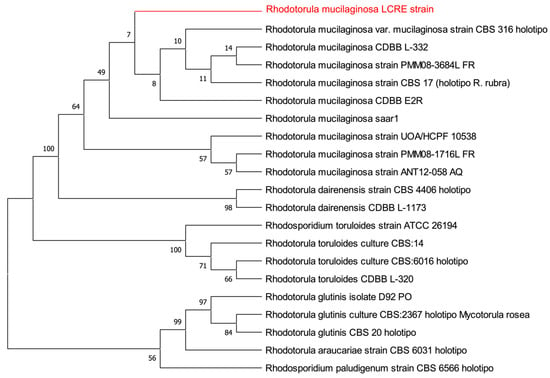
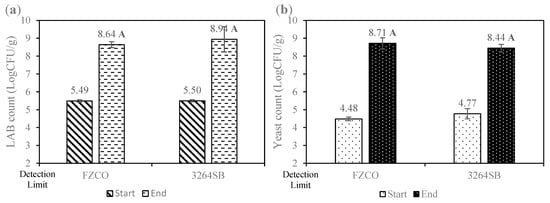
Yeast Biotechnology
A topical collection in Fermentation (ISSN 2311-5637). This collection belongs to the section "Microbial Metabolism, Physiology & Genetics".
Viewed by 1636Editor
Interests: yeast biotechnology; cell immobilization; beer brewing biochemistry and fermentation; mini- and microbioreactors; Saccharomyces cerevisiae; Candida; yeast space biology (bioreactors for microgravity research); yeast adhesins; yeast systems biology; glycobiology; nanobiotechnology; atomic force microscopy; protein crystallization; yeast protein structural biology
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
Yeasts are truly fascinating microorganisms. Due to their diverse and dynamic activities, they have been used to produce many interesting products, such as beer, wine, bread, biofuels and biopharmaceuticals. Saccharomyces cerevisiae (bakers’ yeast) is likely the most human-exploited yeast species. Saccharomyces is a popular choice for industrial applications, although its use in beer production dates to at least the sixth millennium BC. Bakers’ yeast represents a cornerstone of modern biotechnology, enabling the development of efficient production processes for antibiotics, biopharmaceuticals, technical enzymes, and ethanol and biofuels.
Today, diverse yeast species are explored for industrial applications, such as, e.g., the Saccharomyces species, Pichia pastoris and other Pichia species, Kluyveromyces marxianus, Hansenula polymorpha, Yarrowia lipolytica, Candida species, Phaffia rhodozyma, wild yeasts for beer brewing and winemaking, and others with proven potential.
Yeast cells can also be benign for humans, since some yeast species can be pathogenic and cause infections, especially in individuals with weakened immune systems. Strategies to fight pathogenic yeasts have been developed through recent endeavors, such as the development of synthetic antifungal agents, the use of natural products with antifungal activity, and the use of yeasts with antimicrobial infections. Other strategies such as biocontrol agents, reactive oxygen species and RNA interference have been developed to combat pathogenic yeasts. These approaches aim to inhibit the growth and survival of pathogenic yeasts, thereby preventing and treating yeast infections.
This Topical Collection, “Yeast Biotechnology”, is a continuation of the Special Issues “Yeast Biotechnology” series of Fermentation (published by MDPI). This installment will compile the current state-of-the-art research and technology in the area of yeast biotechnology, and highlight the prominent research directions for hot topics, such as recently developed techniques for characterizing yeast and their physiology (including omics and nanobiotechnology techniques), methods for adapting industrial strains (including metabolic, synthetic and evolutionary engineering) and the use of yeasts as microbial cell factories to produce biopharmaceuticals, enzymes, alcohols, organic acids, flavors and fine chemicals, advances in yeast fermentation technology and industrial fermentation processes, as well as yeast biotechnology strategies in fighting pathogenic yeasts.
Topics of interest include, but are not limited to:
Yeast characterization and analysis:
Brewing yeasts (including wild yeasts), wine yeasts and baker’s yeasts;
Evolution and variation in industrial yeast genomes;
Yeast systems biology: genomics, proteomics, fluxomics, metabolomics, and omics integration;
Yeast nanobiotechnology (nano-analysis techniques, construction of nanostructures, etc.).
Yeast strain engineering:
Yeast metabolic engineering: production of biofuels, secondary metabolites, commodity chemicals, proteins, biopharmaceuticals and material precursors;
Yeast synthetic biology: yeasts as cell factories, tools for controlling enzyme expression levels, strategies for regulating spatial localization of enzymes in yeast, regulatory networks and biomolecular logic gates;
Strain improvement via evolutionary engineering.
Fermentation technology:
Industrial bioreactors;
Mini- and micro-bioreactors: single-cell analysis, high-throughput screening and microfluidic bioreactors;
Process intensification: high-density fermentations, high-gravity fermentation, yeast cell immobilization;
Fermentative stress adaptation.
Industrial fermentation processes:
Production of food (bread, etc.) and beverages (beer, wine, cider, etc.);
Production of bakers’ yeast;
Production of biofuels (bioethanol, 1-butanol, biodiesel, jetfuels), commodity chemicals, pharmaceuticals, material precursors, and secondary metabolites.
Fight against pathogenic yeasts:
Antifungal agents, yeast killer toxins, probiotic yeasts, and biocontrol agent development and assessment.
Prof. Dr. Ronnie Willaert
Guest Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Fermentation is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2600 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- systems biology
- genomics
- proteomics
- fluxomics
- metabolomics
- synthetic yeast biology
- metabolic engineering
- evolutionary engineering
- industrial yeast products
- beer
- wine
- bread
- biofuels
- commodity chemical
- biopharmaceuticals
- material precursors
- yeast fermentation technology
- industrial bioreactors
- mini- and microbioreactors
- high-density fermentations
- yeast stress adaptation
- yeast biotechnology to fight pathogenic yeasts
Related Special Issues
- Yeast Biotechnology 1.0 in Fermentation (11 articles)
- Yeast Biotechnology 2.0 in Fermentation (14 articles)
- Yeast Biotechnology 3.0 in Fermentation (16 articles)
- Yeast Biotechnology 4.0 in Fermentation (10 articles)
- Yeast Biotechnology 5.0 in Fermentation (15 articles)
- Yeast Biotechnology 6.0 in Fermentation (11 articles)