Journal Description
Electrochem
Electrochem
is an international, peer-reviewed, open access journal on electrochemistry published quarterly online by MDPI.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within Scopus, CAPlus / SciFinder, and other databases.
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 22.3 days after submission; acceptance to publication is undertaken in 6.9 days (median values for papers published in this journal in the second half of 2023).
- Recognition of Reviewers: APC discount vouchers, optional signed peer review, and reviewer names published annually in the journal.
Latest Articles
Modelling Prospects of Bio-Electrochemical Immunosensing Platforms
Electrochem 2024, 5(2), 146-161; https://doi.org/10.3390/electrochem5020010 (registering DOI) - 24 Apr 2024
Abstract
Electrochemistry is a hotspot in today’s research arena. Many different domains have been extended for their role towards the Internet of Things, digital health, personalized nutrition, and/or wellness using electrochemistry. These advances have led to a substantial increase in the power and popularity
[...] Read more.
Electrochemistry is a hotspot in today’s research arena. Many different domains have been extended for their role towards the Internet of Things, digital health, personalized nutrition, and/or wellness using electrochemistry. These advances have led to a substantial increase in the power and popularity of electroanalysis and its expansion into new phases and environments. The recent COVID-19 pandemic, which turned our lives upside down, has helped us to understand the need for miniaturized electrochemical diagnostic platforms. It also accelerated the role of mobile and wearable, implantable sensors as telehealth systems. The major principle behind these platforms is the role of electrochemical immunoassays, which help in overshadowing the classical gold standard methods (reverse transcriptase polymerase chain reaction) in terms of accuracy, time, manpower, and, most importantly, economics. Many research groups have endeavoured to use electrochemical and bio-electrochemical tools to overcome the limitations of classical assays (in terms of accuracy, accessibility, portability, and response time). This review mainly focuses on the electrochemical technologies used for immunosensing platforms, their fabrication requirements, mechanistic objectives, electrochemical techniques involved, and their subsequent output signal amplifications using a tagged and non-tagged system. The combination of various techniques (optical spectroscopy, Raman scattering, column chromatography, HPLC, and X-ray diffraction) has enabled the construction of high-performance electrodes. Later in the review, these combinations and their utilization will be explained in terms of their mechanistic platform along with chemical bonding and their role in signal output in the later part of article. Furthermore, the market study in terms of real prototypes will be elaborately discussed.
Full article
Open AccessArticle
High-Rate Performance of a Designed Si Nanoparticle–Graphite Nanosheet Composite as the Anode for Lithium-Ion Batteries
by
Vahide Ghanooni Ahmadabadi, Md Mokhlesur Rahman and Ying Chen
Electrochem 2024, 5(2), 133-145; https://doi.org/10.3390/electrochem5020009 - 09 Apr 2024
Abstract
A silicon nanoparticle–graphite nanosheet composite was prepared via a facile ball milling process for use as the anode for high-rate lithium-ion batteries. The size effect of Si nanoparticles on the structure and on the lithium-ion battery performance of the composite is evaluated. SEM
[...] Read more.
A silicon nanoparticle–graphite nanosheet composite was prepared via a facile ball milling process for use as the anode for high-rate lithium-ion batteries. The size effect of Si nanoparticles on the structure and on the lithium-ion battery performance of the composite is evaluated. SEM and TEM analyses show a structural alteration of the composites from Si nanoparticle-surrounded graphite nanosheets to Si nanoparticle-embedded graphite nanosheets by decreasing the size of Si nanoparticles from 250 nm to 40 nm. The composites with finer Si nanoparticles provide an effective nanostructure containing encapsulated Si and free space. This structure facilitates the indirect exposure of Si to electrolyte and Si expansion during cycling, which leads to a stable solid–electrolyte interphase and elevated conductivity. An enhanced rate capability was obtained for the 40 nm Si nanoparticle–graphite nanosheet composite, delivering a specific capacity of 276 mAh g−1 at a current density of 1 C after 1000 cycles and a rate capacity of 205 mAh g−1 at 8 C.
Full article
(This article belongs to the Collection Feature Papers in Electrochemistry)
►▼
Show Figures
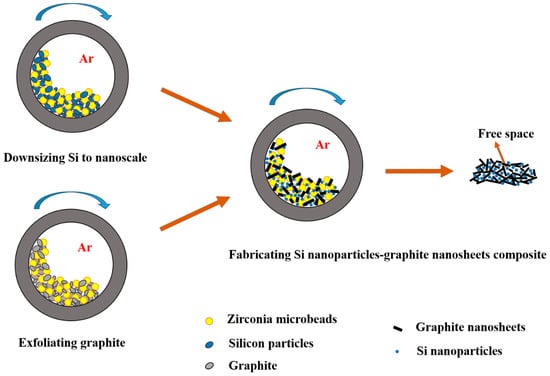
Figure 1
Open AccessCommunication
Electrodeposition of Silicon Fibers from KI–KF–KCl–K2SiF6 Melt and Their Electrochemical Performance during Lithiation/Delithiation
by
Anastasia Leonova, Natalia Leonova, Lyudmila Minchenko and Andrey Suzdaltsev
Electrochem 2024, 5(1), 124-132; https://doi.org/10.3390/electrochem5010008 - 07 Mar 2024
Abstract
►▼
Show Figures
The possibility of using Si-based anodes in lithium-ion batteries is actively investigated due to the increased lithium capacity of silicon. The paper reports the preparation of submicron silicon fibers on glassy carbon in the KI–KF–KCl–K2SiF6 melt at 720 °C. For
[...] Read more.
The possibility of using Si-based anodes in lithium-ion batteries is actively investigated due to the increased lithium capacity of silicon. The paper reports the preparation of submicron silicon fibers on glassy carbon in the KI–KF–KCl–K2SiF6 melt at 720 °C. For this purpose, the parameters of silicon electrodeposition in the form of fibers were determined using cyclic voltammetry, and experimental samples of ordered silicon fibers with an average diameter from 0.1 to 0.3 μm were obtained under galvanostatic electrolysis conditions. Using the obtained silicon fibers, anode half-cells of a lithium-ion battery were fabricated, and its electrochemical performance under multiple lithiations and delithiations was studied. By means of voltametric studies, it is observed that charging and discharging the anode based on the obtained silicon fibers occurs at potentials from 0.2 to 0.05 V and from 0.2 to 0.5 V, respectively. A change in discharge capacity from 520 to 200 mAh g−1 during the first 50 charge/discharge cycles at a charge current of 0.1 C and a Coulombic efficiency of 98–100% was shown. The possibility of charging silicon-based anode samples at charging currents up to 2 C was also noted; the discharge capacity ranged from 25 to 250 mAh g−1.
Full article
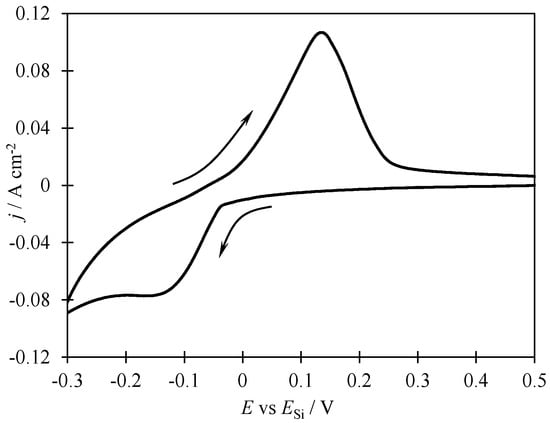
Figure 1
Open AccessArticle
Determining the Oxidation Stability of Electrolytes for Lithium-Ion Batteries Using Quantum Chemistry and Molecular Dynamics
by
Elizaveta Y. Evshchik, Sophia S. Borisevich, Margarita G. Ilyina, Edward M. Khamitov, Alexander V. Chernyak, Tatiana A. Pugacheva, Valery G. Kolmakov, Olga V. Bushkova and Yuri A. Dobrovolsky
Electrochem 2024, 5(1), 107-123; https://doi.org/10.3390/electrochem5010007 - 04 Mar 2024
Abstract
►▼
Show Figures
Determining the oxidation potential (OP) of lithium-ion battery (LIB) electrolytes using theoretical methods will significantly speed up and simplify the process of creating a new generation high-voltage battery. The algorithm for calculating OP should be not only accurate but also fast. Our work
[...] Read more.
Determining the oxidation potential (OP) of lithium-ion battery (LIB) electrolytes using theoretical methods will significantly speed up and simplify the process of creating a new generation high-voltage battery. The algorithm for calculating OP should be not only accurate but also fast. Our work proposes theoretical principles for evaluating the OP of LIB electrolytes by considering LiDFOB solutions with different salt concentrations in EC/DMC solvent mixtures. The advantage of the new algorithm compared to previous versions of the theoretical determination of the oxidation potential of electrolyte solutions used in lithium-ion batteries for calculations of statistically significant complexes, the structure of which was determined by the molecular dynamics method. This approach significantly reduces the number of atomic–molecular systems whose geometric parameters need to be optimized using quantum chemical methods. Due to this, it is possible to increase the speed of calculations and reduce the power requirements of the computer performing the calculations. The theoretical calculations included a set of approaches based on the methods of classical molecular mechanics and quantum chemistry. To select statistically significant complexes that can make a significant contribution to the stability of the electrochemical system, a thorough analysis of molecular dynamics simulation trajectories was performed. Their geometric parameters (including oxidized forms) were optimized by QM methods. As a result, oxidation potentials were assessed, and their dependence on salt concentration was described. Here, we once again emphasize that it is difficult to obtain, by calculation methods, the absolute OP values that would be equal (or close) to the OP values estimated by experimental methods. Nevertheless, a trend can be identified. The results of theoretical calculations are in full agreement with the experimental ones.
Full article
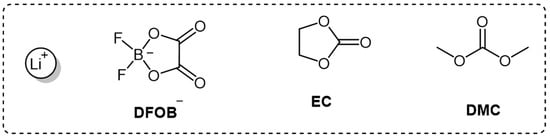
Figure 1
Open AccessArticle
The Effect of Bulk Modification of the MF-4SK Membrane with Phosphorylated Hyper-Branched Dendrimer Bolthorn H20 on the Mechanisms of Electroconvection/Dissociation of Water and Specific Selectivity to Divalent Ions
by
Aslan Achoh, Denis Bondarev, Elena Nosova and Stanislav Melnikov
Electrochem 2024, 5(1), 84-106; https://doi.org/10.3390/electrochem5010006 - 20 Feb 2024
Abstract
This study focuses on the modification of ion-exchange membranes by incorporating a phosphorylated dendrimer into sulfonated polytetrafluoroethylene membranes to enhance the specific selectivity between mono-/divalent ions, using the Ca2+/Na+ pair as an example. This research employs mechanical, physicochemical, and electrochemical
[...] Read more.
This study focuses on the modification of ion-exchange membranes by incorporating a phosphorylated dendrimer into sulfonated polytetrafluoroethylene membranes to enhance the specific selectivity between mono-/divalent ions, using the Ca2+/Na+ pair as an example. This research employs mechanical, physicochemical, and electrochemical analyses to explore the effects of P-H20 incorporation on membrane properties. Bulk modification significantly increases membrane selectivity towards calcium ions (the specific permselectivity coefficient rises from 1.5 to 7.2), while maintaining the same level of the limiting current density. Other findings indicate that bulk modification significantly changes the transport-channel structure of the membrane and alters the mechanism of over-limiting mass transfer. The over-limiting current for the pristine membrane is mainly due to non-equilibrium electroconvection, while modified membranes actively participate in the water-splitting reaction, leading to the suppression of the electroconvection. Despite this drawback, the decrease of the over-limiting potential drop results in a decrease in specific energy consumption from 0.11 to 0.07 kWh/mol. In the underlimiting current mode, the specific energy consumption for all studied membranes remains within the same limits of 0.02–0.03 kWh/mol.
Full article
(This article belongs to the Topic Advances in Chemistry and Chemical Engineering)
►▼
Show Figures
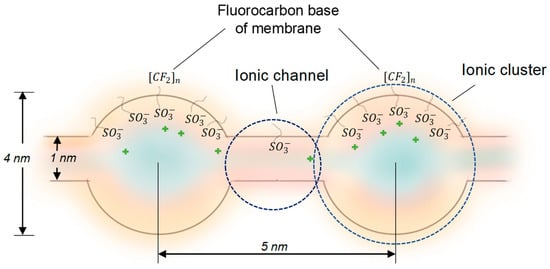
Figure 1
Open AccessArticle
The Electrocatalytic Oxygen Evolution Reaction Activity of Rationally Designed NiFe-Based Glycerates
by
Vivek Kumar Singh, Bibhudatta Malik, Rajashree Konar, Efrat Shawat Avraham and Gilbert Daniel Nessim
Electrochem 2024, 5(1), 70-83; https://doi.org/10.3390/electrochem5010005 - 04 Feb 2024
Abstract
The electrocatalytic oxygen evolution reaction (OER) is an arduous step in water splitting due to its slow reaction rate and large overpotential. Herein, we synthesized glycerate-anion-intercalated nickel–iron glycerates (NiFeGs) using a one-step solvothermal reaction. We designed various NiFeGs by tuning the molar ratio
[...] Read more.
The electrocatalytic oxygen evolution reaction (OER) is an arduous step in water splitting due to its slow reaction rate and large overpotential. Herein, we synthesized glycerate-anion-intercalated nickel–iron glycerates (NiFeGs) using a one-step solvothermal reaction. We designed various NiFeGs by tuning the molar ratio between Ni and Fe to obtain Ni4Fe1G, Ni3Fe1G, Ni3Fe2G, and Ni1Fe1G, which we tested for their OER performance. We initially analyzed the catalytic performance of powder samples immobilized on glassy carbon electrodes using a binder. Ni3Fe2G outperformed the other NiFeG compositions, including NiFe layered double hydroxide (LDH). It exhibited an overpotential of 320 mV at a current density of 10 mA cm–2 in an electrolytic solution of pH 14. We then synthesized carbon paper (CP)-modified Ni3Fe2G as a self-supported electrode (Ni3Fe2G/CP), and it exhibited a high current density (100 mA cm−2) at a low overpotential of 300 mV. The redox peak analysis for the NiFeGs revealed that the initial step of the OER is the formation of γ-NiOOH, which was further confirmed by a post-Raman analysis. We extensively analyzed the catalyst’s stability and lifetime, the nature of the active sites, and the role of the Fe content to enhance the OER performance. This work may provide the motivation to study metal-alkoxide-based efficient OER electrocatalysts that can be used for alkaline water electrolyzer applications.
Full article
(This article belongs to the Collection Feature Papers in Electrochemistry)
►▼
Show Figures

Figure 1
Open AccessArticle
Comparison of Different Electrochemical Methodologies for Electrode Reactions: A Case Study of Paracetamol
by
Zaheer Masood, Haji Muhammad and Iftikhar Ahmed Tahiri
Electrochem 2024, 5(1), 57-69; https://doi.org/10.3390/electrochem5010004 - 31 Jan 2024
Abstract
►▼
Show Figures
Understanding electrochemical reactions at the surface of electrodes requires the accurate calculation of key parameters—the transfer coefficient (α), diffusion coefficient (D0), and heterogeneous electron transfer rate constant (k0). The choice of method to calculate these parameters requires
[...] Read more.
Understanding electrochemical reactions at the surface of electrodes requires the accurate calculation of key parameters—the transfer coefficient (α), diffusion coefficient (D0), and heterogeneous electron transfer rate constant (k0). The choice of method to calculate these parameters requires careful consideration based on the nature of the electrochemical reaction. In this study, we conducted the cyclic voltammetry of paracetamol to calculate the values of these parameters using different methods and present a comparative analysis. Our results demonstrate that the Ep − Ep/2 equation for α and the modified Randles–Ševčík equation for D0 is particularly effective for the calculations of these two parameters. The Kochi and Gileadi methods are reliable alternatives for the calculation of k0. Nicholson and Shain’s method using the equation k0 = Ψ(πnD0Fν/RT)1/2 gives the overestimated values of k0. However, the value of k0 calculated using the plot of ν−1/2 versus Ψ (from the Nicholson and Shain equation, where ν is scan rate) agrees well with the values calculated from the Kochi and Gilaedi methods. This study not only identifies optimal methodologies for quasi-reversible reactions but also contributes to a deeper understanding of electrochemical reactions involving complex electron transfer and coupled chemical reactions, which can be broadly applicable in various electrochemical studies.
Full article
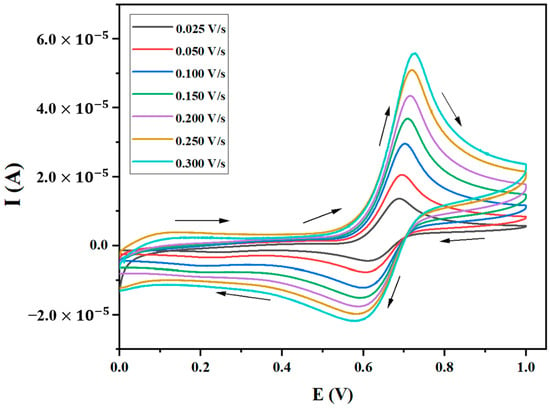
Figure 1
Open AccessArticle
Micromolar Levofloxacin Sensor by Incorporating Highly Crystalline Co3O4 into a Carbon Paste Electrode Structure
by
Tijana Mutić, Dalibor Stanković, Dragan Manojlović, Djordje Petrić, Ferenc Pastor, Vyacheslav V. Avdin, Miloš Ognjanović and Vesna Stanković
Electrochem 2024, 5(1), 45-56; https://doi.org/10.3390/electrochem5010003 - 23 Jan 2024
Cited by 1
Abstract
►▼
Show Figures
In this work, we successfully prepared a modified cobalt oxide (Co3O4) carbon paste electrode to detect Levofloxacin (LEV). By synthesizing Co3O4 nanoparticles through the chemical coprecipitation method, the electrochemical properties of the electrode and LEV were
[...] Read more.
In this work, we successfully prepared a modified cobalt oxide (Co3O4) carbon paste electrode to detect Levofloxacin (LEV). By synthesizing Co3O4 nanoparticles through the chemical coprecipitation method, the electrochemical properties of the electrode and LEV were thoroughly investigated using CV, SWV, and EIS, while material properties were scrutinized using ICP-OES, TEM, SEM, and XRD. The results showed that the prepared electrode displayed a better electrocatalytic response than the bare carbon paste electrode. After optimizing SWV, the electrode exhibited a wide linear working range from 1 to 85 μM at pH 5 of BRBS as the supporting electrolyte. The selectivity of the proposed method was satisfactory, with good repeatability and reproducibility, strongly suggesting a potential application for determining LEV in real samples, particularly in pharmaceutical formulations. The practicality of the approach was demonstrated through good recoveries, and the morphology of the materials was found to be closely related to other parameters, indicating that the developed method can provide a cost-effective, rapid, selective, and sensitive means for LEV monitoring. Overall, this project has made significant progress towards developing a reliable method for detecting LEV and has opened up new opportunities for future research in this field.
Full article
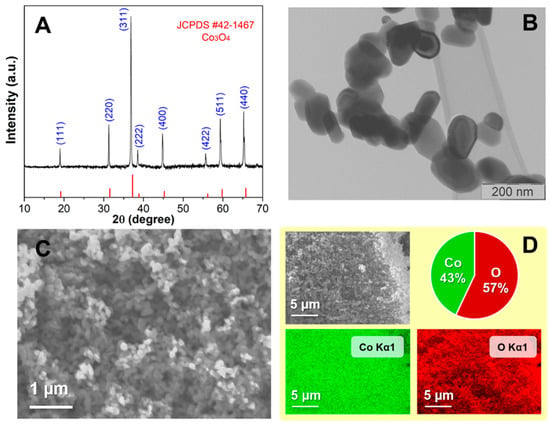
Figure 1
Open AccessArticle
Artificial Intelligence for Electrochemical Prediction and Optimization of Direct Carbon Fuel Cells Fueled with Biochar
by
Adam Cherni and Kamel Halouani
Electrochem 2024, 5(1), 29-44; https://doi.org/10.3390/electrochem5010002 - 04 Jan 2024
Abstract
At present, direct carbon fuel cells constitute an emerging energy technology that electrochemically converts solid carbon to electricity with high efficiency. The recent trend of DCFCs fueled with biochar from biomass carbonization as green fuel has reinforced the environmental benefits of DCFCs as
[...] Read more.
At present, direct carbon fuel cells constitute an emerging energy technology that electrochemically converts solid carbon to electricity with high efficiency. The recent trend of DCFCs fueled with biochar from biomass carbonization as green fuel has reinforced the environmental benefits of DCFCs as a clean and sustainable technology. However, there remain new challenges related to some complex unknown kinetic parameters,
(This article belongs to the Special Issue Advances in Electrochemical Energy Storage Systems)
►▼
Show Figures
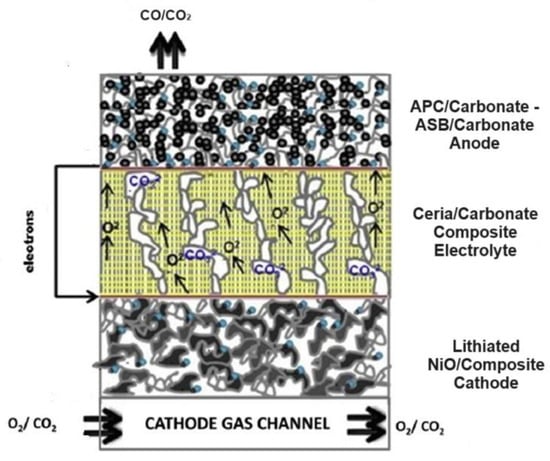
Figure 1
Open AccessArticle
Identification of the Safe Variation Limits for the Optimization of the Measurements in Low-Cost Electrochemical Air Quality Sensors
by
Ioannis Christakis, Elena Sarri, Odysseas Tsakiridis and Ilias Stavrakas
Electrochem 2024, 5(1), 1-28; https://doi.org/10.3390/electrochem5010001 - 21 Dec 2023
Cited by 2
Abstract
Nowadays, the study of air quality has become an increasingly prominent field of research, particularly in large urban centers, given its significant impact on human health. In many countries, government departments and research centers use official high-cost scientific instruments to monitor air quality
[...] Read more.
Nowadays, the study of air quality has become an increasingly prominent field of research, particularly in large urban centers, given its significant impact on human health. In many countries, government departments and research centers use official high-cost scientific instruments to monitor air quality in their regions. Meanwhile, concerned citizens interested in studying the air quality of their local areas often employ low-cost air quality sensors for monitoring purposes. The optimization and evaluation of low-cost sensors have been a field of research by many research groups. This paper presents an extensive study to identify the safe percentage change limits that low-cost electrochemical air quality sensors can have, in order to optimize their measurements. For this work, three low-cost air quality monitoring stations were used, which include an electrochemical sensor for nitrogen dioxide (NO2) (Alphasense NO2-B43F) and an electrochemical sensor for ozone (O3) (Alphasense OX-B431). The aim of this work is to explore the variance of the aforementioned sensors and how this variability can be used to optimize the measurements of low-cost electrochemical sensors, closer to real ones. The analysis is conducted by employing diagrams, boxplot and violin curves of the groups of sensors used, with satisfactory results.
Full article
(This article belongs to the Collection Feature Papers in Electrochemistry)
►▼
Show Figures
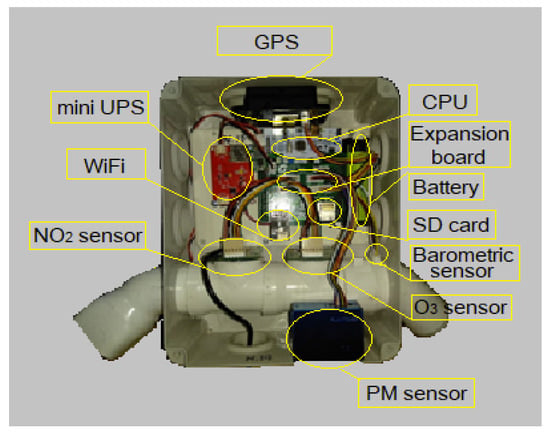
Figure 1
Open AccessReview
Relevant Aspects in the Development of Electrochemical Aptasensors for the Determination of Antibiotics—A Review
by
Daniela Nunes da Silva and Arnaldo César Pereira
Electrochem 2023, 4(4), 553-567; https://doi.org/10.3390/electrochem4040035 - 12 Dec 2023
Abstract
Aptamers are three-dimensional structures of DNA or RNA that present high affinity and selectivity to specific targets, obtained through in vitro screening. Aptamers are used as biological recognizers in electrochemical biosensors, the so-called aptasensors, providing greater specificity in recognizing the most diverse analytes.
[...] Read more.
Aptamers are three-dimensional structures of DNA or RNA that present high affinity and selectivity to specific targets, obtained through in vitro screening. Aptamers are used as biological recognizers in electrochemical biosensors, the so-called aptasensors, providing greater specificity in recognizing the most diverse analytes. Electrochemical aptasensors have extremely relevant characteristics, such as high sensitivity, low cost compared to other biorecognizers such as antibodies, and excellent compatibility, being considered one of the most promising alternative methods in several areas, such as biomedical diagnosis and monitoring environmental contaminants. In this sense, the present work reviews the relevant aspects of methodologies based on electrochemical aptasensors and their applications in determining antibiotics, seeking to foster innovation in electrochemical biosensors.
Full article
(This article belongs to the Collection Feature Papers in Electrochemistry)
►▼
Show Figures
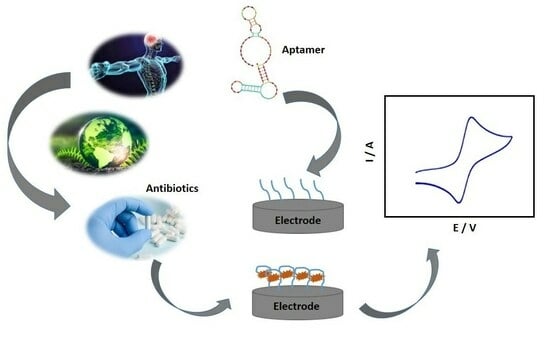
Graphical abstract
Open AccessReview
Electrochemical-Based Biosensor Platforms in Lab-Chip Models for Point-of-Need Toxicant Analysis
by
Mohana Marimuthu, Vinoth Krishnan, Shailendra Devi Sudhakaran, Sevakumaran Vigneswari, Shanmugam Senthilkumar and Murugan Veerapandian
Electrochem 2023, 4(4), 537-552; https://doi.org/10.3390/electrochem4040034 - 21 Nov 2023
Abstract
►▼
Show Figures
The global hazardous waste management market is expected to reach USD 987.51 million by 2027 at a CAGR of 14.48%. The early detection of corrosive, flammable, and infectious toxicants from natural sources or manmade contaminants from different environments is crucial to ensure the
[...] Read more.
The global hazardous waste management market is expected to reach USD 987.51 million by 2027 at a CAGR of 14.48%. The early detection of corrosive, flammable, and infectious toxicants from natural sources or manmade contaminants from different environments is crucial to ensure the safety and security of the global living system. Even though the emergence of advanced science and technology continuously offers a more comfortable lifestyle, there are two sides of the coin in terms of opportunities and challenges, demanding solutions for greener applications and waste-to-wealth strategies. A modern analytical technique based on an electrochemical approach and microfluidics is one such emerging advanced solution for the early and effective detection of toxicants. This review attempts to highlight the different studies performed in the field of toxicant analysis, especially the fusion of electrochemistry and lab-chip model systems, promising for point-of-need analysis. The contents of this report are organised by classifying the types of toxicants and trends in electrochemical-integrated lab-chip assays that test for heavy-metal ions, food-borne pathogens, pesticides, physiological reactive oxygen/nitrogen species, and microbial metabolites. Future demands in toxicant analysis and possible suggestions in the field of microanalysis-mediated electrochemical (bio)sensing are summarised.
Full article
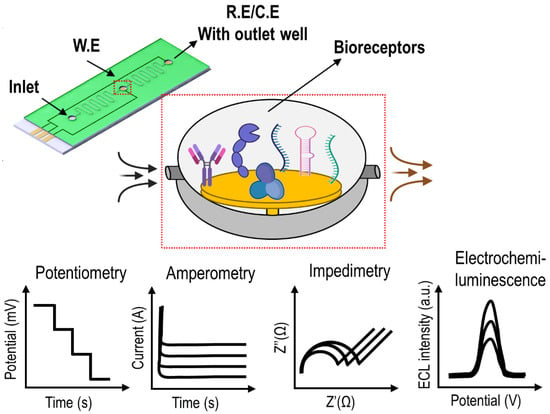
Figure 1
Open AccessArticle
A Disposable Carbon-Based Electrochemical Cell Modified with Carbon Black and Ag/δ-FeOOH for Non-Enzymatic H2O2 Electrochemical Sensing
by
Wiviane E. R. de Melo, Karoline S. Nantes, Ana L. H. K. Ferreira, Márcio C. Pereira, Luiz H. C. Mattoso, Ronaldo C. Faria and André S. Afonso
Electrochem 2023, 4(4), 523-536; https://doi.org/10.3390/electrochem4040033 - 14 Nov 2023
Abstract
►▼
Show Figures
Hydrogen peroxide (H2O2) is an essential analyte for detecting neurodegenerative diseases and inflammatory processes and plays a crucial role in pharmaceuticals, the food industry, and environmental monitoring. However, conventional H2O2 detection methods have drawbacks such as
[...] Read more.
Hydrogen peroxide (H2O2) is an essential analyte for detecting neurodegenerative diseases and inflammatory processes and plays a crucial role in pharmaceuticals, the food industry, and environmental monitoring. However, conventional H2O2 detection methods have drawbacks such as lengthy analysis times, high costs, and bulky equipment. Non-enzymatic sensors have emerged as promising alternatives to overcome these limitations. In this research, we introduce a simple, portable, and cost-effective non-enzymatic sensor that uses carbon black (CB) and silver nanoparticle-modified δ-FeOOH (Ag/δ-FeOOH) integrated into a disposable electrochemical cell (DCell). Scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDS), and electrochemical impedance spectroscopy (EIS) confirmed successful CB and Ag/δ-FeOOH immobilization on the DCell working electrode. Electrochemical investigations revealed that the DCell-CB//Ag/δ-FeOOH sensor exhibited an approximately twofold higher apparent heterogeneous electron transfer rate constant than the DCell-Ag/δ-FeOOH sensor, capitalizing on CB’s advantages. Moreover, the sensor displayed an excellent electrochemical response for H2O2 reduction, boasting a low detection limit of 22 µM and a high analytical sensitivity of 214 μA mM−1 cm−2. Notably, the DCell-CB//Ag/δ-FeOOH sensor exhibited outstanding selectivity for H2O2 detection, even in potential interferents such as dopamine, uric acid, and ascorbic acid. Furthermore, the sensor has the right qualities for monitoring H2O2 in complex biological samples, as evidenced by H2O2 recoveries ranging from 92% to 103% in 10% fetal bovine serum. These findings underscore the considerable potential of the DCell-CB//Ag/δ-FeOOH sensor for precise and reliable H2O2 monitoring in various biomedical and environmental applications.
Full article
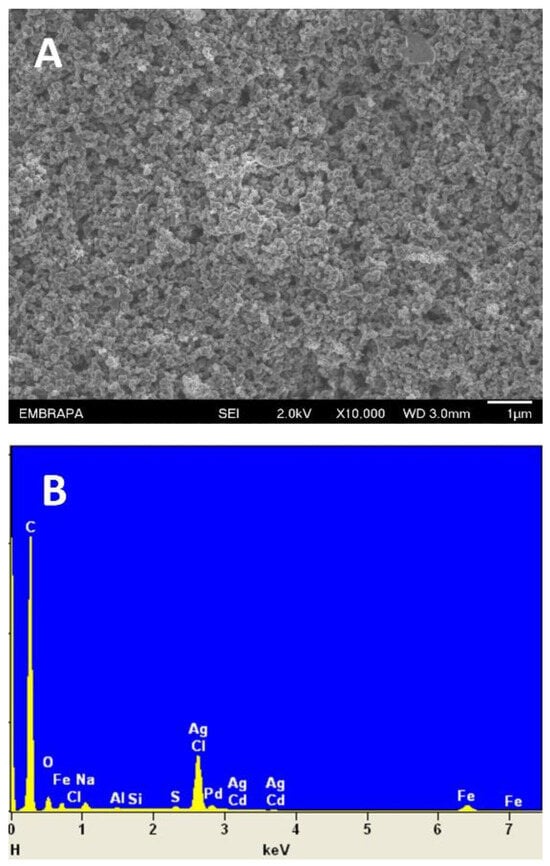
Figure 1
Open AccessReview
Separator Materials for Lithium Sulfur Battery—A Review
by
Ryohei Mori
Electrochem 2023, 4(4), 485-522; https://doi.org/10.3390/electrochem4040032 - 13 Nov 2023
Abstract
In the recent rechargeable battery industry, lithium sulfur batteries (LSBs) have demonstrated to be a promising candidate battery to serve as the next-generation secondary battery, owing to its enhanced theoretical specific energy, economy, and environmental friendliness. Its inferior cyclability, however, which is primarily
[...] Read more.
In the recent rechargeable battery industry, lithium sulfur batteries (LSBs) have demonstrated to be a promising candidate battery to serve as the next-generation secondary battery, owing to its enhanced theoretical specific energy, economy, and environmental friendliness. Its inferior cyclability, however, which is primarily due to electrode deterioration caused by the lithium polysulfide shuttle effect, is still a major problem for the real industrial usage of LSBs. The optimization of the separator and functional barrier layer is an effective strategy for remedying these issues. In this article, the current progress based on the classification and modification of functional separators is summarized. We will also describe their working mechanisms as well as the resulting LSB electrochemical properties. In addition, necessary performance for separators will also be mentioned in order to gain optimized LSB performance.
Full article
(This article belongs to the Topic Electrochemical Energy Storage Materials)
►▼
Show Figures
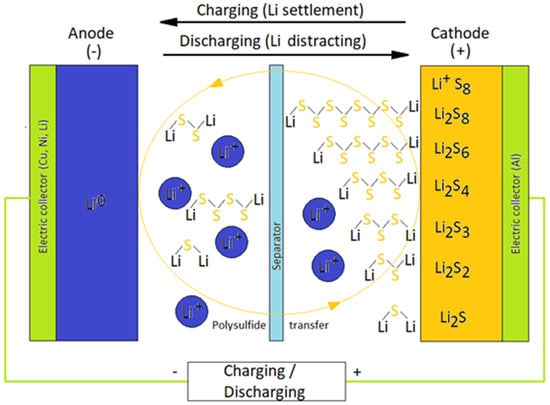
Figure 1
Open AccessArticle
Graphene-Oxide-Coated CoP2@C Anode Enables High Capacity of Lithium-Ion Batteries
by
Wei Zhang, Hangxuan Xie, Zirui Dou, Zhentao Hao, Qianhui Huang, Ziqi Guo, Chao Wang, Kanghua Miao and Xiongwu Kang
Electrochem 2023, 4(4), 473-484; https://doi.org/10.3390/electrochem4040031 - 26 Oct 2023
Cited by 1
Abstract
Cobalt diphosphides (CoP2) show a high theoretical capacity and hold great promise as anode materials for lithium-ion batteries (LIBs). However, the large variation in the volume and structure of CoP2 caused during lithium-ion insertion and extraction results in electrode fragmentation
[...] Read more.
Cobalt diphosphides (CoP2) show a high theoretical capacity and hold great promise as anode materials for lithium-ion batteries (LIBs). However, the large variation in the volume and structure of CoP2 caused during lithium-ion insertion and extraction results in electrode fragmentation and a compromised solid electrolyte interface, ultimately leading to poor cycling performance. Herein, a composite of CoP2 nanoparticles encapsulated in carbon matrix has been successfully synthesized by carbonization of Co-MOF-based zeolitic imidazolate frameworks (ZIF-67) and sequential phosphorization and further wrapped in graphene oxide (CoP2@C@GO). The formation of CoP2 was confirmed by X-ray diffraction, high-resolution transmission electron microscopy and X-ray photoelectron spectroscopy. The morphology of CoP2@C with and without GO wrapping was examined by scanning electron microscopy and transmission electron spectroscopy. It was demonstrated that the decoration of GO significantly reduces the polarization of CoP2@C electrodes, enhancing their charge capacity and cycling stability as an anode material for LIBs. After 200 cycles, they deliver a capacity of 450 mAh·g−1.
Full article
(This article belongs to the Collection Feature Papers in Electrochemistry)
►▼
Show Figures
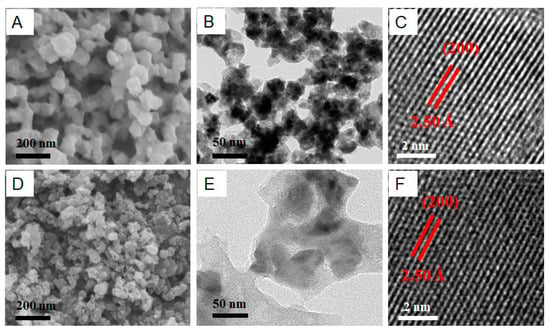
Figure 1
Open AccessArticle
The Difference in the Effects of IR-Drop from the Negative Capacitance of Fast Cyclic Voltammograms
by
Yuanyuan Liu, Koichi Jeremiah Aoki and Jingyuan Chen
Electrochem 2023, 4(4), 460-472; https://doi.org/10.3390/electrochem4040030 - 23 Oct 2023
Cited by 2
Abstract
Diffusion-controlled cyclic voltammograms at fast scan rates show peak shifts, as well as decreases in the peak currents from predicted diffusion-controlled currents, especially when the currents are large in a low concentration of supporting electrolytes. This has been conventionally recognized as an IR
[...] Read more.
Diffusion-controlled cyclic voltammograms at fast scan rates show peak shifts, as well as decreases in the peak currents from predicted diffusion-controlled currents, especially when the currents are large in a low concentration of supporting electrolytes. This has been conventionally recognized as an IR-drop effect due to solution resistance on the peaks, as well as a heterogeneously kinetic effect. It is also brought about by the negatively capacitive currents associated with charge transfer reactions. The reaction product generates dipoles with counterions to yield a capacitance, the current of which flows oppositely to that of the double-layer capacitance. The three effects are specified here in the oxidation of a ferrocenyl derivative using fast scan voltammetry. The expression for voltammograms complicated with IR-drop is derived analytically and yields deformed voltammograms. The peak shift is approximately linear with the IR-voltage, but exhibits a convex variation. The dependence of some parameters on the peaks due to the IR-drop is compared with those due to the negative capacitance. The latter is more conspicuous than the former under conventional conditions. The two effects cannot be distinguished specifically except for variations in the conductance of the solution.
Full article
(This article belongs to the Special Issue Electrochemistry Modulated Interfacial Processes: Fundamental and Application)
►▼
Show Figures
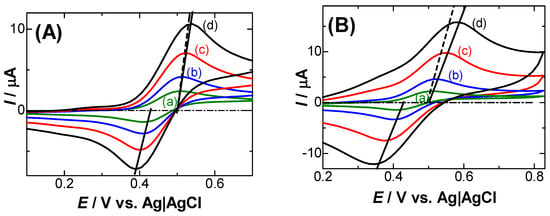
Figure 1
Open AccessArticle
New Analytical Expressions of Concentrations in Packed Bed Immobilized-Cell Electrochemical Photobioreactor
by
Ponraj Jeyabarathi, Marwan Abukhaled, Murugesan Kannan, Lakshmanan Rajendran and Michael E. G. Lyons
Electrochem 2023, 4(4), 447-459; https://doi.org/10.3390/electrochem4040029 - 29 Sep 2023
Abstract
►▼
Show Figures
An electrochemical photobioreactor with a packed bed containing transparent gel granules and immobilized photosynthetic bacterial cells is shown with a one-dimensional two-phase flow and transport model. We consider the biological/chemical events in the electrochemical photobioreactor, the intrinsically connected two-phase flow and mass transport,
[...] Read more.
An electrochemical photobioreactor with a packed bed containing transparent gel granules and immobilized photosynthetic bacterial cells is shown with a one-dimensional two-phase flow and transport model. We consider the biological/chemical events in the electrochemical photobioreactor, the intrinsically connected two-phase flow and mass transport, and other factors. This model is based on a system of nonlinear equations. This paper applies Akbari-Ganji’s and Taylor series methods to find analytical solutions to nonlinear differential equations that arise in an immobilized-cell electrochemical photobioreactor. Approximate analytical expressions of the concentration of glucose and hydrogen are obtained in liquid and gas phases for different parameter values. Numerical simulations are presented to validate the theoretical investigations.
Full article
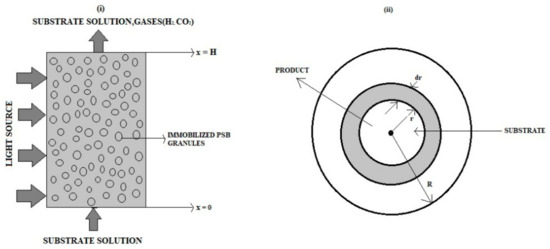
Figure 1
Open AccessArticle
Molecularly Imprinted Electrochemical Sensor Based on Poly (O-Phenylenediamine) for Sensitive Detection of Oxycodone in Water
by
Pranaya Charkravarthula and Amos Mugweru
Electrochem 2023, 4(4), 435-446; https://doi.org/10.3390/electrochem4040028 - 28 Sep 2023
Abstract
►▼
Show Figures
This work was aimed at the development of a sensitive electrochemical detection method for oxycodone in water. Molecularly imprinted electrodes were formed by electro-polymerization process using o-phenylenediamine as a monomer. The electro-polymerization was performed on glassy carbon electrodes in the presence of oxycodone
[...] Read more.
This work was aimed at the development of a sensitive electrochemical detection method for oxycodone in water. Molecularly imprinted electrodes were formed by electro-polymerization process using o-phenylenediamine as a monomer. The electro-polymerization was performed on glassy carbon electrodes in the presence of oxycodone before the extraction of entrapped oxycodone molecules. Various electrochemical techniques were employed to monitor the polymerization and response of the fabricated electrodes toward oxycodone. These techniques included cyclic voltammetry (CV), square wave voltammetry (SWV), differential pulse voltammetry (DPV) and electrochemical impedance spectroscopy (EIS). The oxycodone concentration was determined using SWV by measuring the change in the oxidation peak current of [Fe(CN)6]3−/4− in a 0.1 mM acetate buffer solution. At the optimal electro-polymerization conditions, a calibration curve of the current versus the concentration of oxycodone indicated a linear response at a region from 0.4 nM to 5.0 nM with a detection limit of 1.8 ± 0.239 nM. The MIP-modified electrode’s binding isotherm was fitted using a Langmuir model and showed an association constant, KA, of 1.12 × 106, indicating a high affinity of oxycodone molecules to binding sites. This sensor has the potential to act as an alternative method suitable for the on-site analysis of oxycodone.
Full article
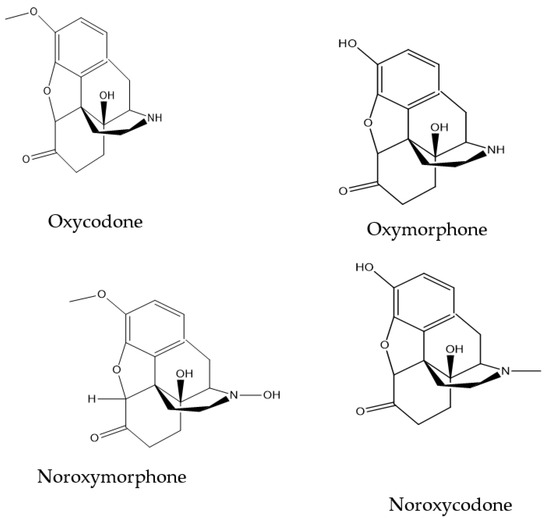
Figure 1
Open AccessArticle
Theory for Electrochemical Heat Sources and Exothermic Explosions: The Akbari–Ganji Method
by
Ramalingam Vanaja, Ponraj Jeyabarathi, Lakshmanan Rajendran and Michael Edward Gerard Lyons
Electrochem 2023, 4(3), 424-434; https://doi.org/10.3390/electrochem4030027 - 05 Sep 2023
Abstract
►▼
Show Figures
A device that transforms chemical energy into electrical energy is an electrochemical cell. The reaction type inside the cell determines whether it is exothermic or endothermic. This paper discusses the mathematical modelling of exothermic explosions in a slab. This model is based on
[...] Read more.
A device that transforms chemical energy into electrical energy is an electrochemical cell. The reaction type inside the cell determines whether it is exothermic or endothermic. This paper discusses the mathematical modelling of exothermic explosions in a slab. This model is based on a nonlinear equation containing a nonlinear term related to Arrhenius, bimolecular, and sensitised laws of reaction kinetics. The absolute temperature can be derived by solving the nonlinear equation using the Akbari–Ganji technique. The mathematical model also numerically solved and simulated in the MATLAB® v2016b software. The new simple theoretical result is validated with previously identified analytical and numerical findings. The influence of the parameters of Frank-Kamenetskii number, activation energy and the numerical exponent on temperature is discussed. The Frank-Kamenetskii number is observed to drop as the temperature is found to decrease, while the activation energy parameter is shown to increase. The numerical exponent has little or no effect on the temperature. An extension of this model to cylinder and sphere geometry is also provided.
Full article
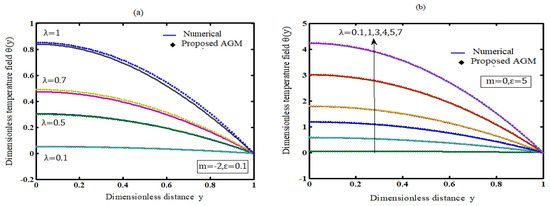
Figure 1
Open AccessReview
Design and Development of Food Waste Inspired Electrochemical Platform for Various Applications
by
Mansi Gandhi
Electrochem 2023, 4(3), 411-423; https://doi.org/10.3390/electrochem4030026 - 14 Aug 2023
Abstract
Plants have a remarkable position among renewable materials because of their abundance, and nearly thousands of tons are consumed worldwide every day. Most unexploited plants and agricultural waste can be a real potential resource system. With increasing environmental awareness and the growing importance
[...] Read more.
Plants have a remarkable position among renewable materials because of their abundance, and nearly thousands of tons are consumed worldwide every day. Most unexploited plants and agricultural waste can be a real potential resource system. With increasing environmental awareness and the growing importance of friendly agricultural waste, crops and fruit waste can be used for efficient conversion into bio-fertilizers, biocarbons, bio-polymers, biosensors and bio-fibers. Global challenges based on limited natural resources and fossil energy reserves simulated keen interest in the development of various electrochemical systems inspired by food and plant scraps, which aid in curbing pollution. The successful adoption of a renewable energy roadmap is dependent on the availability of a cheaper means of storage. In order to cut down the cost of storage units, an improvement on energy storage devices having better stability, power, and energy density with low post-maintenance cost is the vital key. Although food and plant scraps have a huge need for energy storage, it has been extended to various sensing platform fabrications, which are eco-friendly and comparable to organic molecule-based sensors. Current research proclivity has witnessed a huge surge in the development of phyto-chemical-based sensors. The state-of-the-art progresses on the subsequent use of plant-waste systems as nano-engineered electrochemical platforms for numerous environmental science and renewable energy applications. Moreover, the relevant rationale behind the use of waste in a well-developed, sustainable future device is also presented in this review.
Full article
(This article belongs to the Collection Feature Papers in Electrochemistry)
►▼
Show Figures
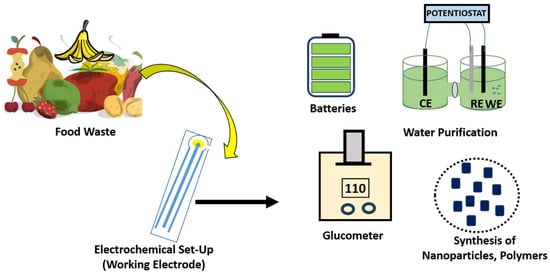
Figure 1
Highly Accessed Articles
Latest Books
E-Mail Alert
News
Topics
Topic in
Batteries, Electrochem, Materials, Nanomaterials, Molecules
Design and Mechanism of Aqueous Batteries
Topic Editors: Chuankun Zhang, Houzhao Wan, Pei Liang, Zhang LiDeadline: 30 April 2024
Topic in
Energies, Nanomaterials, Materials, Electrochem, Batteries
Electrochemical Energy Storage Materials
Topic Editors: Huang Zhang, Yuan MaDeadline: 30 June 2024
Topic in
Batteries, Electrochem, Energies, Materials, Polymers
Advances in Energy Storage Materials/Devices and Solid-State Batteries
Topic Editors: Claudio Gerbaldi, Federico Poli, Cataldo Simari, Akiko Tsurumaki, Francesca Soavi, Alessandro PiovanoDeadline: 31 August 2024

Conferences
Special Issues
Special Issue in
Electrochem
Fuel Cells: Performance and Durability
Guest Editors: Fatemeh Gholami, Martin TomasDeadline: 31 October 2024
Special Issue in
Electrochem
Emerging Trends of Electrochemical Sensors in Food Analysis
Guest Editor: Ítala MarxDeadline: 31 December 2024
Special Issue in
Electrochem
Silicon Electrochemistry: Fundamentals and Modern Applications
Guest Editor: Andrey SuzdaltsevDeadline: 7 July 2025




