Impact of Zinc Transport Mechanisms on Embryonic and Brain Development
Abstract
:1. Introduction
2. Sources, Intakes, and Absorption Cycle of Zinc
3. Epidemiology of Zinc Deficiency
4. Impact of Zinc on Health, Disease, and Development
4.1. Clinical Presentation of Zn Deficiency
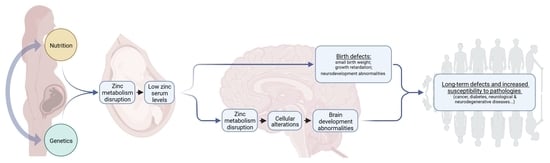
4.2. Impact of Zn Deficiency on Early Embryonic Development
4.3. Origin of Late Developmental Effects
4.4. Long-Term Consequences of Zn Deficiency on Brain Function during Development
5. Impact of Zinc Transporter and Channels on Zinc Deficiency and Development
5.1. ZnTs Transporters: SLC30A Family
5.1.1. ZnT1 (SLC30A1)
5.1.2. ZnT2 (SLC30A2)
5.1.3. ZnT3 (SLC30A3)
5.1.4. ZnT4 (SLC30A4)
5.1.5. ZnT5 (SLC30A5)
5.1.6. ZnT6 (SLC30A6)
5.1.7. ZnT7 (SLC30A7)
5.1.8. ZnT8 (SLC30A8)
5.1.9. ZnT9 (SLC30A9)
5.1.10. ZnT10 (SLC30A10)
5.2. ZnTs Transporters: SLC30A Family
5.2.1. ZIP1 (SLC39A1)
5.2.2. ZIP2 (SLC39A2)
5.2.3. ZIP3 (SLC39A3)
5.2.4. ZIP4 (SLC39A4)
5.2.5. ZIP5 (SLC39A5)
5.2.6. ZIP6 (SLC39A6)
5.2.7. ZIP7 (SLC39A7)
5.2.8. ZIP8 (SLC39A8)
5.2.9. ZIP9 (SLC39A9)
5.2.10. ZIP10 (SLC39A10)
5.2.11. ZIP11 (SLC39A11)
5.2.12. ZIP12 (SLC39A12)
5.2.13. ZIP13 (SLC39A13)
5.2.14. ZIP14 (SLC39A14)
6. TRPM7: A New Player in Zinc Homeostasis
7. Conclusions and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s Disease |
| AE | Acrodermatitis enteropathica |
| ALS | Amyotrophic Lateral Sclerosis |
| ASD | Autism Spectrum Disorders |
| BDNF | Brain-Derived Neurotrophic Factor |
| BIGM103 | Bacillus calmette-guerin-Induced Gene in Monocyte clone 103 |
| Cd | Cadmium |
| CHO | Chinese Hamster Ovary cells |
| CNS | Central Nervous System |
| Cu | Copper |
| DRIs | Dietary Reference Intakes |
| DV | Daily Value |
| EMT | Epithelial–Mesenchymal Transition |
| ER | Endoplasmic Reticulum |
| ESCs | Embryonic Stem Cells |
| FAK | Focal Adhesion Kinase |
| FDC | Food Data Central |
| Fe | Iron |
| FNB | Food and Nutrition Board |
| FXTAS | Fragile X-associated tremor/ataxia syndrome |
| GO | Gene Ontology |
| HD | Huntington’s disease |
| HEK-293 | Human Embryonic Kidney 293 cells |
| HUEL | Human Embryonic Lung Protein |
| IDE | Insulin-Degrading Enzyme |
| KO | Knockout |
| MAPK | Mitogen-Activated Protein Kinase |
| Mg | Magnesium |
| miRNA | micro-Ribonucleic Acid |
| MMP | Matrix Metallopeptidase |
| Mn | Manganese |
| MRI | Magnetic Resonance Imaging |
| MT | Metallothionein |
| MTR | Methionine Synthase |
| NCL | Neuronal Ceroid Lipofuscinosistube |
| NGF | Nerve Growth Factor |
| NMDA | N-methyl-D-aspartate |
| NTDs | Neural Tube Defects |
| PBMCs | Peripheral Blood Mono-nuclear Cells |
| PD | Parkinson’s disease |
| PSD-95 | PostSynaptic Density protein 95 |
| SCD-EDS | Spondylocheirodysplastic form of Ehlers-Danlos syndrome |
| SLC | Solute Carrier |
| SNP | Single Nucleotide Polymorphisms |
| SOD | Superoxide Dismutase |
| SOX9 | Sex determining region Y transcription factor 9 |
| T1D | Type 1 Diabetes |
| T2D | Type 1 Diabetes |
| TGF-β1 | Transforming Growth Factor-Beta 1 |
| TIZD | Transient Infantile Zn Deficiency |
| TNZD | Transient Neonatal Zinc Deficiency |
| TPEN | N,N,N′,N′-tetrakis(2-pyridinylmethyl)-1,2-ethanediamine |
| TRP | Transient Receptor Potential |
| USDA | U.S. Department of Agriculture |
| ZIP | Zinc/Iron-regulated transporter-like Proteins |
| Zn | Zinc |
| ZnT | Zinc transporter protein |
References
- Bafaro, E.; Liu, Y.; Xu, Y.; Dempski, R.E. The Emerging Role of Zinc Transporters in Cellular Homeostasis and Cancer. Signal Transduct. Target. Ther. 2017, 2, 17029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Noh, S.J.; Pronto, J.R.; Jeong, Y.J.; Kim, H.K.; Song, I.S.; Xu, Z.; Kwon, H.Y.; Kang, S.C.; Sohn, E.-H.; et al. The Critical Roles of Zinc: Beyond Impact on Myocardial Signaling. Korean J. Physiol. Pharmacol. 2015, 19, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Hirano, T. Intracellular Zinc Homeostasis and Zinc Signaling. Cancer Sci. 2008, 99, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Andreini, C.; Banci, L.; Bertini, I.; Rosato, A. Counting the Zinc-Proteins Encoded in the Human Genome. J. Proteome Res. 2006, 5, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Wang, J.; Guo, Z.; Li, M.; Li, M.; Liu, S.; Liu, H.; Li, W.; Yin, X.; Tao, J.; et al. Emerging Function and Potential Diagnostic Value of Circular RNAs in Cancer. Mol. Cancer 2018, 17, 123. [Google Scholar] [CrossRef] [Green Version]
- Bertini, I.; Manganl, S.; Viezzoli, M.S. Structure and Properties of Copper-Zinc Superoxide Dismutases. Adv. Inorg. Chem. 1998, 45, 127–250. [Google Scholar]
- Hulse, R.E.; Ralat, L.A.; Tang, W.-J. Structure, Function, and Regulation of Insulin-Degrading Enzyme. Vitam. Horm. 2009, 80, 635–648. [Google Scholar] [CrossRef] [Green Version]
- Ferrer, J.-L.; Ravanel, S.; Robert, M.; Dumas, R. Crystal Structures of Cobalamin-Independent Methionine Synthase Complexed with Zinc, Homocysteine, and Methyltetrahydrofolate. J. Biol. Chem. 2004, 279, 44235–44238. [Google Scholar] [CrossRef] [Green Version]
- Costello, L.C.; Fenselau, C.C.; Franklin, R.B. Evidence for Operation of the Direct Zinc Ligand Exchange Mechanism for Trafficking, Transport, and Reactivity of Zinc in Mammalian Cells. J. Inorg. Biochem. 2011, 105, 589–599. [Google Scholar] [CrossRef] [Green Version]
- Yuan, N.; Wang, Y.-H.; Li, K.-J.; Zhao, Y.; Hu, X.; Mao, L.; Zhao, W.-J.; Lian, H.-Z.; Zheng, W.-J. Effects of Exogenous Zinc on the Cellular Zinc Distribution and Cell Cycle of A549 Cells. Biosci. Biotechnol. Biochem. 2012, 76, 2014–2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hara, T.; Takeda, T.-A.; Takagishi, T.; Fukue, K.; Kambe, T.; Fukada, T. Physiological Roles of Zinc Transporters: Molecular and Genetic Importance in Zinc Homeostasis. J. Physiol. Sci. JPS 2017, 67, 283–301. [Google Scholar] [CrossRef] [PubMed]
- Bozym, R.A.; Chimienti, F.; Giblin, L.J.; Gross, G.W.; Korichneva, I.; Li, Y.; Libert, S.; Maret, W.; Parviz, M.; Frederickson, C.J.; et al. Free Zinc Ions Outside a Narrow Concentration Range Are Toxic to a Variety of Cells in Vitro. Exp. Biol. Med. Maywood NJ 2010, 235, 741–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.-Y.; Zhang, D.-L.; Liu, X.-L.; Li, X.-S.; Cheng, X.-Q.; Chen, J.; Du, H.-N.; Liang, Y. Pathological Concentration of Zinc Dramatically Accelerates Abnormal Aggregation of Full-Length Human Tau and Thereby Significantly Increases Tau Toxicity in Neuronal Cells. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 414–427. [Google Scholar] [CrossRef] [PubMed]
- McCance, R.A.; Widdowson, E.M. The Absorption and Excretion of Zinc. Biochem. J. 1942, 36, 692–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kambe, T.; Hashimoto, A.; Fujimoto, S. Current Understanding of ZIP and ZnT Zinc Transporters in Human Health and Diseases. Cell. Mol. Life Sci. 2014, 71, 3281–3295. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.G. Zinc Transport in Mammalian Cells. Am. J. Physiol. 1996, 270, C401–C410. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering, and Medicine; Oria, M.; Harrison, M.; Stallings, V.A. Dietary Reference Intakes (DRIs): Recommended Dietary Allowances and Adequate Intakes, Elements, Food and Nutrition Board, National Academies. Available online: https://www.ncbi.nlm.nih.gov/books/NBK545442/table/appJ_tab3/ (accessed on 10 May 2022).
- Food Labeling: Revision of the Nutrition and Supplement Facts Labels. Available online: https://www.federalregister.gov/documents/2016/05/27/2016-11867/food-labeling-revision-of-the-nutrition-and-supplement-facts-labels (accessed on 10 May 2022).
- SR11-SR28: USDA ARS. Available online: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/methods-and-application-of-food-composition-laboratory/mafcl-site-pages/sr11-sr28/ (accessed on 10 May 2022).
- Lee, H.H.; Prasad, A.S.; Brewer, G.J.; Owyang, C. Zinc Absorption in Human Small Intestine. Am. J. Physiol. 1989, 256, G87–G91. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.M.; Bacon, J.R.; Aggett, P.J.; Bremner, I. Homeostatic Regulation of Zinc Absorption and Endogenous Losses in Zinc-Deprived Men. Am. J. Clin. Nutr. 1991, 53, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhou, B.; Kuo, Y.-M.; Zemansky, J.; Gitschier, J. A Novel Member of a Zinc Transporter Family Is Defective in Acrodermatitis Enteropathica. Am. J. Hum. Genet. 2002, 71, 66–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittermeier, L.; Demirkhanyan, L.; Stadlbauer, B.; Breit, A.; Recordati, C.; Hilgendorff, A.; Matsushita, M.; Braun, A.; Simmons, D.G.; Zakharian, E.; et al. TRPM7 Is the Central Gatekeeper of Intestinal Mineral Absorption Essential for Postnatal Survival. Proc. Natl. Acad. Sci. USA 2019, 116, 4706–4715. [Google Scholar] [CrossRef] [Green Version]
- Coyle, P.; Philcox, J.C.; Carey, L.C.; Rofe, A.M. Metallothionein: The Multipurpose Protein. Cell. Mol. Life Sci. CMLS 2002, 59, 627–647. [Google Scholar] [CrossRef]
- Wellenreuther, G.; Cianci, M.; Tucoulou, R.; Meyer-Klaucke, W.; Haase, H. The Ligand Environment of Zinc Stored in Vesicles. Biochem. Biophys. Res. Commun. 2009, 380, 198–203. [Google Scholar] [CrossRef] [PubMed]
- King, J.C.; Brown, K.H.; Gibson, R.S.; Krebs, N.F.; Lowe, N.M.; Siekmann, J.H.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND)-Zinc Review. J. Nutr. 2015, 146, 858S–885S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, J.C.; Shames, D.M.; Woodhouse, L.R. Zinc Homeostasis in Humans. J. Nutr. 2000, 130, 1360S–1366S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hambidge, M.; Krebs, N.F. Interrelationships of Key Variables of Human Zinc Homeostasis: Relevance to Dietary Zinc Requirements. Annu. Rev. Nutr. 2001, 21, 429–452. [Google Scholar] [CrossRef]
- Wessells, K.R.; Brown, K.H. Estimating the Global Prevalence of Zinc Deficiency: Results Based on Zinc Availability in National Food Supplies and the Prevalence of Stunting. PLoS ONE 2012, 7, e50568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer Walker, C.L.; Ezzati, M.; Black, R.E. Global and Regional Child Mortality and Burden of Disease Attributable to Zinc Deficiency. Eur. J. Clin. Nutr. 2009, 63, 591–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nriagu, J.O. Zinc Deficiency in Human Health, Encyclopedia of Environmental Health; Elsevier: Amsterdam, The Netherlands, 2019; pp. 489–499. [Google Scholar]
- Dassoni, F.; Abebe, Z.; Ricceri, F.; Morrone, A.; Albertin, C.; Naafs, B. High Frequency of Symptomatic Zinc Deficiency in Infants in Northern Ethiopia. Available online: https://www.hindawi.com/journals/drp/2014/719701/ (accessed on 29 April 2020).
- Ackland, M.L.; Michalczyk, A. Zinc Deficiency and Its Inherited Disorders—A Review. Genes Nutr. 2006, 1, 41–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, A.S.; Halsted, J.A.; Nadimi, M. Syndrome of Iron Deficiency Anemia, Hepatosplenomegaly, Hypogonadism, Dwarfism and Geophagia. Am. J. Med. 1961, 31, 532–546. [Google Scholar] [CrossRef]
- Devirgiliis, C.; Zalewski, P.D.; Perozzi, G.; Murgia, C. Zinc Fluxes and Zinc Transporter Genes in Chronic Diseases. Mutat. Res. 2007, 622, 84–93. [Google Scholar] [CrossRef]
- Maret, W.; Sandstead, H.H. Zinc Requirements and the Risks and Benefits of Zinc Supplementation. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. GMS 2006, 20, 3–18. [Google Scholar] [CrossRef]
- Sandstead, H.H. Human Zinc Deficiency: Discovery to Initial Translation. Adv. Nutr. Bethesda Md 2013, 4, 76–81. [Google Scholar] [CrossRef] [Green Version]
- Takeda, A.; Tamano, H. Insight into Zinc Signaling from Dietary Zinc Deficiency. Brain Res. Rev. 2009, 62, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Bashiri, R.; Ghadiri-Anari, A.; Nadjarzadeh, A. Antioxidant Supplements and Semen Parameters: An Evidence Based Review. Int. J. Reprod. Biomed. 2016, 14, 729–736. [Google Scholar] [CrossRef]
- Giahi, L.; Mohammadmoradi, S.; Javidan, A.; Sadeghi, M.R. Nutritional Modifications in Male Infertility: A Systematic Review Covering 2 Decades. Nutr. Rev. 2016, 74, 118–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beigi Harchegani, A.; Dahan, H.; Tahmasbpour, E.; Bakhtiari Kaboutaraki, H.; Shahriary, A. Effects of Zinc Deficiency on Impaired Spermatogenesis and Male Infertility: The Role of Oxidative Stress, Inflammation and Apoptosis. Hum. Fertil. Camb. Engl. 2020, 23, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Whittle, N.; Lubec, G.; Singewald, N. Zinc Deficiency Induces Enhanced Depression-like Behaviour and Altered Limbic Activation Reversed by Antidepressant Treatment in Mice. Amino Acids 2009, 36, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Aizenman, E.; Stout, A.K.; Hartnett, K.A.; Dineley, K.E.; McLaughlin, B.; Reynolds, I.J. Induction of Neuronal Apoptosis by Thiol Oxidation: Putative Role of Intracellular Zinc Release. J. Neurochem. 2000, 75, 1878–1888. [Google Scholar] [CrossRef] [Green Version]
- Smart, T.G.; Hosie, A.M.; Miller, P.S. Zn2+ Ions: Modulators of Excitatory and Inhibitory Synaptic Activity. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2004, 10, 432–442. [Google Scholar] [CrossRef]
- Aguirre, S.; Veiras, L.; Sánchez, R.; Cardelli Alcalde, D.L.; Elesgaray, R.; Costa, M.A.; Arranz, C.T.; Tomat, A.L. Fetal Programming of Hypertension Induced by Moderate Zinc Restriction during Prenatal Life and Lactation: Early Morphological and Functional Alterations in Cardiovascular System in Both Sexes. Argent. J. Cardiol. 2011, 79, 322–328. [Google Scholar] [CrossRef]
- Marciniak, A.; Patro-Małysza, J.; Kimber-Trojnar, Ż.; Marciniak, B.; Oleszczuk, J.; Leszczyńska-Gorzelak, B. Fetal Programming of the Metabolic Syndrome. Taiwan. J. Obstet. Gynecol. 2017, 56, 133–138. [Google Scholar] [CrossRef]
- Fukada, T.; Yamasaki, S.; Nishida, K.; Murakami, M.; Hirano, T. Zinc Homeostasis and Signaling in Health and Diseases. J. Biol. Inorg. Chem. 2011, 16, 1123–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szewczyk, B. Zinc Homeostasis and Neurodegenerative Disorders. Front. Aging Neurosci. 2013, 5, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Zhao, H.; Xu, Z.; Cheng, X. Zinc Dysregulation in Cancers and Its Potential as a Therapeutic Target. Cancer Biol. Med. 2020, 17, 612–625. [Google Scholar] [CrossRef] [PubMed]
- Vela, G.; Stark, P.; Socha, M.; Sauer, A.K.; Hagmeyer, S.; Grabrucker, A.M. Zinc in Gut-Brain Interaction in Autism and Neurological Disorders. Neural Plast. 2015, 2015, 972791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golan, Y.; Kambe, T.; Assaraf, Y.G. The Role of the Zinc Transporter SLC30A2/ZnT2 in Transient Neonatal Zinc Deficiency. Met. Integr. Biometal Sci. 2017, 9, 1352–1366. [Google Scholar] [CrossRef] [PubMed]
- Aggett, P.J.; Delves, H.T.; Thorn, J.M.; Atherton, D.J.; Harris, J.T.; Bangham, A.D. The Therapeutic Effect of Amphotericin in Acrodermatitis Enteropathica: Hypothesis and Implications. Eur. J. Pediatr. 1981, 137, 23–25. [Google Scholar] [CrossRef]
- Bleck, O.; Ashton, G.H.; Mallipeddi, R.; South, A.P.; Whittock, N.V.; McLean, W.H.; Atherton, D.J.; McGrath, J.A. Genomic Localization, Organization and Amplification of the Human Zinc Transporter Protein Gene, ZNT4, and Exclusion as a Candidate Gene in Different Clinical Variants of Acrodermatitis Enteropathica. Arch. Dermatol. Res. 2001, 293, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Küry, S.; Devilder, M.C.; Avet-Loiseau, H.; Dreno, B.; Moisan, J.P. Expression Pattern, Genomic Structure and Evaluation of the Human SLC30A4 Gene as a Candidate for Acrodermatitis Enteropathica. Hum. Genet. 2001, 109, 178–185. [Google Scholar] [CrossRef]
- Küry, S.; Dréno, B.; Bézieau, S.; Giraudet, S.; Kharfi, M.; Kamoun, R.; Moisan, J.-P. Identification of SLC39A4, a Gene Involved in Acrodermatitis Enteropathica. Nat. Genet. 2002, 31, 239–240. [Google Scholar] [CrossRef] [PubMed]
- Jagadeesan, S.; Kaliyadan, F. Acrodermatitis Enteropathica. In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2020. [Google Scholar]
- Fritz, J.; Walia, C.; Elkadri, A.; Pipkorn, R.; Dunn, R.K.; Sieracki, R.; Goday, P.S.; Cabrera, J.M. A Systematic Review of Micronutrient Deficiencies in Pediatric Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019, 25, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Terrin, G.; Berni Canani, R.; Di Chiara, M.; Pietravalle, A.; Aleandri, V.; Conte, F.; De Curtis, M. Zinc in Early Life: A Key Element in the Fetus and Preterm Neonate. Nutrients 2015, 7, 10427–10446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beaver, L.M.; Truong, L.; Barton, C.L.; Chase, T.T.; Gonnerman, G.D.; Wong, C.P.; Tanguay, R.L.; Ho, E. Combinatorial Effects of Zinc Deficiency and Arsenic Exposure on Zebrafish (Danio Rerio) Development. PLoS ONE 2017, 12, e0183831. [Google Scholar] [CrossRef] [PubMed]
- Beaver, L.M.; Nkrumah-Elie, Y.M.; Truong, L.; Barton, C.L.; Knecht, A.L.; Gonnerman, G.D.; Wong, C.P.; Tanguay, R.L.; Ho, E. Adverse Effects of Parental Zinc Deficiency on Metal Homeostasis and Embryonic Development in a Zebrafish Model. J. Nutr. Biochem. 2017, 43, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Bian, Z.; Liu, H.-B.; Chi, Z.-H. Zinc deficiency induces exfoliation of round spermatids by decreasing the expressions of TGF-β1 and FAK in the mouse testis. Zhonghua Nan Ke Xue Natl. J. Androl. 2019, 25, 9–15. [Google Scholar]
- Kothari, R.P.; Chaudhari, A.R. Zinc Levels in Seminal Fluid in Infertile Males and Its Relation with Serum Free Testosterone. J. Clin. Diagn. Res. JCDR 2016, 10, CC05–CC08. [Google Scholar] [CrossRef]
- Narasimhaiah, M.; Arunachalam, A.; Sellappan, S.; Mayasula, V.K.; Guvvala, P.R.; Ghosh, S.K.; Chandra, V.; Ghosh, J.; Kumar, H. Organic Zinc and Copper Supplementation on Antioxidant Protective Mechanism and Their Correlation with Sperm Functional Characteristics in Goats. Reprod. Domest. Anim. Zuchthyg. 2018, 53, 644–654. [Google Scholar] [CrossRef]
- Huang, L.; Li, X.; Wang, W.; Yang, L.; Zhu, Y. The Role of Zinc in Poultry Breeder and Hen Nutrition: An Update. Biol. Trace Elem. Res. 2019, 192, 308–318. [Google Scholar] [CrossRef]
- Kumari, D.; Nair, N.; Bedwal, R.S. Testicular Apoptosis after Dietary Zinc Deficiency: Ultrastructural and TUNEL Studies. Syst. Biol. Reprod. Med. 2011, 57, 233–243. [Google Scholar] [CrossRef] [Green Version]
- Omu, A.E.; Al-Azemi, M.K.; Kehinde, E.O.; Anim, J.T.; Oriowo, M.A.; Mathew, T.C. Indications of the Mechanisms Involved in Improved Sperm Parameters by Zinc Therapy. Med. Princ. Pract. Int. J. Kuwait Univ. Health Sci. Cent. 2008, 17, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Duncan, F.E.; Que, E.L.; O’Halloran, T.V.; Woodruff, T.K. The Fertilization-Induced Zinc Spark Is a Novel Biomarker of Mouse Embryo Quality and Early Development. Sci. Rep. 2016, 6, 22772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurley, L.S.; Swenerton, H. Congenital Malformations Resulting from Zinc Deficiency in Rats. Proc. Soc. Exp. Biol. Med. Soc. Exp. Biol. Med. N. Y. N 1966, 123, 692–696. [Google Scholar] [CrossRef]
- Wang, H.; Hu, Y.-F.; Hao, J.-H.; Chen, Y.-H.; Su, P.-Y.; Wang, Y.; Yu, Z.; Fu, L.; Xu, Y.-Y.; Zhang, C.; et al. Maternal Zinc Deficiency during Pregnancy Elevates the Risks of Fetal Growth Restriction: A Population-Based Birth Cohort Study. Sci. Rep. 2015, 5, 11262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.-G.; Choi, H.-N.; Yang, H.-R.; Yim, J.-E. Effects of Zinc Supplementation on Catch-up Growth in Children with Failure to Thrive. Nutr. Res. Pract. 2017, 11, 487–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solomons, N.W.; Rosenfield, R.L.; Jacob, R.A.; Sandstead, H.H. Growth Retardation and Zinc Nutrition. Pediatr. Res. 1976, 10, 923–927. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, M.; Ashrafzadeh, S.; Rassi, S.; Naseh, A. Maternal Zinc Deficiency and Congenital Anomalies in Newborns. Pediatr. Int. Off. J. Jpn. Pediatr. Soc. 2017, 59, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Beach, R.S.; Gershwin, M.E.; Hurley, L.S. Persistent Immunological Consequences of Gestation Zinc Deprivation. Am. J. Clin. Nutr. 1983, 38, 579–590. [Google Scholar] [CrossRef] [Green Version]
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a Gatekeeper of Immune Function. Nutrients 2017, 9, 1286. [Google Scholar] [CrossRef] [Green Version]
- Jameson, S. Effects of Zinc Deficiency in Human Reproduction. Acta Med. Scand. Suppl. 1976, 593, 1–89. [Google Scholar] [PubMed]
- Uriu-Adams, J.Y.; Keen, C.L. Zinc and Reproduction: Effects of Zinc Deficiency on Prenatal and Early Postnatal Development. Birth Defects Res. B. Dev. Reprod. Toxicol. 2010, 89, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Anthony, K.; Neuberger, T.; Diaz, F.J. Preconception Zinc Deficiency Disrupts Postimplantation Fetal and Placental Development in Mice. Biol. Reprod. 2014, 90, 83. [Google Scholar] [CrossRef] [PubMed]
- Corniola, R.S.; Tassabehji, N.M.; Hare, J.; Sharma, G.; Levenson, C.W. Zinc Deficiency Impairs Neuronal Precursor Cell Proliferation and Induces Apoptosis via P53-Mediated Mechanisms. Brain Res. 2008, 1237, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, J.; Niswander, L. Zinc Deficiency Causes Neural Tube Defects through Attenuation of P53 Ubiquitylation. Dev. Camb. Engl. 2018, 145, dev169797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanna, L.A.; Clegg, M.S.; Momma, T.Y.; Daston, G.P.; Rogers, J.M.; Keen, C.L. Zinc Influences the in Vitro Development of Peri-Implantation Mouse Embryos. Birt. Defects Res. A. Clin. Mol. Teratol. 2003, 67, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Mnatsakanyan, H.; Sabater i Serra, R.; Salmeron-Sanchez, M.; Rico, P. Zinc Maintains Embryonic Stem Cell Pluripotency and Multilineage Differentiation Potential via AKT Activation. Front. Cell Dev. Biol. 2019, 7, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, B.T.; Dasinger, J.H.; Intapad, S. Fetal Programming and Cardiovascular Pathology. Compr. Physiol. 2015, 5, 997–1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faa, G.; Manchia, M.; Pintus, R.; Gerosa, C.; Marcialis, M.A.; Fanos, V. Fetal Programming of Neuropsychiatric Disorders. Birth Defects Res. Part C Embryo Today Rev. 2016, 108, 207–223. [Google Scholar] [CrossRef]
- Nesan, D.; Sewell, L.C.; Kurrasch, D.M. Opening the Black Box of Endocrine Disruption of Brain Development: Lessons from the Characterization of Bisphenol A. Horm. Behav. 2018, 101, 50–58. [Google Scholar] [CrossRef] [PubMed]
- van de Bor, M. Fetal Toxicology. Handb. Clin. Neurol. 2019, 162, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Cusick, S.E.; Georgieff, M.K. The Role of Nutrition in Brain Development: The Golden Opportunity of the “First 1000 Days”. J. Pediatr. 2016, 175, 16–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Cruz, R.S.; de Oliveira Andrade, F.; de Oca Carioni, V.M.; Rosim, M.P.; Miranda, M.L.P.; Fontelles, C.C.; de Oliveira, P.V.; Barbisan, L.F.; Castro, I.A.; Ong, T.P. Dietary Zinc Deficiency or Supplementation during Gestation Increases Breast Cancer Susceptibility in Adult Female Mice Offspring Following a J-Shaped Pattern and through Distinct Mechanisms. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2019, 134, 110813. [Google Scholar] [CrossRef] [PubMed]
- Juriol, L.V.; Gobetto, M.N.; Mendes Garrido Abregú, F.; Dasso, M.E.; Pineda, G.; Güttlein, L.; Carranza, A.; Podhajcer, O.; Toblli, J.E.; Elesgaray, R.; et al. Cardiac Changes in Apoptosis, Inflammation, Oxidative Stress, and Nitric Oxide System Induced by Prenatal and Postnatal Zinc Deficiency in Male and Female Rats. Eur. J. Nutr. 2018, 57, 569–583. [Google Scholar] [CrossRef] [PubMed]
- Lucassen, P.J.; Naninck, E.F.G.; van Goudoever, J.B.; Fitzsimons, C.; Joels, M.; Korosi, A. Perinatal Programming of Adult Hippocampal Structure and Function; Emerging Roles of Stress, Nutrition and Epigenetics. Trends Neurosci. 2013, 36, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Röhr, D.; Halfter, H.; Schulz, J.B.; Young, P.; Gess, B. Sodium-Dependent Vitamin C Transporter 2 Deficiency Impairs Myelination and Remyelination after Injury: Roles of Collagen and Demethylation. Glia 2017, 65, 1186–1200. [Google Scholar] [CrossRef] [PubMed]
- Willekens, J.; Hergalant, S.; Pourié, G.; Marin, F.; Alberto, J.-M.; Georges, L.; Paoli, J.; Nemos, C.; Daval, J.-L.; Guéant, J.-L.; et al. Wnt Signaling Pathways Are Dysregulated in Rat Female Cerebellum Following Early Methyl Donor Deficiency. Mol. Neurobiol. 2019, 56, 892–906. [Google Scholar] [CrossRef]
- Birnbaum, R.; Weinberger, D.R. Genetic Insights into the Neurodevelopmental Origins of Schizophrenia. Nat. Rev. Neurosci. 2017, 18, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.S.; Derkits, E.J. Prenatal Infection and Schizophrenia: A Review of Epidemiologic and Translational Studies. Am. J. Psychiatry 2010, 167, 261–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- St Clair, D.; Xu, M.; Wang, P.; Yu, Y.; Fang, Y.; Zhang, F.; Zheng, X.; Gu, N.; Feng, G.; Sham, P.; et al. Rates of Adult Schizophrenia Following Prenatal Exposure to the Chinese Famine of 1959–1961. JAMA 2005, 294, 557–562. [Google Scholar] [CrossRef] [Green Version]
- Susser, E.S.; Lin, S.P. Schizophrenia after Prenatal Exposure to the Dutch Hunger Winter of 1944–1945. Arch. Gen. Psychiatry 1992, 49, 983–988. [Google Scholar] [CrossRef]
- Niemelä, S.; Sourander, A.; Surcel, H.-M.; Hinkka-Yli-Salomäki, S.; McKeague, I.W.; Cheslack-Postava, K.; Brown, A.S. Prenatal Nicotine Exposure and Risk of Schizophrenia Among Offspring in a National Birth Cohort. Am. J. Psychiatry 2016, 173, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Abib, R.T.; Gaman, A.; Dargél, A.A.; Tamouza, R.; Kapczinski, F.; Gottfried, C.; Leboyer, M. Intracellular Pathogen Infections and Immune Response in Autism. Neuroimmunomodulation 2018, 25, 271–279. [Google Scholar] [CrossRef]
- Bölte, S.; Girdler, S.; Marschik, P.B. The Contribution of Environmental Exposure to the Etiology of Autism Spectrum Disorder. Cell. Mol. Life Sci. CMLS 2019, 76, 1275–1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.-Y.; Xu, L.-L.; Shao, L.; Xia, R.-M.; Yu, Z.-H.; Ling, Z.-X.; Yang, F.; Deng, M.; Ruan, B. Maternal Infection during Pregnancy and Risk of Autism Spectrum Disorders: A Systematic Review and Meta-Analysis. Brain. Behav. Immun. 2016, 58, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Depino, A.M. Early Prenatal Exposure to LPS Results in Anxiety- and Depression-Related Behaviors in Adulthood. Neuroscience 2015, 299, 56–65. [Google Scholar] [CrossRef]
- Khan, D.; Fernando, P.; Cicvaric, A.; Berger, A.; Pollak, A.; Monje, F.J.; Pollak, D.D. Long-Term Effects of Maternal Immune Activation on Depression-like Behavior in the Mouse. Transl. Psychiatry 2014, 4, e363. [Google Scholar] [CrossRef] [Green Version]
- Majidi-Zolbanin, J.; Doosti, M.-H.; Kosari-Nasab, M.; Salari, A.-A. Prenatal Maternal Immune Activation Increases Anxiety- and Depressive-like Behaviors in Offspring with Experimental Autoimmune Encephalomyelitis. Neuroscience 2015, 294, 69–81. [Google Scholar] [CrossRef]
- Ronovsky, M.; Berger, S.; Zambon, A.; Reisinger, S.N.; Horvath, O.; Pollak, A.; Lindtner, C.; Berger, A.; Pollak, D.D. Maternal Immune Activation Transgenerationally Modulates Maternal Care and Offspring Depression-like Behavior. Brain. Behav. Immun. 2017, 63, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Sauer, A.K.; Grabrucker, A.M. Zinc Deficiency During Pregnancy Leads to Altered Microbiome and Elevated Inflammatory Markers in Mice. Front. Neurosci. 2019, 13, 1295. [Google Scholar] [CrossRef]
- Curtis, L.T.; Patel, K. Nutritional and Environmental Approaches to Preventing and Treating Autism and Attention Deficit Hyperactivity Disorder (ADHD): A Review. J. Altern. Complement. Med. N. Y. N 2008, 14, 79–85. [Google Scholar] [CrossRef]
- Yasuda, H.; Yoshida, K.; Yasuda, Y.; Tsutsui, T. Infantile Zinc Deficiency: Association with Autism Spectrum Disorders. Sci. Rep. 2011, 1, 129. [Google Scholar] [CrossRef] [PubMed]
- Dvergsten, C.L.; Johnson, L.A.; Sandstead, H.H. Alterations in the Postnatal Development of the Cerebellar Cortex Due to Zinc Deficiency. III. Impaired Dendritic Differentiation of Basket and Stellate Cells. Brain Res. 1984, 318, 21–26. [Google Scholar] [CrossRef]
- Supasai, S.; Adamo, A.M.; Mathieu, P.; Marino, R.C.; Hellmers, A.C.; Cremonini, E.; Oteiza, P.I. Gestational Zinc Deficiency Impairs Brain Astrogliogenesis in Rats through Multistep Alterations of the JAK/STAT3 Signaling Pathway. Redox Biol. 2021, 44, 102017. [Google Scholar] [CrossRef] [PubMed]
- Sandstead, H.H.; Strobel, D.A.; Logan, G.M.; Marks, E.O.; Jacob, R.A. Zinc Deficiency in Pregnant Rhesus Monkeys: Effects on Behavior of Infants. Am. J. Clin. Nutr. 1978, 31, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Halas, E.S.; Hunt, C.D.; Eberhardt, M.J. Learning and Memory Disabilities in Young Adult Rats from Mildly Zinc Deficient Dams. Physiol. Behav. 1986, 37, 451–458. [Google Scholar] [CrossRef]
- Tahmasebi Boroujeni, S.; Naghdi, N.; Shahbazi, M.; Farrokhi, A.; Bagherzadeh, F.; Kazemnejad, A.; Javadian, M. The Effect of Severe Zinc Deficiency and Zinc Supplement on Spatial Learning and Memory. Biol. Trace Elem. Res. 2009, 130, 48–61. [Google Scholar] [CrossRef]
- Yu, X.; Jin, L.; Zhang, X.; Yu, X. Effects of Maternal Mild Zinc Deficiency and Zinc Supplementation in Offspring on Spatial Memory and Hippocampal Neuronal Ultrastructural Changes. Nutr. Burbank Los Angel. Cty. Calif 2013, 29, 457–461. [Google Scholar] [CrossRef]
- Golub, M.S.; Gershwin, M.E.; Hurley, L.S.; Hendrickx, A.G.; Saito, W.Y. Studies of Marginal Zinc Deprivation in Rhesus Monkeys: Infant Behavior. Am. J. Clin. Nutr. 1985, 42, 1229–1239. [Google Scholar] [CrossRef] [Green Version]
- Golub, M.S.; Takeuchi, P.T.; Keen, C.L.; Gershwin, M.E.; Hendrickx, A.G.; Lonnerdal, B. Modulation of Behavioral Performance of Prepubertal Monkeys by Moderate Dietary Zinc Deprivation. Am. J. Clin. Nutr. 1994, 60, 238–243. [Google Scholar] [CrossRef]
- Golub, M.S.; Keen, C.L.; Gershwin, M.E.; Hendrickx, A.G. Developmental Zinc Deficiency and Behavior. J. Nutr. 1995, 125, 2263S–2271S. [Google Scholar] [CrossRef]
- Golub, M.S.; Takeuchi, P.T.; Keen, C.L.; Hendrickx, A.G.; Gershwin, M.E. Activity and Attention in Zinc-Deprived Adolescent Monkeys. Am. J. Clin. Nutr. 1996, 64, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Chowanadisai, W.; Kelleher, S.L.; Lönnerdal, B. Maternal Zinc Deficiency Reduces NMDA Receptor Expression in Neonatal Rat Brain, Which Persists into Early Adulthood. J. Neurochem. 2005, 94, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Gao, H.-L.; Zheng, W.; Xin, N.; Chi, Z.-H.; Bai, S.-L.; Wang, Z.-Y. Lactational Zinc Deficiency-Induced Hippocampal Neuronal Apoptosis by a BDNF-Independent TrkB Signaling Pathway. Hippocampus 2011, 21, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, J.R.; Supasai, S.; Kha, J.; Vaeth, B.M.; Mackenzie, G.G.; Adamo, A.M.; Oteiza, P.I. Gestational Marginal Zinc Deficiency Impaired Fetal Neural Progenitor Cell Proliferation by Disrupting the ERK1/2 Signaling Pathway. J. Nutr. Biochem. 2015, 26, 1116–1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamo, A.M.; Liu, X.; Mathieu, P.; Nuttall, J.R.; Supasai, S.; Oteiza, P.I. Early Developmental Marginal Zinc Deficiency Affects Neurogenesis Decreasing Neuronal Number and Altering Neuronal Specification in the Adult Rat Brain. Front. Cell. Neurosci. 2019, 13, 62. [Google Scholar] [CrossRef] [Green Version]
- Mackenzie, G.G.; Zago, M.P.; Keen, C.L.; Oteiza, P.I. Low Intracellular Zinc Impairs the Translocation of Activated NF-Kappa B to the Nuclei in Human Neuroblastoma IMR-32 Cells. J. Biol. Chem. 2002, 277, 34610–34617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aimo, L.; Mackenzie, G.G.; Keenan, A.H.; Oteiza, P.I. Gestational Zinc Deficiency Affects the Regulation of Transcription Factors AP-1, NF-ΚB and NFAT in Fetal Brain. J. Nutr. Biochem. 2010, 21, 1069–1075. [Google Scholar] [CrossRef] [Green Version]
- Bromberg, J.; Darnell, J.E. The Role of STATs in Transcriptional Control and Their Impact on Cellular Function. Oncogene 2000, 19, 2468–2473. [Google Scholar] [CrossRef] [Green Version]
- Cattaneo, E.; Conti, L.; De-Fraja, C. Signalling through the JAK-STAT Pathway in the Developing Brain. Trends Neurosci. 1999, 22, 365–369. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, M.-S. STAT1 as a Key Modulator of Cell Death. Cell. Signal. 2007, 19, 454–465. [Google Scholar] [CrossRef]
- Mui, A.L. The Role of STATs in Proliferation, Differentiation, and Apoptosis. Cell. Mol. Life Sci. CMLS 1999, 55, 1547–1558. [Google Scholar] [CrossRef] [PubMed]
- Supasai, S.; Aimo, L.; Adamo, A.M.; Mackenzie, G.G.; Oteiza, P.I. Zinc Deficiency Affects the STAT1/3 Signaling Pathways in Part through Redox-Mediated Mechanisms. Redox Biol. 2017, 11, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.D.; Bian, W.; Kong, L.W.; Zhao, F.J.; Guo, J.S.; Jing, N.H. Maternal Zinc Deficiency Impairs Brain Nestin Expression in Prenatal and Postnatal Mice. Cell Res. 2001, 11, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Oteiza, P.I.; Cuellar, S.; Lönnerdal, B.; Hurley, L.S.; Keen, C.L. Influence of Maternal Dietary Zinc Intake on in Vitro Tubulin Polymerization in Fetal Rat Brain. Teratology 1990, 41, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Oteiza, P.I.; Gershwin, M.E.; Golub, M.S.; Keen, C.L. Effects of Maternal Marginal Zinc Deficiency on Myelin Protein Profiles in the Suckling Rat and Infant Rhesus Monkey. Biol. Trace Elem. Res. 1992, 34, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Grabrucker, S.; Jannetti, L.; Eckert, M.; Gaub, S.; Chhabra, R.; Pfaender, S.; Mangus, K.; Reddy, P.P.; Rankovic, V.; Schmeisser, M.J.; et al. Zinc Deficiency Dysregulates the Synaptic ProSAP/Shank Scaffold and Might Contribute to Autism Spectrum Disorders. Brain J. Neurol. 2014, 137, 137–152. [Google Scholar] [CrossRef] [Green Version]
- Pfaender, S.; Sauer, A.K.; Hagmeyer, S.; Mangus, K.; Linta, L.; Liebau, S.; Bockmann, J.; Huguet, G.; Bourgeron, T.; Boeckers, T.M.; et al. Zinc Deficiency and Low Enterocyte Zinc Transporter Expression in Human Patients with Autism Related Mutations in SHANK3. Sci. Rep. 2017, 7, 45190. [Google Scholar] [CrossRef] [Green Version]
- Schoen, M.; Asoglu, H.; Bauer, H.F.; Müller, H.-P.; Abaei, A.; Sauer, A.K.; Zhang, R.; Song, T.-J.; Bockmann, J.; Kassubek, J.; et al. Shank3 Transgenic and Prenatal Zinc-Deficient Autism Mouse Models Show Convergent and Individual Alterations of Brain Structures in MRI. Front. Neural Circuits 2019, 13, 6. [Google Scholar] [CrossRef]
- Liuzzi, J.P.; Cousins, R.J. Mammalian Zinc Transporters. Annu. Rev. Nutr. 2004, 24, 151–172. [Google Scholar] [CrossRef]
- Guerinot, M.L. The ZIP Family of Metal Transporters. Biochim. Biophys. Acta 2000, 1465, 190–198. [Google Scholar] [CrossRef] [Green Version]
- Palmiter, R.D.; Findley, S.D. Cloning and Functional Characterization of a Mammalian Zinc Transporter That Confers Resistance to Zinc. EMBO J. 1995, 14, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-Based Map of the Human Proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Andrews, G.K.; Wang, H.; Dey, S.K.; Palmiter, R.D. Mouse Zinc Transporter 1 Gene Provides an Essential Function during Early Embryonic Development. Genes. N. Y. N 2000 2004, 40, 74–81. [Google Scholar] [CrossRef]
- Schröder, B.; Wrocklage, C.; Pan, C.; Jäger, R.; Kösters, B.; Schäfer, H.; Elsässer, H.-P.; Mann, M.; Hasilik, A. Integral and Associated Lysosomal Membrane Proteins. Traffic Cph. Den. 2007, 8, 1676–1686. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Lichten, L.A.; Ryu, M.-S.; Liuzzi, J.P.; Wang, F.; Cousins, R.J. STAT5-Glucocorticoid Receptor Interaction and MTF-1 Regulate the Expression of ZnT2 (Slc30a2) in Pancreatic Acinar Cells. Proc. Natl. Acad. Sci. USA 2010, 107, 2818–2823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelleher, S.L.; McCormick, N.H.; Velasquez, V.; Lopez, V. Zinc in Specialized Secretory Tissues: Roles in the Pancreas, Prostate, and Mammary Gland. Adv. Nutr. Bethesda Md 2011, 2, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Kirschke, C.P.; Huang, L. Expression of the ZNT (SLC30) Family Members in the Epithelium of the Mouse Prostate during Sexual Maturation. J. Mol. Histol. 2008, 39, 359–370. [Google Scholar] [CrossRef]
- Ranaldi, G.; Perozzi, G.; Truong-Tran, A.; Zalewski, P.; Murgia, C. Intracellular Distribution of Labile Zn(II) and Zinc Transporter Expression in Kidney and MDCK Cells. Am. J. Physiol. Ren. Physiol. 2002, 283, F1365–F1375. [Google Scholar] [CrossRef] [Green Version]
- Liuzzi, J.P.; Bobo, J.A.; Cui, L.; McMahon, R.J.; Cousins, R.J. Zinc Transporters 1, 2 and 4 Are Differentially Expressed and Localized in Rats during Pregnancy and Lactation. J. Nutr. 2003, 133, 342–351. [Google Scholar] [CrossRef] [Green Version]
- Lavoie, N.; Jeyaraju, D.V.; Peralta, M.R.; Seress, L.; Pellegrini, L.; Tóth, K. Vesicular Zinc Regulates the Ca2+ Sensitivity of a Subpopulation of Presynaptic Vesicles at Hippocampal Mossy Fiber Terminals. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 18251–18265. [Google Scholar] [CrossRef]
- Chowanadisai, W.; Lönnerdal, B.; Kelleher, S.L. Identification of a Mutation in SLC30A2 (ZnT-2) in Women with Low Milk Zinc Concentration That Results in Transient Neonatal Zinc Deficiency. J. Biol. Chem. 2006, 281, 39699–39707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, T.B.; Wenzel, H.J.; Kafer, K.E.; Schwartzkroin, P.A.; Palmiter, R.D. Elimination of Zinc from Synaptic Vesicles in the Intact Mouse Brain by Disruption of the ZnT3 Gene. Proc. Natl. Acad. Sci. USA 1999, 96, 1716–1721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAllister, B.B.; Dyck, R.H. Zinc Transporter 3 (ZnT3) and Vesicular Zinc in Central Nervous System Function. Neurosci. Biobehav. Rev. 2017, 80, 329–350. [Google Scholar] [CrossRef]
- Palmiter, R.D.; Cole, T.B.; Quaife, C.J.; Findley, S.D. ZnT-3, a Putative Transporter of Zinc into Synaptic Vesicles. Proc. Natl. Acad. Sci. USA 1996, 93, 14934–14939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sensi, S.L.; Paoletti, P.; Bush, A.I.; Sekler, I. Zinc in the Physiology and Pathology of the CNS. Nat. Rev. Neurosci. 2009, 10, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Shen, H. Re.: “Zinc Distribution and Expression Pattern of ZnT3 in Mouse Brain” by Shen et al., Biol Trace Elem Res 119:166–174 (2007). Biol. Trace Elem. Res. 2008, 121, 288. [Google Scholar] [CrossRef] [PubMed]
- Martel, G.; Hevi, C.; Friebely, O.; Baybutt, T.; Shumyatsky, G.P. Zinc Transporter 3 Is Involved in Learned Fear and Extinction, but Not in Innate Fear. Learn. Mem. Cold Spring Harb. N 2010, 17, 582–590. [Google Scholar] [CrossRef] [Green Version]
- Martel, G.; Hevi, C.; Kane-Goldsmith, N.; Shumyatsky, G.P. Zinc Transporter ZnT3 Is Involved in Memory Dependent on the Hippocampus and Perirhinal Cortex. Behav. Brain Res. 2011, 223, 233–238. [Google Scholar] [CrossRef] [Green Version]
- Sindreu, C.; Palmiter, R.D.; Storm, D.R. Zinc Transporter ZnT-3 Regulates Presynaptic Erk1/2 Signaling and Hippocampus-Dependent Memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3366–3370. [Google Scholar] [CrossRef] [Green Version]
- Yoo, M.H.; Kim, T.-Y.; Yoon, Y.H.; Koh, J.-Y. Autism Phenotypes in ZnT3 Null Mice: Involvement of Zinc Dyshomeostasis, MMP-9 Activation and BDNF Upregulation. Sci. Rep. 2016, 6, 28548. [Google Scholar] [CrossRef] [Green Version]
- McCormick, N.H.; Kelleher, S.L. ZnT4 Provides Zinc to Zinc-Dependent Proteins in the Trans-Golgi Network Critical for Cell Function and Zn Export in Mammary Epithelial Cells. Am. J. Physiol. Cell Physiol. 2012, 303, C291–C297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ackland, M.L.; Mercer, J.F. The Murine Mutation, Lethal Milk, Results in Production of Zinc-Deficient Milk. J. Nutr. 1992, 122, 1214–1218. [Google Scholar] [CrossRef] [PubMed]
- Piletz, J.E.; Ganschow, R.E. Zinc Deficiency in Murine Milk Underlies Expression of the Lethal Milk (Lm) Mutation. Science 1978, 199, 181–183. [Google Scholar] [CrossRef] [PubMed]
- Liuzzi, J.P.; Blanchard, R.K.; Cousins, R.J. Differential Regulation of Zinc Transporter 1, 2, and 4 MRNA Expression by Dietary Zinc in Rats. J. Nutr. 2001, 131, 46–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Gitschier, J. A Novel Gene Involved in Zinc Transport Is Deficient in the Lethal Milk Mouse. Nat. Genet. 1997, 17, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Kambe, T.; Narita, H.; Yamaguchi-Iwai, Y.; Hirose, J.; Amano, T.; Sugiura, N.; Sasaki, R.; Mori, K.; Iwanaga, T.; Nagao, M. Cloning and Characterization of a Novel Mammalian Zinc Transporter, Zinc Transporter 5, Abundantly Expressed in Pancreatic Beta Cells. J. Biol. Chem. 2002, 277, 19049–19055. [Google Scholar] [CrossRef] [Green Version]
- Jackson, K.A.; Helston, R.M.; McKay, J.A.; O’Neill, E.D.; Mathers, J.C.; Ford, D. Splice Variants of the Human Zinc Transporter ZnT5 (SLC30A5) Are Differentially Localized and Regulated by Zinc through Transcription and MRNA Stability. J. Biol. Chem. 2007, 282, 10423–10431. [Google Scholar] [CrossRef] [Green Version]
- Thornton, J.K.; Taylor, K.M.; Ford, D.; Valentine, R.A. Differential Subcellular Localization of the Splice Variants of the Zinc Transporter ZnT5 Is Dictated by the Different C-Terminal Regions. PLoS ONE 2011, 6, e23878. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T.; Ishihara, K.; Migaki, H.; Matsuura, W.; Kohda, A.; Okumura, K.; Nagao, M.; Yamaguchi-Iwai, Y.; Kambe, T. Zinc Transporters, ZnT5 and ZnT7, Are Required for the Activation of Alkaline Phosphatases, Zinc-Requiring Enzymes That Are Glycosylphosphatidylinositol-Anchored to the Cytoplasmic Membrane. J. Biol. Chem. 2005, 280, 637–643. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.Y.; Kirschke, C.P.; Huang, L. Immunohistochemical Analysis of ZnT1, 4, 5, 6, and 7 in the Mouse Gastrointestinal Tract. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2007, 55, 223–234. [Google Scholar] [CrossRef] [Green Version]
- Lieberwirth, J.K.; Joset, P.; Heinze, A.; Hentschel, J.; Stein, A.; Iannaccone, A.; Steindl, K.; Kuechler, A.; Jamra, R.A. Correction: Bi-Allelic Loss of Function Variants in SLC30A5 as Cause of Perinatal Lethal Cardiomyopathy. Eur. J. Hum. Genet. EJHG 2021, 29, 887. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Matsuda, K.; Itoh, M.; Kawaguchi, H.; Tomoike, H.; Aoyagi, T.; Nagai, R.; Hori, M.; Nakamura, Y.; Tanaka, T. Osteopenia and Male-Specific Sudden Cardiac Death in Mice Lacking a Zinc Transporter Gene, Znt5. Hum. Mol. Genet. 2002, 11, 1775–1784. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Kirschke, C.P.; Gitschier, J. Functional Characterization of a Novel Mammalian Zinc Transporter, ZnT6. J. Biol. Chem. 2002, 277, 26389–26395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirschke, C.P.; Huang, L. ZnT7, a Novel Mammalian Zinc Transporter, Accumulates Zinc in the Golgi Apparatus. J. Biol. Chem. 2003, 278, 4096–4102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Yu, Y.Y.; Kirschke, C.P.; Gertz, E.R.; Lloyd, K.K.C. Znt7 (Slc30a7)-Deficient Mice Display Reduced Body Zinc Status and Body Fat Accumulation. J. Biol. Chem. 2007, 282, 37053–37063. [Google Scholar] [CrossRef] [Green Version]
- Chimienti, F.; Devergnas, S.; Favier, A.; Seve, M. Identification and Cloning of a Beta-Cell-Specific Zinc Transporter, ZnT-8, Localized into Insulin Secretory Granules. Diabetes 2004, 53, 2330–2337. [Google Scholar] [CrossRef] [Green Version]
- Murgia, C.; Devirgiliis, C.; Mancini, E.; Donadel, G.; Zalewski, P.; Perozzi, G. Diabetes-Linked Zinc Transporter ZnT8 Is a Homodimeric Protein Expressed by Distinct Rodent Endocrine Cell Types in the Pancreas and Other Glands. Nutr. Metab. Cardiovasc. Dis. NMCD 2009, 19, 431–439. [Google Scholar] [CrossRef]
- Lemaire, K.; Ravier, M.A.; Schraenen, A.; Creemers, J.W.M.; Van de Plas, R.; Granvik, M.; Van Lommel, L.; Waelkens, E.; Chimienti, F.; Rutter, G.A.; et al. Insulin Crystallization Depends on Zinc Transporter ZnT8 Expression, but Is Not Required for Normal Glucose Homeostasis in Mice. Proc. Natl. Acad. Sci. USA 2009, 106, 14872–14877. [Google Scholar] [CrossRef] [Green Version]
- Pound, L.D.; Sarkar, S.A.; Benninger, R.K.P.; Wang, Y.; Suwanichkul, A.; Shadoan, M.K.; Printz, R.L.; Oeser, J.K.; Lee, C.E.; Piston, D.W.; et al. Deletion of the Mouse Slc30a8 Gene Encoding Zinc Transporter-8 Results in Impaired Insulin Secretion. Biochem. J. 2009, 421, 371–376. [Google Scholar] [CrossRef] [Green Version]
- Wijesekara, N.; Dai, F.F.; Hardy, A.B.; Giglou, P.R.; Bhattacharjee, A.; Koshkin, V.; Chimienti, F.; Gaisano, H.Y.; Rutter, G.A.; Wheeler, M.B. Beta Cell-Specific Znt8 Deletion in Mice Causes Marked Defects in Insulin Processing, Crystallisation and Secretion. Diabetologia 2010, 53, 1656–1668. [Google Scholar] [CrossRef] [Green Version]
- Mao, Z.; Lin, H.; Su, W.; Li, J.; Zhou, M.; Li, Z.; Zhou, B.; Yang, Q.; Zhou, M.; Pan, K.; et al. Deficiency of ZnT8 Promotes Adiposity and Metabolic Dysfunction by Increasing Peripheral Serotonin Production. Diabetes 2019, 68, 1197–1209. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Kim, J.H.; Stallcup, M.R. GAC63, a GRIP1-Dependent Nuclear Receptor Coactivator. Mol. Cell. Biol. 2005, 25, 5965–5972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sim, D.L.; Chow, V.T. The Novel Human HUEL (C4orf1) Gene Maps to Chromosome 4p12-P13 and Encodes a Nuclear Protein Containing the Nuclear Receptor Interaction Motif. Genomics 1999, 59, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Sim, D.L.C.; Yeo, W.M.; Chow, V.T.K. The Novel Human HUEL (C4orf1) Protein Shares Homology with the DNA-Binding Domain of the XPA DNA Repair Protein and Displays Nuclear Translocation in a Cell Cycle-Dependent Manner. Int. J. Biochem. Cell Biol. 2002, 34, 487–504. [Google Scholar] [CrossRef]
- Perez, Y.; Shorer, Z.; Liani-Leibson, K.; Chabosseau, P.; Kadir, R.; Volodarsky, M.; Halperin, D.; Barber-Zucker, S.; Shalev, H.; Schreiber, R.; et al. SLC30A9 Mutation Affecting Intracellular Zinc Homeostasis Causes a Novel Cerebro-Renal Syndrome. Brain J. Neurol. 2017, 140, 928–939. [Google Scholar] [CrossRef] [Green Version]
- Bosomworth, H.J.; Thornton, J.K.; Coneyworth, L.J.; Ford, D.; Valentine, R.A. Efflux Function, Tissue-Specific Expression and Intracellular Trafficking of the Zn Transporter ZnT10 Indicate Roles in Adult Zn Homeostasis. Met. Integr. Biometal Sci. 2012, 4, 771–779. [Google Scholar] [CrossRef]
- Taylor, C.A.; Hutchens, S.; Liu, C.; Jursa, T.; Shawlot, W.; Aschner, M.; Smith, D.R.; Mukhopadhyay, S. SLC30A10 Transporter in the Digestive System Regulates Brain Manganese under Basal Conditions While Brain SLC30A10 Protects against Neurotoxicity. J. Biol. Chem. 2019, 294, 1860–1876. [Google Scholar] [CrossRef] [Green Version]
- Quadri, M.; Federico, A.; Zhao, T.; Breedveld, G.J.; Battisti, C.; Delnooz, C.; Severijnen, L.-A.; Di Toro Mammarella, L.; Mignarri, A.; Monti, L.; et al. Mutations in SLC30A10 Cause Parkinsonism and Dystonia with Hypermanganesemia, Polycythemia, and Chronic Liver Disease. Am. J. Hum. Genet. 2012, 90, 467–477. [Google Scholar] [CrossRef] [Green Version]
- Tuschl, K.; Clayton, P.T.; Gospe, S.M.; Gulab, S.; Ibrahim, S.; Singhi, P.; Aulakh, R.; Ribeiro, R.T.; Barsottini, O.G.; Zaki, M.S.; et al. Syndrome of Hepatic Cirrhosis, Dystonia, Polycythemia, and Hypermanganesemia Caused by Mutations in SLC30A10, a Manganese Transporter in Man. Am. J. Hum. Genet. 2016, 99, 521. [Google Scholar] [CrossRef] [Green Version]
- Gaither, L.A.; Eide, D.J. The Human ZIP1 Transporter Mediates Zinc Uptake in Human K562 Erythroleukemia Cells. J. Biol. Chem. 2001, 276, 22258–22264. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Kirschke, C.P. A Di-Leucine Sorting Signal in ZIP1 (SLC39A1) Mediates Endocytosis of the Protein. FEBS J. 2007, 274, 3986–3997. [Google Scholar] [CrossRef] [PubMed]
- Milon, B.; Dhermy, D.; Pountney, D.; Bourgeois, M.; Beaumont, C. Differential Subcellular Localization of HZip1 in Adherent and Non-Adherent Cells. FEBS Lett. 2001, 507, 241–246. [Google Scholar] [CrossRef] [Green Version]
- Belloni-Olivi, L.; Marshall, C.; Laal, B.; Andrews, G.K.; Bressler, J. Localization of Zip1 and Zip4 MRNA in the Adult Rat Brain. J. Neurosci. Res. 2009, 87, 3221–3230. [Google Scholar] [CrossRef] [Green Version]
- Dufner-Beattie, J.; Huang, Z.L.; Geiser, J.; Xu, W.; Andrews, G.K. Mouse ZIP1 and ZIP3 Genes Together Are Essential for Adaptation to Dietary Zinc Deficiency during Pregnancy. Genes. N. Y. N 2000 2006, 44, 239–251. [Google Scholar] [CrossRef]
- Kambe, T.; Geiser, J.; Lahner, B.; Salt, D.E.; Andrews, G.K. Slc39a1 to 3 (Subfamily II) Zip Genes in Mice Have Unique Cell-Specific Functions during Adaptation to Zinc Deficiency. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R1474–R1481. [Google Scholar] [CrossRef] [Green Version]
- Michalczyk, A.A.; Ackland, M.L. HZip1 (HSLC39A1) Regulates Zinc Homoeostasis in Gut Epithelial Cells. Genes Nutr. 2013, 8, 475–486. [Google Scholar] [CrossRef] [Green Version]
- Peters, J.L.; Dufner-Beattie, J.; Xu, W.; Geiser, J.; Lahner, B.; Salt, D.E.; Andrews, G.K. Targeting of the Mouse Slc39a2 (Zip2) Gene Reveals Highly Cell-Specific Patterns of Expression, and Unique Functions in Zinc, Iron, and Calcium Homeostasis. Genes. N. Y. N 2000 2007, 45, 339–352. [Google Scholar] [CrossRef]
- Wang, F.; Dufner-Beattie, J.; Kim, B.-E.; Petris, M.J.; Andrews, G.; Eide, D.J. Zinc-Stimulated Endocytosis Controls Activity of the Mouse ZIP1 and ZIP3 Zinc Uptake Transporters*. J. Biol. Chem. 2004, 279, 24631–24639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dufner-Beattie, J.; Huang, Z.L.; Geiser, J.; Xu, W.; Andrews, G.K. Generation and Characterization of Mice Lacking the Zinc Uptake Transporter ZIP3. Mol. Cell. Biol. 2005, 25, 5607–5615. [Google Scholar] [CrossRef] [Green Version]
- Dufner-Beattie, J.; Wang, F.; Kuo, Y.-M.; Gitschier, J.; Eide, D.; Andrews, G.K. The Acrodermatitis Enteropathica Gene ZIP4 Encodes a Tissue-Specific, Zinc-Regulated Zinc Transporter in Mice. J. Biol. Chem. 2003, 278, 33474–33481. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.-E.; Wang, F.; Dufner-Beattie, J.; Andrews, G.K.; Eide, D.J.; Petris, M.J. Zn2+-Stimulated Endocytosis of the MZIP4 Zinc Transporter Regulates Its Location at the Plasma Membrane. J. Biol. Chem. 2004, 279, 4523–4530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liuzzi, J.P.; Bobo, J.A.; Lichten, L.A.; Samuelson, D.A.; Cousins, R.J. Responsive Transporter Genes within the Murine Intestinal-Pancreatic Axis Form a Basis of Zinc Homeostasis. Proc. Natl. Acad. Sci. USA 2004, 101, 14355–14360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dufner-Beattie, J.; Langmade, S.J.; Wang, F.; Eide, D.; Andrews, G.K. Structure, Function, and Regulation of a Subfamily of Mouse Zinc Transporter Genes. J. Biol. Chem. 2003, 278, 50142–50150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dufner-Beattie, J.; Kuo, Y.-M.; Gitschier, J.; Andrews, G.K. The Adaptive Response to Dietary Zinc in Mice Involves the Differential Cellular Localization and Zinc Regulation of the Zinc Transporters ZIP4 and ZIP5. J. Biol. Chem. 2004, 279, 49082–49090. [Google Scholar] [CrossRef] [Green Version]
- De Benedictis, C.A.; Haffke, C.; Hagmeyer, S.; Sauer, A.K.; Grabrucker, A.M. Expression Analysis of Zinc Transporters in Nervous Tissue Cells Reveals Neuronal and Synaptic Localization of ZIP4. Int. J. Mol. Sci. 2021, 22, 4511. [Google Scholar] [CrossRef]
- Dufner-Beattie, J.; Weaver, B.P.; Geiser, J.; Bilgen, M.; Larson, M.; Xu, W.; Andrews, G.K. The Mouse Acrodermatitis Enteropathica Gene Slc39a4 (Zip4) Is Essential for Early Development and Heterozygosity Causes Hypersensitivity to Zinc Deficiency. Hum. Mol. Genet. 2007, 16, 1391–1399. [Google Scholar] [CrossRef] [Green Version]
- Geiser, J.; Venken, K.J.T.; De Lisle, R.C.; Andrews, G.K. A Mouse Model of Acrodermatitis Enteropathica: Loss of Intestine Zinc Transporter ZIP4 (Slc39a4) Disrupts the Stem Cell Niche and Intestine Integrity. PLoS Genet. 2012, 8, e1002766. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Kim, B.-E.; Petris, M.J.; Eide, D.J. The Mammalian Zip5 Protein Is a Zinc Transporter That Localizes to the Basolateral Surface of Polarized Cells. J. Biol. Chem. 2004, 279, 51433–51441. [Google Scholar] [CrossRef] [Green Version]
- Weaver, B.P.; Dufner-Beattie, J.; Kambe, T.; Andrews, G.K. Novel Zinc-Responsive Post-Transcriptional Mechanisms Reciprocally Regulate Expression of the Mouse Slc39a4 and Slc39a5 Zinc Transporters (Zip4 and Zip5). Biol. Chem. 2007, 388, 1301–1312. [Google Scholar] [CrossRef] [Green Version]
- Wex, T.; Grungreiff, K.; Schutte, K.; Stengritt, M.; Reinhold, D. Expression Analysis of Zinc Transporters in Resting and Stimulated Human Peripheral Blood Mononuclear Cells. Biomed. Rep. 2014, 2, 217–222. [Google Scholar] [CrossRef] [Green Version]
- Geiser, J.; De Lisle, R.C.; Andrews, G.K. The Zinc Transporter Zip5 (Slc39a5) Regulates Intestinal Zinc Excretion and Protects the Pancreas against Zinc Toxicity. PLoS ONE 2013, 8, e82149. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.M.; Morgan, H.E.; Johnson, A.; Hadley, L.J.; Nicholson, R.I. Structure-Function Analysis of LIV-1, the Breast Cancer-Associated Protein That Belongs to a New Subfamily of Zinc Transporters. Biochem. J. 2003, 375, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.M.; Morgan, H.E.; Johnson, A.; Nicholson, R.I. Structure-Function Analysis of HKE4, a Member of the New LIV-1 Subfamily of Zinc Transporters. Biochem. J. 2004, 377, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Anzilotti, C.; Swan, D.J.; Boisson, B.; Deobagkar-Lele, M.; Oliveira, C.; Chabosseau, P.; Engelhardt, K.R.; Xu, X.; Chen, R.; Alvarez, L.; et al. An Essential Role for the Zn2+ Transporter ZIP7 in B Cell Development. Nat. Immunol. 2019, 20, 350–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, G.; Zhang, Y.; Yu, J.; Yu, Y.; Zhang, F.; Zhang, Z.; Wu, A.; Yan, X.; Zhou, Y.; Wang, F. Slc39a7/Zip7 Plays a Critical Role in Development and Zinc Homeostasis in Zebrafish. PLoS ONE 2012, 7, e42939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Schneider, S.N.; Dragin, N.; Girijashanker, K.; Dalton, T.P.; He, L.; Miller, M.L.; Stringer, K.F.; Soleimani, M.; Richardson, D.D.; et al. Enhanced Cadmium-Induced Testicular Necrosis and Renal Proximal Tubule Damage Caused by Gene-Dose Increase in a Slc39a8-Transgenic Mouse Line. Am. J. Physiol. Cell Physiol. 2007, 292, C1523–C1535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Li, H.; Soleimani, M.; Girijashanker, K.; Reed, J.M.; He, L.; Dalton, T.P.; Nebert, D.W. Cd2+ versus Zn2+ Uptake by the ZIP8 HCO3--Dependent Symporter: Kinetics, Electrogenicity and Trafficking. Biochem. Biophys. Res. Commun. 2008, 365, 814–820. [Google Scholar] [CrossRef] [Green Version]
- Fujishiro, H.; Hamao, S.; Isawa, M.; Himeno, S. Segment-Specific and Direction-Dependent Transport of Cadmium and Manganese in Immortalized S1, S2, and S3 Cells Derived from Mouse Kidney Proximal Tubules. J. Toxicol. Sci. 2019, 44, 611–619. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Gálvez-Peralta, M.; Zhang, X.; Deng, J.; Liu, Z.; Nebert, D.W. In Utero Gene Expression in the Slc39a8(Neo/Neo) Knockdown Mouse. Sci. Rep. 2018, 8, 10703. [Google Scholar] [CrossRef] [Green Version]
- Gálvez-Peralta, M.; He, L.; Jorge-Nebert, L.F.; Wang, B.; Miller, M.L.; Eppert, B.L.; Afton, S.; Nebert, D.W. ZIP8 Zinc Transporter: Indispensable Role for Both Multiple-Organ Organogenesis and Hematopoiesis in Utero. PLoS ONE 2012, 7, e36055. [Google Scholar] [CrossRef] [Green Version]
- Dickinson, M.E.; Flenniken, A.M.; Ji, X.; Teboul, L.; Wong, M.D.; White, J.K.; Meehan, T.F.; Weninger, W.J.; Westerberg, H.; Adissu, H.; et al. High-Throughput Discovery of Novel Developmental Phenotypes. Nature 2016, 537, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Boycott, K.M.; Beaulieu, C.L.; Kernohan, K.D.; Gebril, O.H.; Mhanni, A.; Chudley, A.E.; Redl, D.; Qin, W.; Hampson, S.; Küry, S.; et al. Autosomal-Recessive Intellectual Disability with Cerebellar Atrophy Syndrome Caused by Mutation of the Manganese and Zinc Transporter Gene SLC39A8. Am. J. Hum. Genet. 2015, 97, 886–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.H.; Hogrebe, M.; Grüneberg, M.; DuChesne, I.; von der Heiden, A.L.; Reunert, J.; Schlingmann, K.P.; Boycott, K.M.; Beaulieu, C.L.; Mhanni, A.A.; et al. SLC39A8 Deficiency: A Disorder of Manganese Transport and Glycosylation. Am. J. Hum. Genet. 2015, 97, 894–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuura, W.; Yamazaki, T.; Yamaguchi-Iwai, Y.; Masuda, S.; Nagao, M.; Andrews, G.K.; Kambe, T. SLC39A9 (ZIP9) Regulates Zinc Homeostasis in the Secretory Pathway: Characterization of the ZIP Subfamily I Protein in Vertebrate Cells. Biosci. Biotechnol. Biochem. 2009, 73, 1142–1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Converse, A.; Thomas, P. The Zinc Transporter ZIP9 (Slc39a9) Regulates Zinc Dynamics Essential to Egg Activation in Zebrafish. Sci. Rep. 2020, 10, 15673. [Google Scholar] [CrossRef]
- Lichten, L.A.; Ryu, M.-S.; Guo, L.; Embury, J.; Cousins, R.J. MTF-1-Mediated Repression of the Zinc Transporter Zip10 Is Alleviated by Zinc Restriction. PLoS ONE 2011, 6, e21526. [Google Scholar] [CrossRef]
- Ryu, M.-S.; Lichten, L.A.; Liuzzi, J.P.; Cousins, R.J. Zinc Transporters ZnT1 (Slc30a1), Zip8 (Slc39a8), and Zip10 (Slc39a10) in Mouse Red Blood Cells Are Differentially Regulated during Erythroid Development and by Dietary Zinc Deficiency. J. Nutr. 2008, 138, 2076–2083. [Google Scholar] [CrossRef] [Green Version]
- Landry, G.M.; Furrow, E.; Holmes, H.L.; Hirata, T.; Kato, A.; Williams, P.; Strohmaier, K.; Gallo, C.J.R.; Chang, M.; Pandey, M.K.; et al. Cloning, Function, and Localization of Human, Canine, and Drosophila ZIP10 (SLC39A10), a Zn2+ Transporter. Am. J. Physiol. Ren. Physiol. 2019, 316, F263–F273. [Google Scholar] [CrossRef]
- Kelleher, S.L.; Velasquez, V.; Croxford, T.P.; McCormick, N.H.; Lopez, V.; MacDavid, J. Mapping the Zinc-Transporting System in Mammary Cells: Molecular Analysis Reveals a Phenotype-Dependent Zinc-Transporting Network during Lactation. J. Cell. Physiol. 2012, 227, 1761–1770. [Google Scholar] [CrossRef] [Green Version]
- Martin, A.B.; Aydemir, T.B.; Guthrie, G.J.; Samuelson, D.A.; Chang, S.-M.; Cousins, R.J. Gastric and Colonic Zinc Transporter ZIP11 (Slc39a11) in Mice Responds to Dietary Zinc and Exhibits Nuclear Localization. J. Nutr. 2013, 143, 1882–1888. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Wu, A.; Zhang, Z.; Yan, G.; Zhang, F.; Zhang, L.; Shen, X.; Hu, R.; Zhang, Y.; Zhang, K.; et al. Characterization of the GufA Subfamily Member SLC39A11/Zip11 as a Zinc Transporter. J. Nutr. Biochem. 2013, 24, 1697–1708. [Google Scholar] [CrossRef] [PubMed]
- Chowanadisai, W.; Graham, D.M.; Keen, C.L.; Rucker, R.B.; Messerli, M.A. Neurulation and Neurite Extension Require the Zinc Transporter ZIP12 (Slc39a12). Proc. Natl. Acad. Sci. USA 2013, 110, 9903–9908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bin, B.-H.; Fukada, T.; Hosaka, T.; Yamasaki, S.; Ohashi, W.; Hojyo, S.; Miyai, T.; Nishida, K.; Yokoyama, S.; Hirano, T. Biochemical Characterization of Human ZIP13 Protein: A Homo-Dimerized Zinc Transporter Involved in the Spondylocheiro Dysplastic Ehlers-Danlos Syndrome. J. Biol. Chem. 2011, 286, 40255–40265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukada, T.; Civic, N.; Furuichi, T.; Shimoda, S.; Mishima, K.; Higashiyama, H.; Idaira, Y.; Asada, Y.; Kitamura, H.; Yamasaki, S.; et al. The Zinc Transporter SLC39A13/ZIP13 Is Required for Connective Tissue Development; Its Involvement in BMP/TGF-Beta Signaling Pathways. PLoS ONE 2008, 3, e3642. [Google Scholar] [CrossRef]
- Giunta, C.; Elçioglu, N.H.; Albrecht, B.; Eich, G.; Chambaz, C.; Janecke, A.R.; Yeowell, H.; Weis, M.; Eyre, D.R.; Kraenzlin, M.; et al. Spondylocheiro Dysplastic Form of the Ehlers-Danlos Syndrome--an Autosomal-Recessive Entity Caused by Mutations in the Zinc Transporter Gene SLC39A13. Am. J. Hum. Genet. 2008, 82, 1290–1305. [Google Scholar] [CrossRef] [Green Version]
- Xiao, G.; Zhou, B. ZIP13: A Study of Drosophila Offers an Alternative Explanation for the Corresponding Human Disease. Front. Genet. 2017, 8, 234. [Google Scholar] [CrossRef] [Green Version]
- Guthrie, G.J.; Aydemir, T.B.; Troche, C.; Martin, A.B.; Chang, S.-M.; Cousins, R.J. Influence of ZIP14 (Slc39A14) on Intestinal Zinc Processing and Barrier Function. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G171–G178. [Google Scholar] [CrossRef] [Green Version]
- Zhao, N.; Gao, J.; Enns, C.A.; Knutson, M.D. ZRT/IRT-like Protein 14 (ZIP14) Promotes the Cellular Assimilation of Iron from Transferrin. J. Biol. Chem. 2010, 285, 32141–32150. [Google Scholar] [CrossRef] [Green Version]
- Tominaga, K.; Kagata, T.; Johmura, Y.; Hishida, T.; Nishizuka, M.; Imagawa, M. SLC39A14, a LZT Protein, Is Induced in Adipogenesis and Transports Zinc. FEBS J. 2005, 272, 1590–1599. [Google Scholar] [CrossRef]
- Tuschl, K.; Meyer, E.; Valdivia, L.E.; Zhao, N.; Dadswell, C.; Abdul-Sada, A.; Hung, C.Y.; Simpson, M.A.; Chong, W.K.; Jacques, T.S.; et al. Mutations in SLC39A14 Disrupt Manganese Homeostasis and Cause Childhood-Onset Parkinsonism-Dystonia. Nat. Commun. 2016, 7, 11601. [Google Scholar] [CrossRef] [Green Version]
- Hojyo, S.; Fukada, T.; Shimoda, S.; Ohashi, W.; Bin, B.-H.; Koseki, H.; Hirano, T. The Zinc Transporter SLC39A14/ZIP14 Controls G-Protein Coupled Receptor-Mediated Signaling Required for Systemic Growth. PLoS ONE 2011, 6, e18059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkitkasemwong, S.; Akinyode, A.; Paulus, E.; Weiskirchen, R.; Hojyo, S.; Fukada, T.; Giraldo, G.; Schrier, J.; Garcia, A.; Janus, C.; et al. SLC39A14 Deficiency Alters Manganese Homeostasis and Excretion Resulting in Brain Manganese Accumulation and Motor Deficits in Mice. Proc. Natl. Acad. Sci. USA 2018, 115, E1769–E1778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekler, I.; Moran, A.; Hershfinkel, M.; Dori, A.; Margulis, A.; Birenzweig, N.; Nitzan, Y.; Silverman, W.F. Distribution of the Zinc Transporter ZnT-1 in Comparison with Chelatable Zinc in the Mouse Brain. J. Comp. Neurol. 2002, 447, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, Y.; Zhou, B. Dietary Zinc Absorption Is Mediated by ZnT1 in Drosophila Melanogaster. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2009, 23, 2650–2661. [Google Scholar] [CrossRef] [PubMed]
- Palmiter, R.D.; Cole, T.B.; Findley, S.D. ZnT-2, a Mammalian Protein That Confers Resistance to Zinc by Facilitating Vesicular Sequestration. EMBO J. 1996, 15, 1784–1791. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, C.; McCormick, N.H.; Croxford, T.P.; Seo, Y.A.; Grider, A.; Kelleher, S.L. Marginal Maternal Zinc Deficiency in Lactating Mice Reduces Secretory Capacity and Alters Milk Composition. J. Nutr. 2012, 142, 655–660. [Google Scholar] [CrossRef] [Green Version]
- Lasry, I.; Seo, Y.A.; Ityel, H.; Shalva, N.; Pode-Shakked, B.; Glaser, F.; Berman, B.; Berezovsky, I.; Goncearenco, A.; Klar, A.; et al. A Dominant Negative Heterozygous G87R Mutation in the Zinc Transporter, ZnT-2 (SLC30A2), Results in Transient Neonatal Zinc Deficiency. J. Biol. Chem. 2012, 287, 29348–29361. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Wang, J.; Yang, Y.; Wang, S. A Novel Homozygous Mutation p.E88K in Maternal SLC30A2 Gene as a Cause of Transient Neonatal Zinc Deficiency. Exp. Dermatol. 2020, 29, 556–561. [Google Scholar] [CrossRef]
- Alam, S.; Hennigar, S.R.; Gallagher, C.; Soybel, D.I.; Kelleher, S.L. Exome Sequencing of SLC30A2 Identifies Novel Loss- and Gain-of-Function Variants Associated with Breast Cell Dysfunction. J. Mammary Gland Biol. Neoplasia 2015, 20, 159–172. [Google Scholar] [CrossRef]
- Kelleher, S.L.; Gagnon, A.; Rivera, O.C.; Hicks, S.D.; Carney, M.C.; Alam, S. Milk-Derived MiRNA Profiles Elucidate Molecular Pathways That Underlie Breast Dysfunction in Women with Common Genetic Variants in SLC30A2. Sci. Rep. 2019, 9, 12686. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Zhou, Y.; Gill, D.L.; Kelleher, S.L. A Genetic Variant in SLC30A2 Causes Breast Dysfunction during Lactation by Inducing ER Stress, Oxidative Stress and Epithelial Barrier Defects. Sci. Rep. 2018, 8, 3542. [Google Scholar] [CrossRef] [PubMed]
- Rivera, O.C.; Hennigar, S.R.; Kelleher, S.L. ZnT2 Is Critical for Lysosome Acidification and Biogenesis during Mammary Gland Involution. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R323–R335. [Google Scholar] [CrossRef] [PubMed]
- Rivera, O.C.; Geddes, D.T.; Barber-Zucker, S.; Zarivach, R.; Gagnon, A.; Soybel, D.I.; Kelleher, S.L. A Common Genetic Variant in ZnT2 (Thr288Ser) Is Present in Women with Low Milk Volume and Alters Lysosome Function and Cell Energetics. Am. J. Physiol. Cell Physiol. 2020, 318, C1166–C1177. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.Y.; Hong, D.K.; Jeong, J.H.; Lee, B.E.; Koh, J.-Y.; Suh, S.W. Zinc Transporter 3 Modulates Cell Proliferation and Neuronal Differentiation in the Adult Hippocampus. Stem Cells Dayt. Ohio 2020, 38, 994–1006. [Google Scholar] [CrossRef]
- Thackray, S.E.; McAllister, B.B.; Dyck, R.H. Behavioral Characterization of Female Zinc Transporter 3 (ZnT3) Knockout Mice. Behav. Brain Res. 2017, 321, 36–49. [Google Scholar] [CrossRef]
- Perez-Becerril, C.; Morris, A.G.; Mortimer, A.; McKenna, P.J.; de Belleroche, J. Allelic Variants in the Zinc Transporter-3 Gene, SLC30A3, a Candidate Gene Identified from Gene Expression Studies, Show Gender-Specific Association with Schizophrenia. Eur. Psychiatry J. Assoc. Eur. Psychiatr. 2014, 29, 172–178. [Google Scholar] [CrossRef]
- Perez-Becerril, C.; Morris, A.G.; Mortimer, A.; McKenna, P.J.; de Belleroche, J. Common Variants in the Chromosome 2p23 Region Containing the SLC30A3 (ZnT3) Gene Are Associated with Schizophrenia in Female but Not Male Individuals in a Large Collection of European Samples. Psychiatry Res. 2016, 246, 335–340. [Google Scholar] [CrossRef]
- Saito, T.; Takahashi, K.; Nakagawa, N.; Hosokawa, T.; Kurasaki, M.; Yamanoshita, O.; Yamamoto, Y.; Sasaki, H.; Nagashima, K.; Fujita, H. Deficiencies of Hippocampal Zn and ZnT3 Accelerate Brain Aging of Rat. Biochem. Biophys. Res. Commun. 2000, 279, 505–511. [Google Scholar] [CrossRef]
- Hancock, S.M.; Portbury, S.D.; Gunn, A.P.; Roberts, B.R.; Bush, A.I.; Adlard, P.A. Zinc Transporter-3 Knockout Mice Demonstrate Age-Dependent Alterations in the Metalloproteome. Int. J. Mol. Sci. 2020, 21, 839. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Cole, T.B.; Palmiter, R.D.; Koh, J.Y. Accumulation of Zinc in Degenerating Hippocampal Neurons of ZnT3-Null Mice after Seizures: Evidence against Synaptic Vesicle Origin. J. Neurosci. Off. J. Soc. Neurosci. 2000, 20, RC79. [Google Scholar]
- Chen, N.-N.; Zhao, D.-J.; Sun, Y.-X.; Wang, D.-D.; Ni, H. Long-Term Effects of Zinc Deficiency and Zinc Supplementation on Developmental Seizure-Induced Brain Damage and the Underlying GPR39/ZnT-3 and MBP Expression in the Hippocampus. Front. Neurosci. 2019, 13, 920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.-Y.; Kim, J.-H.; Palmiter, R.D.; Koh, J.-Y. Zinc Released from Metallothionein-Iii May Contribute to Hippocampal CA1 and Thalamic Neuronal Death Following Acute Brain Injury. Exp. Neurol. 2003, 184, 337–347. [Google Scholar] [CrossRef]
- Kaneko, M.; Noguchi, T.; Ikegami, S.; Sakurai, T.; Kakita, A.; Toyoshima, Y.; Kambe, T.; Yamada, M.; Inden, M.; Hara, H.; et al. Zinc Transporters ZnT3 and ZnT6 Are Downregulated in the Spinal Cords of Patients with Sporadic Amyotrophic Lateral Sclerosis. J. Neurosci. Res. 2015, 93, 370–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, L.; Li, L.; Yang, S.; Wang, W.; Ye, C.; Li, H. Disruption of Zinc Transporter ZnT3 Transcriptional Activity and Synaptic Vesicular Zinc in the Brain of Huntington’s Disease Transgenic Mouse. Cell Biosci. 2020, 10, 106. [Google Scholar] [CrossRef]
- Sikora, J.; Kieffer, B.L.; Paoletti, P.; Ouagazzal, A.-M. Synaptic Zinc Contributes to Motor and Cognitive Deficits in 6-Hydroxydopamine Mouse Models of Parkinson’s Disease. Neurobiol. Dis. 2020, 134, 104681. [Google Scholar] [CrossRef]
- Beyer, N.; Coulson, D.T.R.; Heggarty, S.; Ravid, R.; Hellemans, J.; Irvine, G.B.; Johnston, J.A. Zinc Transporter MRNA Levels in Alzheimer’s Disease Postmortem Brain. J. Alzheimers Dis. JAD 2012, 29, 863–873. [Google Scholar] [CrossRef]
- Enache, D.; Pereira, J.B.; Jelic, V.; Winblad, B.; Nilsson, P.; Aarsland, D.; Bereczki, E. Increased Cerebrospinal Fluid Concentration of ZnT3 Is Associated with Cognitive Impairment in Alzheimer’s Disease. J. Alzheimers Dis. JAD 2020, 77, 1143–1155. [Google Scholar] [CrossRef]
- Yue, C.; Shan, Z.; Tan, Y.; Yao, C.; Liu, Y.; Liu, Q.; Tan, X.; Du, X. His-Rich Domain of Selenoprotein P Ameliorates Neuropathology and Cognitive Deficits by Regulating TrkB Pathway and Zinc Homeostasis in an Alzheimer Model of Mice. ACS Chem. Neurosci. 2020, 11, 4098–4110. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, T.; Yu, D.; Feng, W.-Y.; Nie, Y.-X.; Stoltenberg, M.; Danscher, G.; Wang, Z.-Y. Elevation of Zinc Transporter ZnT3 Protein in the Cerebellar Cortex of the AbetaPP/PS1 Transgenic Mouse. J. Alzheimers Dis. JAD 2010, 20, 323–331. [Google Scholar] [CrossRef]
- Michalczyk, A.; Varigos, G.; Catto-Smith, A.; Blomeley, R.C.; Ackland, M.L. Analysis of Zinc Transporter, HZnT4 ( Slc30A4), Gene Expression in a Mammary Gland Disorder Leading to Reduced Zinc Secretion into Milk. Hum. Genet. 2003, 113, 202–210. [Google Scholar] [CrossRef]
- Erway, L.C.; Grider, A. Zinc Metabolism in Lethal-Milk Mice. Otolith, Lactation, and Aging Effects. J. Hered. 1984, 75, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Todd, W.R.; Elvehjem, C.A.; Hart, E.B. Zinc in the Nutrition of the Rat. Am. J. Physiol.-Leg. Content 1933, 107, 146–156. [Google Scholar] [CrossRef]
- Suzuki, T.; Ishihara, K.; Migaki, H.; Ishihara, K.; Nagao, M.; Yamaguchi-Iwai, Y.; Kambe, T. Two Different Zinc Transport Complexes of Cation Diffusion Facilitator Proteins Localized in the Secretory Pathway Operate to Activate Alkaline Phosphatases in Vertebrate Cells. J. Biol. Chem. 2005, 280, 30956–30962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cragg, R.A.; Phillips, S.R.; Piper, J.M.; Varma, J.S.; Campbell, F.C.; Mathers, J.C.; Ford, D. Homeostatic Regulation of Zinc Transporters in the Human Small Intestine by Dietary Zinc Supplementation. Gut 2005, 54, 469–478. [Google Scholar] [CrossRef]
- Valentine, R.A.; Jackson, K.A.; Christie, G.R.; Mathers, J.C.; Taylor, P.M.; Ford, D. ZnT5 Variant B Is a Bidirectional Zinc Transporter and Mediates Zinc Uptake in Human Intestinal Caco-2 Cells. J. Biol. Chem. 2007, 282, 14389–14393. [Google Scholar] [CrossRef] [Green Version]
- Kumánovics, A.; Poruk, K.E.; Osborn, K.A.; Ward, D.M.; Kaplan, J. YKE4 (YIL023C) Encodes a Bidirectional Zinc Transporter in the Endoplasmic Reticulum of Saccharomyces Cerevisiae. J. Biol. Chem. 2006, 281, 22566–22574. [Google Scholar] [CrossRef] [Green Version]
- Devergnas, S.; Chimienti, F.; Naud, N.; Pennequin, A.; Coquerel, Y.; Chantegrel, J.; Favier, A.; Seve, M. Differential Regulation of Zinc Efflux Transporters ZnT-1, ZnT-5 and ZnT-7 Gene Expression by Zinc Levels: A Real-Time RT-PCR Study. Biochem. Pharmacol. 2004, 68, 699–709. [Google Scholar] [CrossRef]
- Florea, D.; Molina-López, J.; Hogstrand, C.; Lengyel, I.; de la Cruz, A.P.; Rodríguez-Elvira, M.; Planells, E. Changes in Zinc Status and Zinc Transporters Expression in Whole Blood of Patients with Systemic Inflammatory Response Syndrome (SIRS). J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. GMS 2018, 49, 202–209. [Google Scholar] [CrossRef]
- Noh, H.; Paik, H.Y.; Kim, J.; Chung, J. The Changes of Zinc Transporter ZnT Gene Expression in Response to Zinc Supplementation in Obese Women. Biol. Trace Elem. Res. 2014, 162, 38–45. [Google Scholar] [CrossRef]
- Noh, H.; Paik, H.Y.; Kim, J.; Chung, J. The Alteration of Zinc Transporter Gene Expression Is Associated with Inflammatory Markers in Obese Women. Biol. Trace Elem. Res. 2014, 158, 1–8. [Google Scholar] [CrossRef]
- Foster, M.; Petocz, P.; Samman, S. Inflammation Markers Predict Zinc Transporter Gene Expression in Women with Type 2 Diabetes Mellitus. J. Nutr. Biochem. 2013, 24, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Barresi, V.; Valenti, G.; Spampinato, G.; Musso, N.; Castorina, S.; Rizzarelli, E.; Condorelli, D.F. Transcriptome Analysis Reveals an Altered Expression Profile of Zinc Transporters in Colorectal Cancer. J. Cell. Biochem. 2018, 119, 9707–9719. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-H.; Wang, X.; Stoltenberg, M.; Danscher, G.; Huang, L.; Wang, Z.-Y. Abundant Expression of Zinc Transporters in the Amyloid Plaques of Alzheimer’s Disease Brain. Brain Res. Bull. 2008, 77, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Overbeck, S.; Uciechowski, P.; Ackland, M.L.; Ford, D.; Rink, L. Intracellular Zinc Homeostasis in Leukocyte Subsets Is Regulated by Different Expression of Zinc Exporters ZnT-1 to ZnT-9. J. Leukoc. Biol. 2008, 83, 368–380. [Google Scholar] [CrossRef]
- Lovell, M.A.; Smith, J.L.; Markesbery, W.R. Elevated Zinc Transporter-6 in Mild Cognitive Impairment, Alzheimer Disease, and Pick Disease. J. Neuropathol. Exp. Neurol. 2006, 65, 489–498. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.L.; Xiong, S.; Markesbery, W.R.; Lovell, M.A. Altered Expression of Zinc Transporters-4 and -6 in Mild Cognitive Impairment, Early and Late Alzheimer’s Disease Brain. Neuroscience 2006, 140, 879–888. [Google Scholar] [CrossRef]
- Napoli, E.; Ross-Inta, C.; Wong, S.; Omanska-Klusek, A.; Barrow, C.; Iwahashi, C.; Garcia-Arocena, D.; Sakaguchi, D.; Berry-Kravis, E.; Hagerman, R.; et al. Altered Zinc Transport Disrupts Mitochondrial Protein Processing/Import in Fragile X-Associated Tremor/Ataxia Syndrome. Hum. Mol. Genet. 2011, 20, 3079–3092. [Google Scholar] [CrossRef] [Green Version]
- Napoli, E.; Ross-Inta, C.; Song, G.; Wong, S.; Hagerman, R.; Gane, L.W.; Smilowitz, J.T.; Tassone, F.; Giulivi, C. Premutation in the Fragile X Mental Retardation 1 (FMR1) Gene Affects Maternal Zn-Milk and Perinatal Brain Bioenergetics and Scaffolding. Front. Neurosci. 2016, 10, 159. [Google Scholar] [CrossRef] [Green Version]
- Lyubartseva, G.; Smith, J.L.; Markesbery, W.R.; Lovell, M.A. Alterations of Zinc Transporter Proteins ZnT-1, ZnT-4 and ZnT-6 in Preclinical Alzheimer’s Disease Brain. Brain Pathol. Zur. Switz. 2010, 20, 343–350. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.-H.; Wang, X.; Zheng, Z.-H.; Ren, H.; Stoltenberg, M.; Danscher, G.; Huang, L.; Rong, M.; Wang, Z.-Y. Altered Expression and Distribution of Zinc Transporters in APP/PS1 Transgenic Mouse Brain. Neurobiol. Aging 2010, 31, 74–87. [Google Scholar] [CrossRef]
- Zhu, B.; Huo, R.; Zhi, Q.; Zhan, M.; Chen, X.; Hua, Z.-C. Increased Expression of Zinc Transporter ZIP4, ZIP11, ZnT1, and ZnT6 Predicts Poor Prognosis in Pancreatic Cancer. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. GMS 2021, 65, 126734. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Ji, X.; Gao, Y.; Zhu, X.; Xiao, G. ZnT7 RNAi Favors RafGOFscrib-/--Induced Tumor Growth and Invasion in Drosophila through JNK Signaling Pathway. Oncogene 2021, 40, 2217–2229. [Google Scholar] [CrossRef] [PubMed]
- Tepaamorndech, S.; Huang, L.; Kirschke, C.P. A Null-Mutation in the Znt7 Gene Accelerates Prostate Tumor Formation in a Transgenic Adenocarcinoma Mouse Prostate Model. Cancer Lett. 2011, 308, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Chimienti, F.; Devergnas, S.; Pattou, F.; Schuit, F.; Garcia-Cuenca, R.; Vandewalle, B.; Kerr-Conte, J.; Van Lommel, L.; Grunwald, D.; Favier, A.; et al. In Vivo Expression and Functional Characterization of the Zinc Transporter ZnT8 in Glucose-Induced Insulin Secretion. J. Cell Sci. 2006, 119, 4199–4206. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Tian, W.; Pratt, E.B.; Dirling, L.B.; Shyng, S.-L.; Meshul, C.K.; Cohen, D.M. Down-Regulation of ZnT8 Expression in INS-1 Rat Pancreatic Beta Cells Reduces Insulin Content and Glucose-Inducible Insulin Secretion. PLoS ONE 2009, 4, e5679. [Google Scholar] [CrossRef] [Green Version]
- Nicolson, T.J.; Bellomo, E.A.; Wijesekara, N.; Loder, M.K.; Baldwin, J.M.; Gyulkhandanyan, A.V.; Koshkin, V.; Tarasov, A.I.; Carzaniga, R.; Kronenberger, K.; et al. Insulin Storage and Glucose Homeostasis in Mice Null for the Granule Zinc Transporter ZnT8 and Studies of the Type 2 Diabetes-Associated Variants. Diabetes 2009, 58, 2070–2083. [Google Scholar] [CrossRef] [Green Version]
- Elmaoğulları, S.; Uçaktürk, S.A.; Elbeg, Ş.; Döğer, E.; Tayfun, M.; Gürbüz, F.; Bideci, A. Prevalence of ZnT8 Antibody in Turkish Children and Adolescents with New Onset Type 1 Diabetes. J. Clin. Res. Pediatr. Endocrinol. 2018, 10, 108–112. [Google Scholar] [CrossRef]
- Wenzlau, J.M.; Juhl, K.; Yu, L.; Moua, O.; Sarkar, S.A.; Gottlieb, P.; Rewers, M.; Eisenbarth, G.S.; Jensen, J.; Davidson, H.W.; et al. The Cation Efflux Transporter ZnT8 (Slc30A8) Is a Major Autoantigen in Human Type 1 Diabetes. Proc. Natl. Acad. Sci. USA 2007, 104, 17040–17045. [Google Scholar] [CrossRef] [Green Version]
- Parsons, D.S.; Hogstrand, C.; Maret, W. The C-Terminal Cytosolic Domain of the Human Zinc Transporter ZnT8 and Its Diabetes Risk Variant. FEBS J. 2018, 285, 1237–1250. [Google Scholar] [CrossRef] [Green Version]
- Tamaki, M.; Fujitani, Y.; Hara, A.; Uchida, T.; Tamura, Y.; Takeno, K.; Kawaguchi, M.; Watanabe, T.; Ogihara, T.; Fukunaka, A.; et al. The Diabetes-Susceptible Gene SLC30A8/ZnT8 Regulates Hepatic Insulin Clearance. J. Clin. Investig. 2013, 123, 4513–4524. [Google Scholar] [CrossRef] [Green Version]
- Bhola, S.; Cave, E.M.; Bhana, S.; Crowther, N.J.; Padoa, C.J. Zinc Transporter 8 (ZnT8) Autoantibody Prevalence in Black South African Participants with Type 1 Diabetes. BMC Endocr. Disord. 2021, 21, 151. [Google Scholar] [CrossRef] [PubMed]
- Gomes, K.F.B.; Semzezem, C.; Batista, R.; Fukui, R.T.; Santos, A.S.; Correia, M.R.; Passos-Bueno, M.R.; Silva, M.E.R. da Importance of Zinc Transporter 8 Autoantibody in the Diagnosis of Type 1 Diabetes in Latin Americans. Sci. Rep. 2017, 7, 207. [Google Scholar] [CrossRef] [PubMed]
- Marchand, L.; Garnier, L.; Thivolet, C.; Nicolino, M.; Fabien, N. Very High Titres of ZnT8 Autoantibodies at Type 1 Diabetes Onset and Presence of Autoantibodies Related to Other Autoimmune Disorders. Diabetes Metab. 2020, 46, 73–74. [Google Scholar] [CrossRef] [PubMed]
- Niechciał, E.; Rogowicz-Frontczak, A.; Piłaciński, S.; Fichna, M.; Skowrońska, B.; Fichna, P.; Zozulińska-Ziółkiewicz, D. Autoantibodies against Zinc Transporter 8 Are Related to Age and Metabolic State in Patients with Newly Diagnosed Autoimmune Diabetes. Acta Diabetol. 2018, 55, 287–294. [Google Scholar] [CrossRef] [Green Version]
- Rogowicz-Frontczak, A.; Pilacinski, S.; Wyka, K.; Wierusz-Wysocka, B.; Zozulinska-Ziolkiewicz, D. Zinc Transporter 8 Autoantibodies (ZnT8-Ab) Are Associated with Higher Prevalence of Multiple Diabetes-Related Autoantibodies in Adults with Type 1 Diabetes. Diabetes Res. Clin. Pract. 2018, 146, 313–320. [Google Scholar] [CrossRef]
- Seman, N.A.; Mohamud, W.N.W.; Östenson, C.-G.; Brismar, K.; Gu, H.F. Increased DNA Methylation of the SLC30A8 Gene Promoter Is Associated with Type 2 Diabetes in a Malay Population. Clin. Epigenetics 2015, 7, 30. [Google Scholar] [CrossRef] [Green Version]
- Mashal, S.; Khanfar, M.; Al-Khalayfa, S.; Srour, L.; Mustafa, L.; Hakooz, N.M.; Zayed, A.A.; Khader, Y.S.; Azab, B. SLC30A8 Gene Polymorphism Rs13266634 Associated with Increased Risk for Developing Type 2 Diabetes Mellitus in Jordanian Population. Gene 2021, 768, 145279. [Google Scholar] [CrossRef]
- Nielsen, L.B.; Vaziri-Sani, F.; Pörksen, S.; Andersen, M.-L.M.; Svensson, J.; Bergholdt, R.; Pociot, F.; Hougaard, P.; de Beaufort, C.; Castaño, L.; et al. Relationship between ZnT8Ab, the SLC30A8 Gene and Disease Progression in Children with Newly Diagnosed Type 1 Diabetes. Autoimmunity 2011, 44, 616–623. [Google Scholar] [CrossRef] [Green Version]
- Thirunavukkarasu, R.; Asirvatham, A.J.; Chitra, A.; Jayalakshmi, M. SLC30A8 Gene Rs13266634 C/T Polymorphism in Children with Type 1 Diabetes in Tamil Nadu, India. J. Clin. Res. Pediatr. Endocrinol. 2019, 11, 55–60. [Google Scholar] [CrossRef]
- Giacconi, R.; Malavolta, M.; Chiodi, L.; Boccoli, G.; Costarelli, L.; Bonfigli, A.R.; Galeazzi, R.; Piacenza, F.; Basso, A.; Gasparini, N.; et al. ZnT8 Arg325Trp Polymorphism Influences Zinc Transporter Expression and Cytokine Production in PBMCs from Patients with Diabetes. Diabetes Res. Clin. Pract. 2018, 144, 102–110. [Google Scholar] [CrossRef]
- Kawasaki, E.; Uga, M.; Nakamura, K.; Kuriya, G.; Satoh, T.; Fujishima, K.; Ozaki, M.; Abiru, N.; Yamasaki, H.; Wenzlau, J.M.; et al. Association between Anti-ZnT8 Autoantibody Specificities and SLC30A8 Arg325Trp Variant in Japanese Patients with Type 1 Diabetes. Diabetologia 2008, 51, 2299–2302. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, M.; Mahrooz, A.; Abediankenari, S.; Hayati Roodbari, N. Association of Rs11558471 in SLC30A8 Gene with Interleukin 17 Serum Levels and Insulin Resistance in Iranian Patients with Type 2 Diabetes. Iran. J. Immunol. IJI 2020, 17, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Sargazi, S.; Heidari Nia, M.; Sargazi, F.M.; Sheervalilou, R.; Saravani, R.; Mirinejad, S. SNPs in the 3’-Untranslated Region of SLC30A8 Confer Risk of Type 2 Diabetes Mellitus in a South-East Iranian Population: Evidences from Case-Control and Bioinformatics Studies. J. Diabetes Metab. Disord. 2020, 19, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.; Wong, R.; Barriga, K.J.; Klingensmith, G.; Ziegler, A.-G.; Rewers, M.J.; Steck, A.K. Rs11203203 Is Associated with Type 1 Diabetes Risk in Population Pre-Screened for High-Risk HLA-DR,DQ Genotypes. Pediatr. Diabetes 2012, 13, 611–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, S.; Molina-López, J.; Parsons, D.; Corpe, C.; Maret, W.; Hogstrand, C. Differential Cytolocation and Functional Assays of the Two Major Human SLC30A8 (ZnT8) Isoforms. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. GMS 2017, 44, 116–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, M.K.; Vadde, R. Insights into the Structure-Function Relationship of Both Wild and Mutant Zinc Transporter ZnT8 in Human: A Computational Structural Biology Approach. J. Biomol. Struct. Dyn. 2020, 38, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Sala, D.; Giachetti, A.; Rosato, A. Insights into the Dynamics of the Human Zinc Transporter ZnT8 by MD Simulations. J. Chem. Inf. Model. 2021, 61, 901–912. [Google Scholar] [CrossRef]
- Ullah, R.; Shehzad, A.; Shah, M.A.; March, M.D.; Ismat, F.; Iqbal, M.; Onesti, S.; Rahman, M.; McPherson, M.J. C-Terminal Domain of the Human Zinc Transporter HZnT8 Is Structurally Indistinguishable from Its Disease Risk Variant (R325W). Int. J. Mol. Sci. 2020, 21, 926. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.; Xie, T.; Zeng, W.; Jiang, Y.; Bai, X.-C. Cryo-EM Structures of Human ZnT8 in Both Outward- and Inward-Facing Conformations. eLife 2020, 9, e58823. [Google Scholar] [CrossRef]
- Baumann, K.; Kesselring, K.; Lampasona, V.; Walschus, U.; Kerner, W.; Wassmuth, R.; Schlosser, M. Autoantibodies against Zinc Transporter 8 Further Stratify the Autoantibody-Defined Risk for Type 1 Diabetes in a General Population of Schoolchildren and Have Distinctive Isoform Binding Patterns in Different Forms of Autoimmune Diabetes: Results from the Karlsburg Type 1 Diabetes Risk Study. Diabet. Med. J. Br. Diabet. Assoc. 2021, 38, e14389. [Google Scholar] [CrossRef]
- Bost, C.; Jordan, T.; Magali, D.; Françoise, F.; Nicole, F. Anti-ZnT8 Autoantibodies: A New Marker to Be Screened in Patients with Anti-Adrenal Antibodies. Clin. Chim. Acta Int. J. Clin. Chem. 2020, 511, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.; Ibrahim, F.; Sobngwi, E.; Gautier, J.F.; Boudou, P. Zinc Transporter 8 Autoantibodies Assessment in Daily Practice. Clin. Biochem. 2017, 50, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Lounici Boudiaf, A.; Bouziane, D.; Smara, M.; Meddour, Y.; Haffaf, E.M.; Oudjit, B.; Chaib Mamouzi, S.; Aouichat Bouguerra, S. Could ZnT8 Antibodies Replace ICA, GAD, IA2 and Insulin Antibodies in the Diagnosis of Type 1 Diabetes? Curr. Res. Transl. Med. 2018, 66, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rochmah, N.; Faizi, M.; Windarti, S.W. Zinc Transporter 8 Autoantibody in the Diagnosis of Type 1 Diabetes in Children. Clin. Exp. Pediatr. 2020, 63, 402–405. [Google Scholar] [CrossRef]
- Merriman, C.; Li, H.; Li, H.; Fu, D. Highly Specific Monoclonal Antibodies for Allosteric Inhibition and Immunodetection of the Human Pancreatic Zinc Transporter ZnT8. J. Biol. Chem. 2018, 293, 16206–16216. [Google Scholar] [CrossRef] [Green Version]
- Dwivedi, O.P.; Lehtovirta, M.; Hastoy, B.; Chandra, V.; Krentz, N.A.J.; Kleiner, S.; Jain, D.; Richard, A.-M.; Abaitua, F.; Beer, N.L.; et al. Loss of ZnT8 Function Protects against Diabetes by Enhanced Insulin Secretion. Nat. Genet. 2019, 51, 1596–1606. [Google Scholar] [CrossRef]
- Flannick, J.; Thorleifsson, G.; Beer, N.L.; Jacobs, S.B.R.; Grarup, N.; Burtt, N.P.; Mahajan, A.; Fuchsberger, C.; Atzmon, G.; Benediktsson, R.; et al. Loss-of-Function Mutations in SLC30A8 Protect against Type 2 Diabetes. Nat. Genet. 2014, 46, 357–363. [Google Scholar] [CrossRef]
- Merriman, C.; Fu, D. Down-Regulation of the Islet-Specific Zinc Transporter-8 (ZnT8) Protects Human Insulinoma Cells against Inflammatory Stress. J. Biol. Chem. 2019, 294, 16992–17006. [Google Scholar] [CrossRef]
- Sun, H.; Li, C.; Li, S.; Li, X.; Wang, J.; Zhou, Z.; Shao, M. Gene Silencing of ZnT8 Attenuates Inflammation and Protects Pancreatic Tissue Injury in T1D. Immunol. Lett. 2018, 198, 1–6. [Google Scholar] [CrossRef]
- Pound, L.D.; Sarkar, S.A.; Ustione, A.; Dadi, P.K.; Shadoan, M.K.; Lee, C.E.; Walters, J.A.; Shiota, M.; McGuinness, O.P.; Jacobson, D.A.; et al. The Physiological Effects of Deleting the Mouse SLC30A8 Gene Encoding Zinc Transporter-8 Are Influenced by Gender and Genetic Background. PLoS ONE 2012, 7, e40972. [Google Scholar] [CrossRef]
- Xu, J.; Wijesekara, N.; Regeenes, R.; Rijjal, D.A.; Piro, A.L.; Song, Y.; Wu, A.; Bhattacharjee, A.; Liu, Y.; Marzban, L.; et al. Pancreatic β Cell-Selective Zinc Transporter 8 Insufficiency Accelerates Diabetes Associated with Islet Amyloidosis. JCI Insight 2021, 6, 143037. [Google Scholar] [CrossRef] [PubMed]
- Hardy, A.B.; Wijesekara, N.; Genkin, I.; Prentice, K.J.; Bhattacharjee, A.; Kong, D.; Chimienti, F.; Wheeler, M.B. Effects of High-Fat Diet Feeding on Znt8-Null Mice: Differences between β-Cell and Global Knockout of Znt8. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E1084–E1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okura, T.; Fujioka, Y.; Nakamura, R.; Anno, M.; Ito, Y.; Kitao, S.; Matsumoto, K.; Shoji, K.; Sumi, K.; Matsuzawa, K.; et al. Hepatic Insulin Clearance Is Increased in Patients with High HbA1c Type 2 Diabetes: A Preliminary Report. BMJ Open Diabetes Res. Care 2020, 8, e001149. [Google Scholar] [CrossRef] [PubMed]
- Barragán-Álvarez, C.P.; Padilla-Camberos, E.; Díaz, N.F.; Cota-Coronado, A.; Hernández-Jiménez, C.; Bravo-Reyna, C.C.; Díaz-Martínez, N.E. Loss of Znt8 Function in Diabetes Mellitus: Risk or Benefit? Mol. Cell. Biochem. 2021, 476, 2703–2718. [Google Scholar] [CrossRef] [PubMed]
- Syring, K.E.; Bosma, K.J.; Goleva, S.B.; Singh, K.; Oeser, J.K.; Lopez, C.A.; Skaar, E.P.; McGuinness, O.P.; Davis, L.K.; Powell, D.R.; et al. Potential Positive and Negative Consequences of ZnT8 Inhibition. J. Endocrinol. 2020, 246, 189–205. [Google Scholar] [CrossRef] [PubMed]
- Hosseini-Esfahani, F.; Mirmiran, P.; Koochakpoor, G.; Daneshpour, M.S.; Guity, K.; Azizi, F. Some Dietary Factors Can Modulate the Effect of the Zinc Transporters 8 Polymorphism on the Risk of Metabolic Syndrome. Sci. Rep. 2017, 7, 1649. [Google Scholar] [CrossRef] [Green Version]
- Daniels, M.J.; Jagielnicki, M.; Yeager, M. Structure/Function Analysis of Human ZnT8 (SLC30A8): A Diabetes Risk Factor and Zinc Transporter. Curr. Res. Struct. Biol. 2020, 2, 144–155. [Google Scholar] [CrossRef]
- Csikós, A.; Kozma, B.; Pór, Á.; Kovács, I.; Lampé, R.; Miklós, I.; Takacs, P. Zinc Transporter 9 (SLC30A9) Expression Is Decreased in the Vaginal Tissues of Menopausal Women. Biol. Trace Elem. Res. 2021, 199, 4011–4019. [Google Scholar] [CrossRef]
- Deng, H.; Qiao, X.; Xie, T.; Fu, W.; Li, H.; Zhao, Y.; Guo, M.; Feng, Y.; Chen, L.; Zhao, Y.; et al. SLC-30A9 Is Required for Zn2+ Homeostasis, Zn2+ Mobilization, and Mitochondrial Health. Proc. Natl. Acad. Sci. USA 2021, 118, e2023909118. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Gbadamosi, O.; Kolor, K.; Sosa, J.; Andrzejczuk, L.; Gibson, G.; St Croix, C.; Chikina, M.; Aizenman, E.; Clark, N.; et al. Evolutionary Rate Covariation Identifies SLC30A9 (ZnT9) as a Mitochondrial Zinc Transporter. Biochem. J. 2021, 478, 3205–3220. [Google Scholar] [CrossRef]
- Gartmann, L.; Wex, T.; Grüngreiff, K.; Reinhold, D.; Kalinski, T.; Malfertheiner, P.; Schütte, K. Expression of Zinc Transporters ZIP4, ZIP14 and ZnT9 in Hepatic Carcinogenesis-An Immunohistochemical Study. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. GMS 2018, 49, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; Malas, K.M.; Tydrick, C.; Siddiqui, I.A.; Iczkowski, K.A.; Ahmad, N. Analysis of Zinc-Exporters Expression in Prostate Cancer. Sci. Rep. 2016, 6, 36772. [Google Scholar] [CrossRef] [Green Version]
- Uhlen, M.; Zhang, C.; Lee, S.; Sjöstedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A Pathology Atlas of the Human Cancer Transcriptome. Science 2017, 357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kambe, T. Molecular Architecture and Function of ZnT Transporters. Curr. Top. Membr. 2012, 69, 199–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukhopadhyay, S. Familial Manganese-Induced Neurotoxicity Due to Mutations in SLC30A10 or SLC39A14. Neurotoxicology 2018, 64, 278–283. [Google Scholar] [CrossRef]
- Bosomworth, H.J.; Adlard, P.A.; Ford, D.; Valentine, R.A. Altered Expression of ZnT10 in Alzheimer’s Disease Brain. PLoS ONE 2013, 8, e65475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmona, A.; Zogzas, C.E.; Roudeau, S.; Porcaro, F.; Garrevoet, J.; Spiers, K.M.; Salomé, M.; Cloetens, P.; Mukhopadhyay, S.; Ortega, R. SLC30A10 Mutation Involved in Parkinsonism Results in Manganese Accumulation within Nanovesicles of the Golgi Apparatus. ACS Chem. Neurosci. 2019, 10, 599–609. [Google Scholar] [CrossRef]
- Leyva-Illades, D.; Chen, P.; Zogzas, C.E.; Hutchens, S.; Mercado, J.M.; Swaim, C.D.; Morrisett, R.A.; Bowman, A.B.; Aschner, M.; Mukhopadhyay, S. SLC30A10 Is a Cell Surface-Localized Manganese Efflux Transporter, and Parkinsonism-Causing Mutations Block Its Intracellular Trafficking and Efflux Activity. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 14079–14095. [Google Scholar] [CrossRef]
- Hutchens, S.; Liu, C.; Jursa, T.; Shawlot, W.; Chaffee, B.K.; Yin, W.; Gore, A.C.; Aschner, M.; Smith, D.R.; Mukhopadhyay, S. Deficiency in the Manganese Efflux Transporter SLC30A10 Induces Severe Hypothyroidism in Mice. J. Biol. Chem. 2017, 292, 9760–9773. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Hutchens, S.; Jursa, T.; Shawlot, W.; Polishchuk, E.V.; Polishchuk, R.S.; Dray, B.K.; Gore, A.C.; Aschner, M.; Smith, D.R.; et al. Hypothyroidism Induced by Loss of the Manganese Efflux Transporter SLC30A10 May Be Explained by Reduced Thyroxine Production. J. Biol. Chem. 2017, 292, 16605–16615. [Google Scholar] [CrossRef] [Green Version]
- Lioumi, M.; Ferguson, C.A.; Sharpe, P.T.; Freeman, T.; Marenholz, I.; Mischke, D.; Heizmann, C.; Ragoussis, J. Isolation and Characterization of Human and Mouse ZIRTL, a Member of the IRT1 Family of Transporters, Mapping within the Epidermal Differentiation Complex. Genomics 1999, 62, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Thokala, S.; Bodiga, V.L.; Kudle, M.R.; Bodiga, S. Comparative Response of Cardiomyocyte ZIPs and ZnTs to Extracellular Zinc and TPEN. Biol. Trace Elem. Res. 2019, 192, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Méndez, R.O.; Santiago, A.; Yepiz-Plascencia, G.; Peregrino-Uriarte, A.B.; de la Barca, A.M.C.; García, H.S. Zinc Fortification Decreases ZIP1 Gene Expression of Some Adolescent Females with Appropriate Plasma Zinc Levels. Nutrients 2014, 6, 2229–2239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharif, R.; Thomas, P.; Zalewski, P.; Fenech, M. Zinc Supplementation Influences Genomic Stability Biomarkers, Antioxidant Activity, and Zinc Transporter Genes in an Elderly Australian Population with Low Zinc Status. Mol. Nutr. Food Res. 2015, 59, 1200–1212. [Google Scholar] [CrossRef] [Green Version]
- Desouki, M.M.; Franklin, R.B.; Costello, L.C.; Fadare, O. Persistent Low Expression of HZip1 in Mucinous Carcinomas of the Ovary, Colon, Stomach and Lung. J. Ovarian Res. 2015, 8, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehvy, A.I.; Horev, G.; Golan, Y.; Glaser, F.; Shammai, Y.; Assaraf, Y.G. Alterations in ZnT1 Expression and Function Lead to Impaired Intracellular Zinc Homeostasis in Cancer. Cell Death Discov. 2019, 5, 144. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, J.; He, S.; Xiao, B.; Peng, X. SLC39A1 Contribute to Malignant Progression and Have Clinical Prognostic Impact in Gliomas. Cancer Cell Int. 2020, 20, 573. [Google Scholar] [CrossRef]
- Franklin, R.B.; Ma, J.; Zou, J.; Guan, Z.; Kukoyi, B.I.; Feng, P.; Costello, L.C. Human ZIP1 Is a Major Zinc Uptake Transporter for the Accumulation of Zinc in Prostate Cells. J. Inorg. Biochem. 2003, 96, 435–442. [Google Scholar] [CrossRef] [Green Version]
- Franklin, R.B.; Feng, P.; Milon, B.; Desouki, M.M.; Singh, K.K.; Kajdacsy-Balla, A.; Bagasra, O.; Costello, L.C. HZIP1 Zinc Uptake Transporter down Regulation and Zinc Depletion in Prostate Cancer. Mol. Cancer 2005, 4, 32. [Google Scholar] [CrossRef] [Green Version]
- Holubova, M.; Axmanova, M.; Gumulec, J.; Raudenska, M.; Sztalmachova, M.; Babula, P.; Adam, V.; Kizek, R.; Masarik, M. KRAS NF-ΚB Is Involved in the Development of Zinc Resistance and Reduced Curability in Prostate Cancer. Met. Integr. Biometal Sci. 2014, 6, 1240–1253. [Google Scholar] [CrossRef]
- Zou, J.; Milon, B.C.; Desouki, M.M.; Costello, L.C.; Franklin, R.B. HZIP1 Zinc Transporter Down-Regulation in Prostate Cancer Involves the Overexpression of Ras Responsive Element Binding Protein-1 (RREB-1). Prostate 2011, 71, 1518–1524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, J.; Bobo, J.A.; Liuzzi, J.P.; Cousins, R.J. Effects of Intracellular Zinc Depletion on Metallothionein and ZIP2 Transporter Expression and Apoptosis. J. Leukoc. Biol. 2001, 70, 559–566. [Google Scholar] [PubMed]
- Cousins, R.J.; Blanchard, R.K.; Popp, M.P.; Liu, L.; Cao, J.; Moore, J.B.; Green, C.L. A Global View of the Selectivity of Zinc Deprivation and Excess on Genes Expressed in Human THP-1 Mononuclear Cells. Proc. Natl. Acad. Sci. USA 2003, 100, 6952–6957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, T.; Wang, X.; Yang, J.; Hu, X.; Wu, W.; Guo, L.; Kang, L.; Zhang, L. Overexpression of Zip-2 MRNA in the Leukocytes of Asthmatic Infants. Pediatr. Pulmonol. 2009, 44, 763–767. [Google Scholar] [CrossRef]
- Tao, Y.; Huang, Q.; Jiang, Y.; Wang, X.; Sun, P.; Tian, Y.; Wu, H.; Zhang, M.; Meng, S.; Wang, Y.; et al. Up-Regulation of Slc39A2(Zip2) MRNA in Peripheral Blood Mononuclear Cells from Patients with Pulmonary Tuberculosis. Mol. Biol. Rep. 2013, 40, 4979–4984. [Google Scholar] [CrossRef]
- Giacconi, R.; Costarelli, L.; Malavolta, M.; Piacenza, F.; Galeazzi, R.; Gasparini, N.; Basso, A.; Mariani, E.; Fulop, T.; Rink, L.; et al. Association among 1267 A/G HSP70-2, -308 G/A TNF-α Polymorphisms and pro-Inflammatory Plasma Mediators in Old ZincAge Population. Biogerontology 2014, 15, 65–79. [Google Scholar] [CrossRef]
- Giacconi, R.; Costarelli, L.; Malavolta, M.; Cardelli, M.; Galeazzi, R.; Piacenza, F.; Gasparini, N.; Basso, A.; Mariani, E.; Fulop, T.; et al. Effect of ZIP2 Gln/Arg/Leu (Rs2234632) Polymorphism on Zinc Homeostasis and Inflammatory Response Following Zinc Supplementation. BioFactors Oxf. Engl. 2015, 41, 414–423. [Google Scholar] [CrossRef]
- Juknat, A.; Rimmerman, N.; Levy, R.; Vogel, Z.; Kozela, E. Cannabidiol Affects the Expression of Genes Involved in Zinc Homeostasis in BV-2 Microglial Cells. Neurochem. Int. 2012, 61, 923–930. [Google Scholar] [CrossRef]
- Hashimoto, A.; Nakagawa, M.; Tsujimura, N.; Miyazaki, S.; Kizu, K.; Goto, T.; Komatsu, Y.; Matsunaga, A.; Shirakawa, H.; Narita, H.; et al. Properties of Zip4 Accumulation during Zinc Deficiency and Its Usefulness to Evaluate Zinc Status: A Study of the Effects of Zinc Deficiency during Lactation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R459–R468. [Google Scholar] [CrossRef]
- Reis, B.Z.; dos Santos Vieira, D.A.; da Costa Maynard, D.; da Silva, D.G.; Mendes-Netto, R.S.; Cozzolino, S.M.F. Zinc Nutritional Status Influences ZnT1 and ZIP4 Gene Expression in Children with a High Risk of Zinc Deficiency. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. GMS 2020, 61, 126537. [Google Scholar] [CrossRef]
- Danbolt, N. Acrodermatitis Enteropathica; Report of Two Additional Cases. Acta Derm. Venereol. 1948, 28, 532–543. [Google Scholar] [PubMed]
- Antala, S.; Dempski, R.E. The Human ZIP4 Transporter Has Two Distinct Binding Affinities and Mediates Transport of Multiple Transition Metals. Biochemistry 2012, 51, 963–973. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Y.; Liu, Z.; Bharadwaj, U.; Wang, H.; Wang, X.; Zhang, S.; Liuzzi, J.P.; Chang, S.-M.; Cousins, R.J.; et al. Aberrant Expression of Zinc Transporter ZIP4 (SLC39A4) Significantly Contributes to Human Pancreatic Cancer Pathogenesis and Progression. Proc. Natl. Acad. Sci. USA 2007, 104, 18636–18641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weaver, B.P.; Zhang, Y.; Hiscox, S.; Guo, G.L.; Apte, U.; Taylor, K.M.; Sheline, C.T.; Wang, L.; Andrews, G.K. Zip4 (Slc39a4) Expression Is Activated in Hepatocellular Carcinomas and Functions to Repress Apoptosis, Enhance Cell Cycle and Increase Migration. PLoS ONE 2010, 5, e13158. [Google Scholar] [CrossRef] [PubMed]
- Manning, D.L.; Daly, R.J.; Lord, P.G.; Kelly, K.F.; Green, C.D. Effects of Oestrogen on the Expression of a 4.4 Kb MRNA in the ZR-75-1 Human Breast Cancer Cell Line. Mol. Cell. Endocrinol. 1988, 59, 205–212. [Google Scholar] [CrossRef]
- El-Tanani, M.K.; Green, C.D. Interaction between Estradiol and Growth Factors in the Regulation of Specific Gene Expression in MCF-7 Human Breast Cancer Cells. J. Steroid Biochem. Mol. Biol. 1997, 60, 269–276. [Google Scholar] [CrossRef]
- el-Tanani, M.K.; Green, C.D. Insulin/IGF-1 Modulation of the Expression of Two Estrogen-Induced Genes in MCF-7 Cells. Mol. Cell. Endocrinol. 1996, 121, 29–35. [Google Scholar] [CrossRef]
- el-Tanani, M.K.; Green, C.D. Interaction between Estradiol and CAMP in the Regulation of Specific Gene Expression. Mol. Cell. Endocrinol. 1996, 124, 71–77. [Google Scholar] [CrossRef]
- McClelland, R.A.; Manning, D.L.; Gee, J.M.; Willsher, P.; Robertson, J.F.; Ellis, I.O.; Blamey, R.W.; Nicholson, R.I. Oestrogen-Regulated Genes in Breast Cancer: Association of PLIV1 with Response to Endocrine Therapy. Br. J. Cancer 1998, 77, 1653–1656. [Google Scholar] [CrossRef] [Green Version]
- Taylor, K.M. LIV-1 Breast Cancer Protein Belongs to New Family of Histidine-Rich Membrane Proteins with Potential to Control Intracellular Zn2+ Homeostasis. IUBMB Life 2000, 49, 249–253. [Google Scholar] [CrossRef]
- Nimmanon, T.; Ziliotto, S.; Ogle, O.; Burt, A.; Gee, J.M.W.; Andrews, G.K.; Kille, P.; Hogstrand, C.; Maret, W.; Taylor, K.M. The ZIP6/ZIP10 Heteromer Is Essential for the Zinc-Mediated Trigger of Mitosis. Cell. Mol. Life Sci. CMLS 2021, 78, 1781–1798. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.M.; Muraina, I.A.; Brethour, D.; Schmitt-Ulms, G.; Nimmanon, T.; Ziliotto, S.; Kille, P.; Hogstrand, C. Zinc Transporter ZIP10 Forms a Heteromer with ZIP6 Which Regulates Embryonic Development and Cell Migration. Biochem. J. 2016, 473, 2531–2544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croxford, T.P.; McCormick, N.H.; Kelleher, S.L. Moderate Zinc Deficiency Reduces Testicular Zip6 and Zip10 Abundance and Impairs Spermatogenesis in Mice. J. Nutr. 2011, 141, 359–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowanadisai, W.; Kelleher, S.L.; Lönnerdal, B. Zinc Deficiency Is Associated with Increased Brain Zinc Import and LIV-1 Expression and Decreased ZnT-1 Expression in Neonatal Rats. J. Nutr. 2005, 135, 1002–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowanadisai, W.; Lönnerdal, B.; Kelleher, S.L. Zip6 (LIV-1) Regulates Zinc Uptake in Neuroblastoma Cells under Resting but Not Depolarizing Conditions. Brain Res. 2008, 1199, 10–19. [Google Scholar] [CrossRef]
- Lee, W.-W.; Cui, D.; Czesnikiewicz-Guzik, M.; Vencio, R.Z.N.; Shmulevich, I.; Aderem, A.; Weyand, C.M.; Goronzy, J.J. Age-Dependent Signature of Metallothionein Expression in Primary CD4 T Cell Responses Is Due to Sustained Zinc Signaling. Rejuvenation Res. 2008, 11, 1001–1011. [Google Scholar] [CrossRef]
- Yu, M.; Lee, W.-W.; Tomar, D.; Pryshchep, S.; Czesnikiewicz-Guzik, M.; Lamar, D.L.; Li, G.; Singh, K.; Tian, L.; Weyand, C.M.; et al. Regulation of T Cell Receptor Signaling by Activation-Induced Zinc Influx. J. Exp. Med. 2011, 208, 775–785. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Tan, J.; Li, D.; Jiang, L.; Li, T.; Yang, Y.; Wang, G.; Shang, Z.; Wang, J.; Zhou, J. SLC39A6/ZIP6 Is Essential for Zinc Homeostasis and T-Cell Development in Zebrafish. Biochem. Biophys. Res. Commun. 2019, 511, 896–902. [Google Scholar] [CrossRef]
- Huang, L.; Kirschke, C.P.; Zhang, Y.; Yu, Y.Y. The ZIP7 Gene (Slc39a7) Encodes a Zinc Transporter Involved in Zinc Homeostasis of the Golgi Apparatus. J. Biol. Chem. 2005, 280, 15456–15463. [Google Scholar] [CrossRef] [Green Version]
- Woodruff, G.; Bouwkamp, C.G.; de Vrij, F.M.; Lovenberg, T.; Bonaventure, P.; Kushner, S.A.; Harrington, A.W. The Zinc Transporter SLC39A7 (ZIP7) Is Essential for Regulation of Cytosolic Zinc Levels. Mol. Pharmacol. 2018, 94, 1092–1100. [Google Scholar] [CrossRef]
- Ollig, J.; Kloubert, V.; Taylor, K.M.; Rink, L. B Cell Activation and Proliferation Increase Intracellular Zinc Levels. J. Nutr. Biochem. 2019, 64, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Li, L.-L.; Zhang, S.-Q.; Ni, H. Long-Term Effects of Ketogenic Diet on Subsequent Seizure-Induced Brain Injury During Early Adulthood: Relationship of Seizure Thresholds to Zinc Transporter-Related Gene Expressions. Biol. Trace Elem. Res. 2016, 174, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-L.; Jin, M.-F.; Ni, H. Zinc/CaMK II Associated-Mitophagy Signaling Contributed to Hippocampal Mossy Fiber Sprouting and Cognitive Deficits Following Neonatal Seizures and Its Regulation by Chronic Leptin Treatment. Front. Neurol. 2018, 9, 802. [Google Scholar] [CrossRef] [PubMed]
- Grubman, A.; Lidgerwood, G.E.; Duncan, C.; Bica, L.; Tan, J.-L.; Parker, S.J.; Caragounis, A.; Meyerowitz, J.; Volitakis, I.; Moujalled, D.; et al. Deregulation of Subcellular Biometal Homeostasis through Loss of the Metal Transporter, Zip7, in a Childhood Neurodegenerative Disorder. Acta Neuropathol. Commun. 2014, 2, 25. [Google Scholar] [CrossRef] [Green Version]
- Kanninen, K.M.; Grubman, A.; Caragounis, A.; Duncan, C.; Parker, S.J.; Lidgerwood, G.E.; Volitakis, I.; Ganio, G.; Crouch, P.J.; White, A.R. Altered Biometal Homeostasis Is Associated with CLN6 MRNA Loss in Mouse Neuronal Ceroid Lipofuscinosis. Biol. Open 2013, 2, 635–646. [Google Scholar] [CrossRef] [Green Version]
- Begum, N.A.; Kobayashi, M.; Moriwaki, Y.; Matsumoto, M.; Toyoshima, K.; Seya, T. Mycobacterium Bovis BCG Cell Wall and Lipopolysaccharide Induce a Novel Gene, BIGM103, Encoding a 7-TM Protein: Identification of a New Protein Family Having Zn-Transporter and Zn-Metalloprotease Signatures. Genomics 2002, 80, 630–645. [Google Scholar] [CrossRef]
- He, L.; Girijashanker, K.; Dalton, T.P.; Reed, J.; Li, H.; Soleimani, M.; Nebert, D.W. ZIP8, Member of the Solute-Carrier-39 (SLC39) Metal-Transporter Family: Characterization of Transporter Properties. Mol. Pharmacol. 2006, 70, 171–180. [Google Scholar] [CrossRef]
- Nebert, D.W.; Gálvez-Peralta, M.; Hay, E.B.; Li, H.; Johansson, E.; Yin, C.; Wang, B.; He, L.; Soleimani, M. ZIP14 and ZIP8 Zinc/Bicarbonate Symporters in Xenopus Oocytes: Characterization of Metal Uptake and Inhibition. Met. Integr. Biometal Sci. 2012, 4, 1218–1225. [Google Scholar] [CrossRef]
- Dalton, T.P.; He, L.; Wang, B.; Miller, M.L.; Jin, L.; Stringer, K.F.; Chang, X.; Baxter, C.S.; Nebert, D.W. Identification of Mouse SLC39A8 as the Transporter Responsible for Cadmium-Induced Toxicity in the Testis. Proc. Natl. Acad. Sci. USA 2005, 102, 3401–3406. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; He, L.; Dong, H.; Dalton, T.P.; Nebert, D.W. Generation of a Slc39a8 Hypomorph Mouse: Markedly Decreased ZIP8 Zn2+/(HCO3−)2 Transporter Expression. Biochem. Biophys. Res. Commun. 2011, 410, 289–294. [Google Scholar] [CrossRef] [Green Version]
- Hermann, E.R.; Chambers, E.; Davis, D.N.; Montgomery, M.R.; Lin, D.; Chowanadisai, W. Brain Magnetic Resonance Imaging Phenome-Wide Association Study With Metal Transporter Gene SLC39A8. Front. Genet. 2021, 12, 647946. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Chen, Q.; Wang, W.; Desrivières, S.; Quinlan, E.B.; Jia, T.; Macare, C.; Robert, G.H.; Cui, J.; Guedj, M.; et al. Association of a Schizophrenia-Risk Nonsynonymous Variant With Putamen Volume in Adolescents: A Voxelwise and Genome-Wide Association Study. JAMA Psychiatry 2019, 76, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Carrera, N.; Arrojo, M.; Sanjuán, J.; Ramos-Ríos, R.; Paz, E.; Suárez-Rama, J.J.; Páramo, M.; Agra, S.; Brenlla, J.; Martínez, S.; et al. Association Study of Nonsynonymous Single Nucleotide Polymorphisms in Schizophrenia. Biol. Psychiatry 2012, 71, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Jian, X.; Chen, J.; Li, Z.; Song, Z.; Zhou, J.; Xu, W.; Liu, Y.; Shen, J.; Wang, Y.; Yi, Q.; et al. SLC39A8 Is a Risk Factor for Schizophrenia in Uygur Chinese: A Case-Control Study. BMC Psychiatry 2019, 19, 293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Wu, D.-D.; Yao, Y.-G.; Huo, Y.-X.; Liu, J.-W.; Su, B.; Chasman, D.I.; Chu, A.Y.; Huang, T.; Qi, L.; et al. Recent Positive Selection Drives the Expansion of a Schizophrenia Risk Nonsynonymous Variant at SLC39A8 in Europeans. Schizophr. Bull. 2016, 42, 178–190. [Google Scholar] [CrossRef] [Green Version]
- McCoy, T.H.; Pellegrini, A.M.; Perlis, R.H. Using Phenome-Wide Association to Investigate the Function of a Schizophrenia Risk Locus at SLC39A8. Transl. Psychiatry 2019, 9, 45. [Google Scholar] [CrossRef] [Green Version]
- Mealer, R.G.; Jenkins, B.G.; Chen, C.-Y.; Daly, M.J.; Ge, T.; Lehoux, S.; Marquardt, T.; Palmer, C.D.; Park, J.H.; Parsons, P.J.; et al. The Schizophrenia Risk Locus in SLC39A8 Alters Brain Metal Transport and Plasma Glycosylation. Sci. Rep. 2020, 10, 13162. [Google Scholar] [CrossRef]
- Tseng, W.C.; Reinhart, V.; Lanz, T.A.; Weber, M.L.; Pang, J.; Le, K.X.V.; Bell, R.D.; O’Donnell, P.; Buhl, D.L. Schizophrenia-Associated SLC39A8 Polymorphism Is a Loss-of-Function Allele Altering Glutamate Receptor and Innate Immune Signaling. Transl. Psychiatry 2021, 11, 136. [Google Scholar] [CrossRef]
- Thomas, P.; Converse, A.; Berg, H.A. ZIP9, a Novel Membrane Androgen Receptor and Zinc Transporter Protein. Gen. Comp. Endocrinol. 2018, 257, 130–136. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Chen, H.; Chen, M.; Mi, S.; Ma, J.; Wang, C.; Sun, H.; Liu, X.; Cui, H. Non-Genomic Mechanisms Mediate Androgen-Induced PSD95 Expression. Aging 2019, 11, 2281–2294. [Google Scholar] [CrossRef]
- Zheng, D.; Feeney, G.P.; Kille, P.; Hogstrand, C. Regulation of ZIP and ZnT Zinc Transporters in Zebrafish Gill: Zinc Repression of ZIP10 Transcription by an Intronic MRE Cluster. Physiol. Genom. 2008, 34, 205–214. [Google Scholar] [CrossRef] [Green Version]
- Kagara, N.; Tanaka, N.; Noguchi, S.; Hirano, T. Zinc and Its Transporter ZIP10 Are Involved in Invasive Behavior of Breast Cancer Cells. Cancer Sci. 2007, 98, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.Y.; Duncan, F.E.; Que, E.L.; Kim, A.M.; O’Halloran, T.V.; Woodruff, T.K. Maternally-Derived Zinc Transporters ZIP6 and ZIP10 Drive the Mammalian Oocyte-to-Egg Transition. Mol. Hum. Reprod. 2014, 20, 1077–1089. [Google Scholar] [CrossRef] [PubMed]
- Brethour, D.; Mehrabian, M.; Williams, D.; Wang, X.; Ghodrati, F.; Ehsani, S.; Rubie, E.A.; Woodgett, J.R.; Sevalle, J.; Xi, Z.; et al. A ZIP6-ZIP10 Heteromer Controls NCAM1 Phosphorylation and Integration into Focal Adhesion Complexes during Epithelial-to-Mesenchymal Transition. Sci. Rep. 2017, 7, 40313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaler, P.; Prasad, R. Molecular Cloning and Functional Characterization of Novel Zinc Transporter RZip10 (Slc39a10) Involved in Zinc Uptake across Rat Renal Brush-Border Membrane. Am. J. Physiol. Ren. Physiol. 2007, 292, F217–F229. [Google Scholar] [CrossRef] [Green Version]
- Bagheri-Fam, S.; Ferraz, C.; Demaille, J.; Scherer, G.; Pfeifer, D. Comparative Genomics of the SOX9 Region in Human and Fugu Rubripes: Conservation of Short Regulatory Sequence Elements within Large Intergenic Regions. Genomics 2001, 78, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.M.; Nicholson, R.I. The LZT Proteins; the LIV-1 Subfamily of Zinc Transporters. Biochim. Biophys. Acta 2003, 1611, 16–30. [Google Scholar] [CrossRef] [Green Version]
- Davis, D.N.; Strong, M.D.; Chambers, E.; Hart, M.D.; Bettaieb, A.; Clarke, S.L.; Smith, B.J.; Stoecker, B.J.; Lucas, E.A.; Lin, D.; et al. A Role for Zinc Transporter Gene SLC39A12 in the Nervous System and Beyond. Gene 2021, 799, 145824. [Google Scholar] [CrossRef]
- Guo, L.; Hu, X.; Xu, T.; Qi, X.; Wan, Y.; Liu, X.; Jiang, Y.; Zhang, L. Over-Expression of Zip-13 MRNA in Kidney and Lung during Dietary Zinc Deficiency in Wistar Rats. Mol. Biol. Rep. 2011, 38, 1869–1874. [Google Scholar] [CrossRef]
- Taylor, K.M.; Morgan, H.E.; Johnson, A.; Nicholson, R.I. Structure-Function Analysis of a Novel Member of the LIV-1 Subfamily of Zinc Transporters, ZIP14. FEBS Lett. 2005, 579, 427–432. [Google Scholar] [CrossRef] [Green Version]
- Jenkitkasemwong, S.; Wang, C.-Y.; Mackenzie, B.; Knutson, M.D. Physiologic Implications of Metal-Ion Transport by ZIP14 and ZIP8. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2012, 25, 643–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liuzzi, J.P.; Lichten, L.A.; Rivera, S.; Blanchard, R.K.; Aydemir, T.B.; Knutson, M.D.; Ganz, T.; Cousins, R.J. Interleukin-6 Regulates the Zinc Transporter Zip14 in Liver and Contributes to the Hypozincemia of the Acute-Phase Response. Proc. Natl. Acad. Sci. USA 2005, 102, 6843–6848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Homma, K.; Fujisawa, T.; Tsuburaya, N.; Yamaguchi, N.; Kadowaki, H.; Takeda, K.; Nishitoh, H.; Matsuzawa, A.; Naguro, I.; Ichijo, H. SOD1 as a Molecular Switch for Initiating the Homeostatic ER Stress Response under Zinc Deficiency. Mol. Cell 2013, 52, 75–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridlo, M.R.; Kim, G.A.; Taweechaipaisankul, A.; Kim, E.H.; Lee, B.C. Zinc Supplementation Alleviates Endoplasmic Reticulum Stress during Porcine Oocyte in Vitro Maturation by Upregulating Zinc Transporters. J. Cell. Physiol. 2021, 236, 2869–2880. [Google Scholar] [CrossRef]
- Anagianni, S.; Tuschl, K. Genetic Disorders of Manganese Metabolism. Curr. Neurol. Neurosci. Rep. 2019, 19, 33. [Google Scholar] [CrossRef] [Green Version]
- Runnels, L.W.; Yue, L.; Clapham, D.E. TRP-PLIK, a Bifunctional Protein with Kinase and Ion Channel Activities. Science 2001, 291, 1043–1047. [Google Scholar] [CrossRef]
- Schmitz, C.; Perraud, A.-L.; Johnson, C.O.; Inabe, K.; Smith, M.K.; Penner, R.; Kurosaki, T.; Fleig, A.; Scharenberg, A.M. Regulation of Vertebrate Cellular Mg2+ Homeostasis by TRPM7. Cell 2003, 114, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Monteilh-Zoller, M.K.; Hermosura, M.C.; Nadler, M.J.S.; Scharenberg, A.M.; Penner, R.; Fleig, A. TRPM7 Provides an Ion Channel Mechanism for Cellular Entry of Trace Metal Ions. J. Gen. Physiol. 2003, 121, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Krapivinsky, G.; Krapivinsky, L.; Manasian, Y.; Clapham, D.E. The TRPM7 Chanzyme Is Cleaved to Release a Chromatin-Modifying Kinase. Cell 2014, 157, 1061–1072. [Google Scholar] [CrossRef] [Green Version]
- Inoue, K.; Branigan, D.; Xiong, Z.-G. Zinc-Induced Neurotoxicity Mediated by Transient Receptor Potential Melastatin 7 Channels. J. Biol. Chem. 2010, 285, 7430–7439. [Google Scholar] [CrossRef] [Green Version]
- Vargas-Poussou, R.; Claverie-Martin, F.; Prot-Bertoye, C.; Carotti, V.; van der Wijst, J.; Perdomo-Ramirez, A.; Fraga-Rodriguez, G.M.; Hureaux, M.; Bos, C.; Latta, F.; et al. Possible Role for Rare TRPM7 Variants in Patients with Hypomagnesemia with Secondary Hypocalcemia. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.—Eur. Ren. Assoc. 2022, gfac182. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Desai, B.N.; Navarro, B.; Donovan, A.; Andrews, N.C.; Clapham, D.E. Deletion of Trpm7 Disrupts Embryonic Development and Thymopoiesis without Altering Mg2+ Homeostasis. Science 2008, 322, 756–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Su, L.-T.; Khadka, D.K.; Mezzacappa, C.; Komiya, Y.; Sato, A.; Habas, R.; Runnels, L.W. TRPM7 Regulates Gastrulation during Vertebrate Embryogenesis. Dev. Biol. 2011, 350, 348–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgiev, P.; Okkenhaug, H.; Drews, A.; Wright, D.; Lambert, S.; Flick, M.; Carta, V.; Martel, C.; Oberwinkler, J.; Raghu, P. TRPM Channels Mediate Zinc Homeostasis and Cellular Growth during Drosophila Larval Development. Cell Metab. 2010, 12, 386–397. [Google Scholar] [CrossRef] [Green Version]


| Age | Male | Female | Pregnancy | Lactation | |
|---|---|---|---|---|---|
| Infants | 0–6 months | 2 mg * | 2 mg * | ||
| 7–12 months | 3 mg | 3 mg | |||
| Children | 1–3 years | 3 mg | 3 mg | ||
| 4–8 years | 5 mg | 5 mg | |||
| 9–13 years | 8 mg | 8 mg | |||
| 14–18 years | 11 mg | 9 mg | 13 mg | 14 mg | |
| ≥18 years | 11 mg | 8 mg | 11 mg | 12 mg |
| Food | Weight (g) | Measure | Milligrams (mg) Per Serving | Percent DV * |
|---|---|---|---|---|
| Oysters, eastern, wild, raw | 84 | 6.0 medium | 33 | 300 |
| Beef chuck, blade roast, separable lean and fat, trimmed to 0” fat, all grades, cooked, braised | 235 | 1.0 piece, cooked, excluding refuse (yield from 1 lb raw meat with refuse) | 20.16 | 183 |
| Soybeans, mature seeds, raw | 186 | 1.0 cup | 9.1 | 83 |
| Lamb, domestic, rib, separable lean only, trimmed to 1/4” fat, choice, cooked, broiled | 147 | 1.0 piece, cooked, excluding refuse (yield from 1 lb raw meat with refuse) | 7.75 | 70 |
| Nuts, cashew nuts, dry roasted, with salt added | 137 | 1.0 cup, halves and whole | 7.67 | 70 |
| Peas, green, split, mature seeds, raw | 197 | 1.0 cup | 6.99 | 63 |
| Oats | 156 | 1.0 cup | 6.19 | 56 |
| Crustaceans, spiny lobster, mixed species, cooked, moist heat | 85 | 3.0 oz | 6.18 | 56 |
| Cocoa dry powder, unsweetened | 86 | 1.0 cup | 5.86 | 53 |
| Desserts, mousse, chocolate, prepared-from-recipe | 808 | 1.0 recipe yield | 5.17 | 47 |
| Cheese, pasteurized process, swiss | 140 | 1.0 cup | 5.05 | 46 |
| Pork, fresh, shoulder, whole, separable lean and fat, cooked, roasted | 135 | 1.0 cup | 5.01 | 46 |
| Chicken, broilers or fryers, dark meat, drumstick, meat only, cooked, roasted | 96 | 1.0 drumstick without skin | 2.46 | 12 |
| Pepperoni, beef and pork, sliced | 85 | 3.0 oz | 2.07 | 19 |
| Yogurt, vanilla, non-fat | 245 | 8 fl oz | 2.03 | 18 |
| Fast foods, taco with beef, cheese and lettuce, soft | 102 | 1.0 each taco | 1.4 | 13 |
| Fish, tuna, light, canned in oil, drained solids | 146 | 1.0 cup | 1.31 | 12 |
| Milk, reduced fat, fluid, 2% milkfat, with added nonfat milk solids and vitamin A and vitamin D | 245 | 1.0 cup | 0.98 | 9 |
| Bananas, raw | 225 | 1.0 cup | 0.34 | 3 |
| Lettuce, butterhead (includes boston and bibb types), raw | 55 | 1.0 cup | 0.11 | 1 |
| Cabbage, common (danish, domestic, and pointed types), stored, raw | 35 | 0.5 cup | 0.06 | 0.5 |
| Protein | Gene | Subcellular Localization | Tissue Expression | Phenotypes of KO or Mutant Animals | Human Inherited Diseases Associated with Mutations |
|---|---|---|---|---|---|
| ZnT1 | SLC30A1 | Plasma membrane (basal pole of enterocytes) [137] | Ubiquitous [138] | KO: embryonic lethal [139] Heterozygous KO: Higher sensitivity to maternal Zn deficiency [139] | |
| ZnT2 | SLC30A2 | Endosome and lysosome [140] | Human: pancreas [141], mammary gland [142], prostate [143], kidney [144], small intestine [138] Rat: enterocytes [145] | KO: Electrophysiological abnormalities of mossy fiber synapses onto CA3 pyramidal neurons [146] | Transient Infantile Zinc Deficiency (TIZD) [147] |
| ZnT3 | SLC30A3 | Synaptic vesicles [148,149] | Brain [150]: synaptic vesicles of glutamatergic neurons of hippocampus [151,152] and cerebral cortex [151,152], testis [150], epididymis [138] | KO: Fear memory decrease [153] Long-term memory impairment in males [154] Spatial navigation and memory defects [154,155] Autism-like phenotype in males: decreased social interaction, decreased locomotion and increased anxiety-like behavior [156] | |
| ZnT4 | SLC30A4 | Plasma membrane, intracellular vesicles [144,157] | Human: wide expression, enriched in brain (hippocampus and caudate), thyroid, lung, testis, heart, skin, pancreas [138] Mouse: mammary gland [158,159] Human and mouse: SLC30A4 transcript expression in small intestine but not protein [138,160] | Mutations: low zinc in breast milk (lethal milk mouse) [158,159,161] | |
| ZnT5 | SLC30A5 | Golgi [162], plasma membrane [163,164], cytoplasmic vesicles [165] | Ubiquitous [138] with higher levels in stomach (parietal cells), duodenum and jejunum [166] | Homozygous loss of function: perinatal death, cardiomyopathy, hydrops fetalis, cystic hygroma [167] KO: growth defects, osteopenia, muscle weakness and male-specific sudden cardiac death [168] | |
| ZnT6 | SLC30A6 | Golgi, intracellular vesicles [169] | Human: ubiquitous [138] Mouse: high levels in brain, lung and small intestine [169] (duodenum and jejunum) [166] | - | |
| ZnT7 | SLC30A7 | Golgi, intracellular vesicles [170] | Human: ubiquitous with higher levels in small intestine and colon, but low levels in the brain [138] Mouse: SLC30A7 transcript expression in liver, kidney, spleen and small intestine [170] but protein only detected in duodenum and jejunum (high) and along the gastrointestinal tract (lower expression) [166] | KO: Irreversible growth retardation, small weight, zinc deficiency [171] | |
| ZnT8 | SLC30A8 | Secretory granules [172,173] | Human: pancreas [172] Mouse: pancreatic beta and alpha islet cells, thyroid, adrenal gland [173] | Pancreatic function defects [172,173,174,175,176] Increased adiposity [177] | |
| ZnT9 | SLC30A9 | Cytoplasm, nucleus [178,179,180]; endoplasmic reticulum [181] | Ubiquitous [138] with higher levels in cerebellum, skeletal muscles, thymus and kidney [181] | No KO model, but (c.1047_1049delGCA, p.(A350del)) mutation causes neurological alterations and severe intellectual disability [181] | |
| ZnT10 | SLC30A10 | Golgi, plasma membrane [182] | Small intestine, liver, cerebral cortex [138,182], and retina [181] | No changes in plasma or tissue Zn concentration, but high manganese contents in plasma, brain and liver [183] | Juvenile-onset dystonia, adult-onset parkinsonism, severe hypermanganesemia, polycythemia, and chronic hepatic disease (steatosis and cirrhosis) [184,185] |
| Protein | Gene | Subcellular Localization | Tissue Expression | Phenotypes of KO or Mutant Animals | Human Inherited Diseases Associated with Mutations |
|---|---|---|---|---|---|
| ZIP1 | SLC39A1 | Plasma membrane [186], endoplasmic reticulum [186], intracellular vesicles [187,188] | Ubiquitous [138] with higher levels in small intestine human [186] and brain (hippocampus, thalamus and cerebral cortex) [189] | KO: higher sensitivity to zinc deficiency causing developmental malformations and increased lethality [190,191,192] | |
| ZIP2 | SLC39A2 | Wide expression: brain (cortex, cerebellum, basal ganglia), endocrine tissues, lungs, gastrointestinal tract, liver, pancreas, kidney, heart [138] | KO: higher sensitivity to zinc deficiency causing developmental malformations [193] | ||
| ZIP3 | SLC39A3 | Golgi (zinc sufficient conditions), plasma membrane (zinc depletion) [194] | Wide expression with higher levels along gastrointestinal tract [138] | KO: higher sensitivity to zinc deficiency causing developmental malformations [195] ZIP1-ZIP3 double-KO: high sensitivity to zinc deficiency and higher rates of developmental malformations than ZIP1 single KO [190] ZIP1-ZIP2-ZIP3 triple-KO: higher sensitivity to zinc deficiency and 80% of developmental malformation (with 60% of severe defects) [191] | |
| ZIP4 | SLC39A4 | Intracellular vesicles (zinc sufficient conditions), plasma membrane (zinc depletion) [196,197,198] | Human: duodenum, jejunum [23,56,138] (Enterocytes [197,198,199,200]), pancreatic β cells Rat: in vitro cultures of astrocytes and neurons [201] | Homozygous KO: early embryonic lethal [202] Heterozygous KO: developmental defects (severe growth retardation, exencephaly), higher sensitivity to zinc deficiency [202] Intestinal epithelium conditional KO: intestinal stem-cell niche disruption; epithelium disorganization [203] | Acrodermatitis enteropathica (AE): “genetic zinc deficiency” [23,54,55,56] |
| ZIP5 | SLC39A5 | Plasma membrane (basolateral pole of polarized cells) [200,204], intracellular vesicles [200,205] | Liver, pancreas [204], peripheral blood mononuclear cells [206] | KO: zinc accumulation in liver and pancreas [207] | |
| ZIP6 | SLC39A6 | Plasma membrane [208] | Ubiquitous with higher levels in cerebellum, adrenal gland, endometrium [138] | - | |
| ZIP7 | SLC39A7 | Golgi, endoplasmic reticulum [209] | Wide expression with higher levels in endocrine tissues, lung, bone marrow, lymphoid tissues, brain (hippocampus, cerebral cortex, basal ganglia, cerebellum), kidney, gastrointestinal tract [138] | Hypomorphic: developmental defects, B-cell maturation arrest [210] KO: embryonic lethal [210] KD (Zebrafish): developmental defects, reduction in brain zinc contents [211] | Hypomorphic mutations of SLC39A7 (autosomal recessive): absent B cells, agammaglobulinemia, early onset infections [210] |
| ZIP8 | SLC39A8 | Plasma membrane (apical pole of polarized cells) [212], cytoplasm [213] | Wide expression with higher levels in lung, kidney [214], gastrointestinal tract [138] | Hypomorphic: growth retardation, developmental failure [215], late developmental death [216] KO: heart morphology defects [217] | Congenital disorder of glycosylation, type IIn (autosomal recessive): intellectual disability, cerebellar atrophy, low blood zinc and manganese, zinc and manganese renal wasting [218,219] |
| ZIP9 | SLC39A9 | Golgi, intracellular vesicles [220] | Wide expression with higher levels in brain (cortex, cerebellum, hippocampus), gastrointestinal tract, kidney [138] | KO (Zebrafish): developmental abnormalities, decreased viability, zinc homeostasis disruption [221] | Autosomal recessive cerebro-renal symptom: early neurological deterioration, severe intellectual disability, ataxia, camptocormia, early-onset nephropathy, hypertension [181] |
| ZIP10 | SLC39A10 | Golgi [182], plasma membrane [182,222,223] | Male (seminal vesicle, prostate) and female (endometrium, fallopian tube) sexual tissues, lung, thyroid [138], kidney [224] | - | |
| ZIP11 | SLC39A11 | Golgi [225], nucleus [226] | Mammary gland [225], colon [226], spleen [227] | - | |
| ZIP12 | SLC39A12 | Golgi (zinc sufficient conditions), plasma membrane (zinc deficient conditions) [228] | Brain [228] | KO (Zebrafish): neural tube closure defects, reduced viability [228] | |
| ZIP13 | SLC39A13 | Golgi, endoplasmic reticulum [229] | Wide expression, with higher levels in esophagus, skeletal muscle, skin, tonsil [138] | KO: osteogenesis and chondrogenesis defects, abnormal maturation of odontoblasts and fibroblasts [230] | Spondylocheirodysplastic form of Ehlers-Danlos syndrome (autosomal recessive): progressive kyphoscoliosis, hypermobility of joints, hyperelasticity of skin, severe hypotonia of skeletal muscles [231,232] |
| ZIP14 | SLC39A14 | Plasma membrane (basolateral pole of enterocytes) [233], endosome, lysosome [233,234] | Wide expression: digestive and gastrointestinal tract [233], pancreas, kidney, muscles, endocrine tissues [138] with highest levels in liver [235] Isoform 1: ubiquitous [236] Isoform 2: not expressed in brain, heart, skeletal muscle, and skin [236] | KO: Growth retardation, impaired gluconeogenesis [237] Intestinal zinc accumulation in endosomes, higher permeability of intestinal membrane, dysregulation of tight junction protein expression [233] Manganese excretion defect, liver and brain (neurotoxicity) manganese accumulation, manganese deficit in other tissues, motor deficit [238] | Childhood-onset dystonia parkinsonism: hypermanganesemia, neurodegeneration [236] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Willekens, J.; Runnels, L.W. Impact of Zinc Transport Mechanisms on Embryonic and Brain Development. Nutrients 2022, 14, 2526. https://doi.org/10.3390/nu14122526
Willekens J, Runnels LW. Impact of Zinc Transport Mechanisms on Embryonic and Brain Development. Nutrients. 2022; 14(12):2526. https://doi.org/10.3390/nu14122526
Chicago/Turabian StyleWillekens, Jeremy, and Loren W. Runnels. 2022. "Impact of Zinc Transport Mechanisms on Embryonic and Brain Development" Nutrients 14, no. 12: 2526. https://doi.org/10.3390/nu14122526