [CrossRef]
- Charron, C.S.; Novotny, J.A.; Jeffery, E.H.; Kramer, M.; Ross, S.A.; Seifried, H.E. Consumption of baby kale increased cytochrome P450 1A2 (CYP1A2) activity and influenced bilirubin metabolism in a randomized clinical trial. J. Funct. Foods 2020, 64, 103624. [Google Scholar] [CrossRef]
- Hasan, K.M.M.; Tamanna, N.; Haque, M.A. Biochemical and histopathological profiling of Wistar rat treated with Brassica napus as a supplementary feed. Food Sci. Hum. Wellness 2018, 7, 77–82. [Google Scholar] [CrossRef]
- Abouzed, T.K.; Althobaiti, F.; Abdelkhlek, N.A.; Eldomany, E.B.; Nasr, N.E.; Sadek, K.M.; El-Shazly, S.A.; Kahilo, K.A.; Dor-ghamm, D.A. Antitumor and Antioxidant Activity of S-Methyl Methionine Sulfonium Chloride against Liver Cancer Induced in Wistar Albino Rats by Diethyl Nitrosamine and Carbon Tertrachloride. Int. J. Environ. Res. Public Health 2021, 18, 9726. [Google Scholar] [CrossRef]
- Mullur, R.; Liu, Y.Y.; Brent, G.A. Thyroid hormone regulation of metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef] [Green Version]
- Duntas, L.H.; Brenta, G. A renewed focus on the association between thyroid hormones and lipid metabolism. Front. Endocrinol. 2018, 9, 511. [Google Scholar] [CrossRef]
- Gao, J.; Cheng, B.; Sun, Y.; Zhao, Y.; Zhao, G. Effects of dietary inclusion with rapeseed cake containing high glucosinolates on nitrogen metabolism and urine nitrous oxide emissions in steers. Anim. Nutr. 2022, 8, 204–215. [Google Scholar] [CrossRef]
- Trepanier, L.A. Idiosyncratic toxicity associated with potentiated sulfonamides in the dog. J. Vet. Pharmacol. Ther. 2004, 27, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Fabris, N. Immunodepression in thyroid-deprived animals. Clin. Exp. Immunol. 1973, 15, 601. [Google Scholar] [PubMed]
- Woyengo, T.A.; Kiarie, E.; Nyachoti, C.M. Growth performance, organ weights, and blood parameters of broilers fed diets containing expeller-extracted canola meal. Poult. Sci. 2011, 90, 2520–2527. [Google Scholar] [CrossRef] [PubMed]
- Munters, E.; Pieters, N.; Cuypers, A.; Penders, J.; Vangronsveld, J.; Nawrot, T. Effects of broccoli sprouts intake on oxidative stress, inflammation, microalbuminuria and platelet function in human volunteers: A cross-over study. Proc. Nutr. Soc. 2010, 69, 590. [Google Scholar] [CrossRef] [Green Version]
- Emont, M.P.; Yu, H.; Wu, J. Transcriptional control and hormonal response of thermogenic fat. J. Endocrinol. 2015, 225, 35–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paśko, P.; Bukowska-Strakova, K.; Gdula-Argasińska, J.; Tyszka-Czochara, M. Rutabaga (Brassica napus L. var. napobrassica) seeds, roots, and sprouts: A novel kind of food with antioxidant properties and proapoptotic potential in Hep G2 hepatoma cell line. J. Med. Food 2013, 16, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Paśko, P.; Galanty, A.; Żmudzki, P.; Gdula-Argasińska, J.; Zagrodzki, P. Influence of different light conditions and time of sprouting on harmful and beneficial aspects of rutabaga sprouts in comparison to their roots and seeds. J. Sci. Food Agric. 2019, 99, 302–308. [Google Scholar] [CrossRef] [Green Version]
- Paśko, P.; Tyszka-Czochara, M.; Galanty, A.; Gdula-Argasińska, J.; Żmudzki, P.; Bartoń, H.; Zagrodzki, P.; Gorinstein, S. Comparative study of predominant phytochemical compounds and proapoptotic potential of broccoli sprouts and florets. Plant Foods Hum. Nutr. 2018, 73, 95–100. [Google Scholar] [CrossRef] [Green Version]



| C | KS | ID | KS/ID | SDM | KS/SDM |
|---|---|---|---|---|---|
| 14.8 ± 3.3 abc | 11.0 ± 2.8 a | 11.75 ± 3.0 de | 13.7 ± 2.9 f | 23.1 ± 3.8 bdefg | 12.3 ± 2.9 cg |
| Parameters | C | KS | ID | KS/ID | SDM | KS/SDM | p Value |
|---|---|---|---|---|---|---|---|
| Hormones | |||||||
| TSH [μIU/L] n = 12 | 9.15 ± 1.55 ab | 9.44 ± 1.51 c | 10.43 ± 2.71 | 9.26 ± 1.52 d | 29.86 ± 19.43 acd | 23.23 ± 13.37 b | a *** d ** bc * |
| fT3 [pg/mL] n = 12 | 4.33 ± 0.70 a | 4.25 ± 0.99 b | 4.52 ± 0.99 c | 4.24 ± 0.99 d | 2.91 ± 0.93 abcd | 3.19 ± 1.17 | ac ** bd * |
| fT4 [ng/dL] n = 12 | 10.78 ± 4.12 | 9.03 ± 1.84 | 10.65 ± 1.81 a | 8.34 ± 1.67 | 9.09 ± 2.81 | 8.06 ± 1.61 a | a * |
| Thyroid Glands | |||||||
| GPX1 [U/g] n = 4 | 2.27 ± 0.99 | 3.58 ± 0.54 | 3.53 ± 1.24 | 2.62 ± 0.76 | 1.54 ± 0.40 | 2.39 ± 0.74 | - |
| TR [mU/mg] n = 4 | 2.54 ± 0.13 a | 5.89 ± 3.20 a | 3.51 ± 2.32 | 4.35 ± 2.62 | 1.86 ± 0.87 | 2.46 ± 0.72 | a * |
| Plasma | |||||||
| GPX3 [U/mg] n = 12 | 0.50 ± 0.17 | 0.53 ± 0.04 | 0.56 ± 0.03 | 0.50 ± 0.03 | 0.51 ± 0.04 | 0.51 ± 0.04 | - |
| FRAP [μmol/L] n = 12 | 434.6 ± 88.9 a | 468.5 ± 66.6 | 391.7 ± 39.0 b | 430.6 ± 36.0 c | 475.7 ± 89.2 | 594.2 ± 148.1 abc | b *** ac ** |
| IL-6 [pg/mL] n = 6 | 20.50± 10.74 a | 31.94 ± 10.93 | 54.73 ± 9.28 a | 26.77 ± 16.53 | 46.04 ± 24.96 | 43.83 ± 3.61 | a * |
| IL-10 [pg/mL] n = 6 | 32.06 ± 8.52 | 37.44 ± 7.13 | 60.76 ± 25.86 | 21.06 ± 4.13 | 78.46 ± 48.12 a | 16.32 ± 9.27 a | a * |
| TNFα [pg/mL] n = 6 | 45.80 ± 32.25 | 65.25 ± 29.10 | 71.67 ± 9.63 a | 13.40 ± 6.24 a | 53.72 ± 29.55 | 42.00 ± 17.62 | a * |
| Body Temperature | |||||||
| TEMP [°C] n = 12 | 35.5 ± 0.6 abc | 33.2 ± 0.7 | 34.3 ± 1.7 | 32.7 ± 1.4 a | 31.7 ± 1.8 b | 32.7 ± 1.3 c | a * b *** c ** |
| Parameters | C | KS | ID | KS/ID | SDM | KS/SDM |
|---|---|---|---|---|---|---|
| Papillary formation [%] | 0% | 0% | 0% | 25% | 60% | 80% |
| Aggregates of lymphocytes [%] | 0% | 25% | 0% | 0% | 0% | 0% |
| Vacuolization of the colloid [%] | 0% | 0% | 40% | 0% | 0% | 0% |
| Shape of epithelial cells [flatted vs. cuboidal] [%] | 100% flatted | 60% cuboidal | 60% cuboidal | 50% cuboidal | 100% cuboidal | 100% cuboidal |
| Follicular epithelial area [mm2] | 1.57 ± 0.14 ab | 1.77 ± 0.86 c | 3.06 ± 0.71 | 1.59 ± 0.82 d | 4.37 ± 1.03 bcd | 3.57 ± 1.68 a |
| Follicular luminal area [mm2] | 0.32 ± 0.06 a | 0.38 ± 0.14 | 0.69 ± 0.24 ab | 0.41 ± 0.20 | 0.41 ± 018 | 0.22 ± 0.06 b |
| Overall thyroid area [mm2] | 1.79 ± 0.25 a | 2.24 ± 0.80 | 3.50 ± 1.13 | 1.97 ± 1.00 b | 4.64 ± 1.40 ab | 3.79 ± 1.72 |
| Parameters | C | KS | ID | KS/ID | SDM | KS/SDM | p-Value |
|---|---|---|---|---|---|---|---|
| n = 12 | Blood Morphology Parameters | ||||||
| RBC [106/μL] | 9.13 ± 0.60 | 9.21 ± 0.92 | 9.89 ± 0.45 a | 9.32 ± 0.81 | 9.13 ± 0.45 a | 9.48 ± 0.83 | a * |
| Hb [g/dL] | 13.82 ± 0.50 | 14.32 ± 1.16 | 15.42 ± 0.54 | 19.69 ± 18.65 | 14.36 ± 0.60 | 21.32 ± 7.93 | - |
| Hct [%] | 44.42 ± 2.57 ab | 47.48 ± 3.34 | 50.84 ± 2.48 ac | 61.88 ± 48.22 | 46.30 ± 2.16 c | 50.10 ± 5.30 b | a *** bc * |
| MCV [fL] | 48.61 ± 1.84 ab | 51.00 ± 1.38 | 51.89 ± 0.91 a | 50.93 ± 1.02 | 50.65 ± 2.22 | 52.86 ± 2.04 b | ab ** |
| MCH [pg/cell] | 15.19 ± 0.79 | 15.58 ± 0.48 | 15.58 ± 0.43 | 15.45 ± 0.37 | 15.75 ± 0.87 | 21.71 ± 7.54 | |
| MCHC [g/dL] | 31.19 ± 1.17 | 30.54 ± 0.40 | 30.05 ± 0.45 a | 30.39 ± 0.60 | 31.02 ± 0.68 a | 41.28 ± 14.69 | a * |
| WBC [103/μL] | 20.85 ± 8.80 abcd | 9.02 ± 3.54 a | 8.73 ± 1.32 a | 9.23 ± 1.05 c | 11.07 ± 2.85 | 9.09 ± 1.53 d | a b *** c * d ** |
| PLT [103/μL] | 946.3 ± 202.8 ab | 637.2 ± 260.8 | 582.9 ± 287.3 a | 733.1 ± 253.3 | 889.4 ± 125.4 c | 506.9 ± 218.5 b c | a* b *** c ** |
| n = 12 | Biochemical Parameters | ||||||
| Glucose [mmoL/L] | 11.51 ± 2.40 | 11.77 ± 1.03 | 11.99 ± 1.10 | 11.54 ± 1.80 | 11.80 ± 1.95 | 11.07 ± 2.18 | - |
| Uric acid [mg/dL] | 22.83 ± 11.29 | 21.00 ± 6.30 | 25.75 ± 6.61 | 23.58 ± 8.63 | 20.83 ± 5.84 | 22.75 ± 5.59 | - |
| Urea [mmol/L] | 8.48 ± 1.35 | 9.79 ± 2.38 | 11.81 ± 2.88 | 11.19 ± 4.00 | 10.17 ± 2.13 | 12.43 ± 4.32 | - |
| Creatinine [μmol/L] | 21.50 ± 4.98 ac | 20.92 ± 3.23 | 17.75 ± 1.96 ab | 17.08 ± 1.68 c | 23.44 ± 4.82 b | 20.10 ± 1.20 | abc * |
| ASPAT [U/L] | 103.2 ± 28.7 abc | 87.2 ± 36.7 | 87.0 ± 19.8 | 69.6 ± 15.4 a | 72.6 ± 16.6 b | 71.9 ± 12.3 c | abc * |
| ALAT [U/L] | 28.88 ± 11.44 abcd | 81.14 ± 36.96 a | 85.86 ± 27.57 be | 87.18 ± 62.90 c | 46.74 ± 18.85 e | 83.34 ± 32.11 d | e * ac ** bd *** |
| TG [mmoL/L] | 0.79 ± 0.14 a | 0.83 ± 0.29 b | 0.48 ± 0.15 abc | 0.77 ± 0.31 c | 0.63 ± 0.13 | 0.66 ± 0.09 | c * b ** a *** |
| TC [mmoL/L] | 2.51 ± 0.53 a | 2.26 ± 0.19 | 1.95 ± 0.25 abcde | 2.36 ± 0.16 b | 2.26 ± 0.59 cd | 2.31 ± 0.45 e | bcde * a *** |
| HDL [mmoL/L] | 0.89 ± 0.07 | 0.96 ± 0.16 | 1.05 ± 0.78 | 1.01 ± 0.63 | 0.95 ± 0.50 | 0.81 ± 0.20 | |
| PAL [U/L] | 104.9 ± 48.7 abc | 147.1 ± 23.4 | 165.3 ± 26.1 b | 167.5 ± 23.2 a | 127.3 ± 35.0 d | 173.4 ± 16.0 cd | abd * c ** |
| Pairs of Correlated Parameters | Correlation Weights | |
|---|---|---|
| MCV | MCH | 0.200 |
| MCV | follicular epithelial area | 0.159 |
| TEMP | fT3 | 0.155 |
| ASPAT | fT3 | 0.150 |
| MCV | overall thyroid area | 0.149 |
| RBC | uric acid | 0.148 |
| RBC | follicular luminal area | 0.144 |
| MCV | ASPAT | −0.143 |
| fT3 | follicular epithelial area | −0.147 |
| IL-6 | fT3 | −0.147 |
| fT3 | overall thyroid area | −0.148 |
| uric acid | Creatinine | −0.154 |
| Creatinine | follicular luminal area | −0.157 |
| RBC | Creatinine | −0.166 |
| MCV | fT3 | −0.188 |
| Parameter | TSH | fT4 | fT3 | Temp | TC | TG | HDL | ASPAT | ALAT | GLU | WBC | PLT | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wistar rats | ID |  |  |  |  |  |  |  |  |  |  |  |  |
| SDM |  |  |  |  |  |  |  |  |  |  |  |  | |
| Fischer rats | ID |  |  |  |  |  |  |  |  |  |  |  |  |
| SDM |  |  |  |  |  |  |  |  |  |  |  |  |
 increase
increase  decrease
decrease  no changes.
no changes.| Parameter | TSH | fT4 | fT3 | Temp | TC | TG | HDL | ASPAT | ALAT | GLU | WBC | PLT | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wistar rats | KS |  |  |  |  |  |  |  |  |  |  |  |  |
| BS |  |  |  |  |  |  |  |  |  |  |  |  | |
| Fischer rats | RS |  |  |  |  |  |  |  |  |  |  |  |  |
 increase
increase  decrease
decrease  no changes.
no changes.Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paśko, P.; Okoń, K.; Prochownik, E.; Krośniak, M.; Francik, R.; Kryczyk-Kozioł, J.; Grudzińska, M.; Tyszka-Czochara, M.; Malinowski, M.; Sikora, J.; et al. The Impact of Kohlrabi Sprouts on Various Thyroid Parameters in Iodine Deficiency- and Sulfadimethoxine-Induced Hypothyroid Rats. Nutrients 2022, 14, 2802. https://doi.org/10.3390/nu14142802
Paśko P, Okoń K, Prochownik E, Krośniak M, Francik R, Kryczyk-Kozioł J, Grudzińska M, Tyszka-Czochara M, Malinowski M, Sikora J, et al. The Impact of Kohlrabi Sprouts on Various Thyroid Parameters in Iodine Deficiency- and Sulfadimethoxine-Induced Hypothyroid Rats. Nutrients. 2022; 14(14):2802. https://doi.org/10.3390/nu14142802
Chicago/Turabian StylePaśko, Paweł, Krzysztof Okoń, Ewelina Prochownik, Mirosław Krośniak, Renata Francik, Jadwiga Kryczyk-Kozioł, Marta Grudzińska, Małgorzata Tyszka-Czochara, Mateusz Malinowski, Jakub Sikora, and et al. 2022. "The Impact of Kohlrabi Sprouts on Various Thyroid Parameters in Iodine Deficiency- and Sulfadimethoxine-Induced Hypothyroid Rats" Nutrients 14, no. 14: 2802. https://doi.org/10.3390/nu14142802