G Protein-Coupled Receptors and Their Kinases in Cell Biology and Disease (Closed)
A topical collection in International Journal of Molecular Sciences (ISSN 1422-0067). This collection belongs to the section "Molecular Biology".
Viewed by 25506Editor
Interests: cardiovascular disease; G protein coupled receptor; neurodegenerative disorders; periodontitis; pharmacology
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
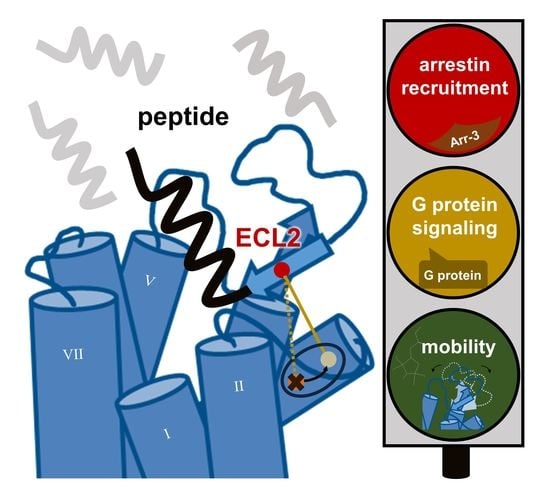
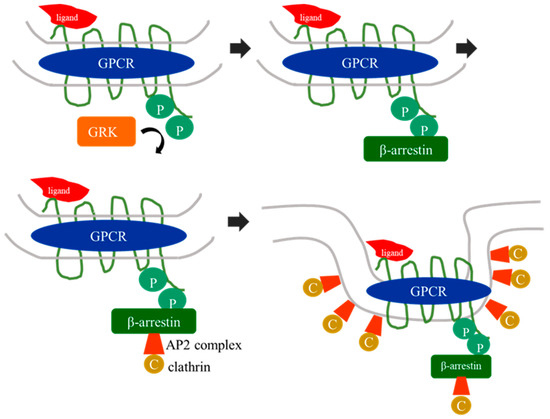
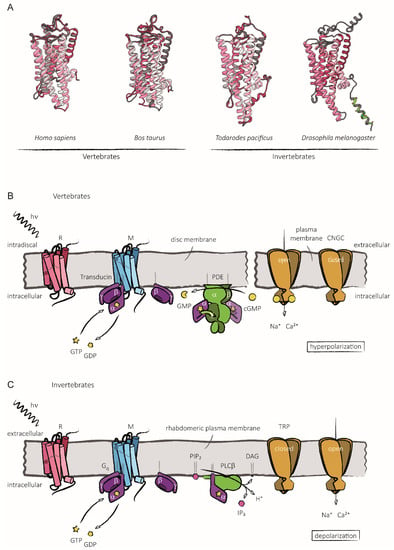
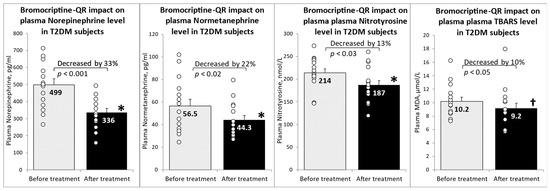
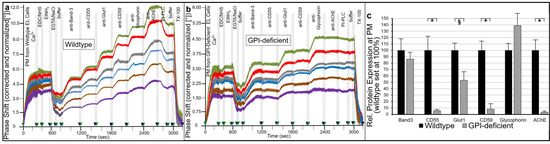
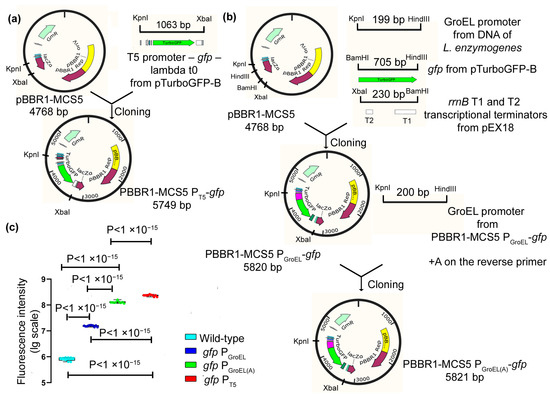
Over the past three decades, since Nobel prize winners Robert Lefkowitz and Brian Kobilka characterized the structure of G protein-coupled receptors (GPCRs), a great deal of clinical and pharmacological evidence has advanced our knowledge of how these receptors and their signaling pathways influence almost every aspect of mammals’ physiology. GPCRs can transduce cellular signals from neurohormones, sensory stimuli, and ions, and their activity is directly modulated by GPCR kinases (GRKs), phosphorylation, and subsequent desensitization. Nevertheless, an alteration in GRKs’ expression, with subsequent GPCR dysfunction, may induce—or at least influence—the development and progression of different systemic disorders. Thus, several drugs which are able to directly inhibit or enhance GPCR signaling have been developed and are currently used in clinical practice.
This Topical Collection calls for both original articles and reviews providing readers of IJMS with a comprehensive elucidation of the GPCR and GRK functions in cell biology that is necessary for developing novel research approaches as well as therapeutic strategies.
Dr. Alessandro Cannavo
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. International Journal of Molecular Sciences is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. There is an Article Processing Charge (APC) for publication in this open access journal. For details about the APC please see here. Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- Cell biology
- Cardiovascular disease
- G protein-coupled receptor
- GRK ischemia
- Neurodegeneration
- Inflammation
- Metabolism
- Pharmacology