Chronic Inflammation, Oxidative Stress and Adult Stem Cells
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Stem Cells".
Viewed by 19263
Share This Topical Collection
Editor
Topical Collection Information
Dear Colleagues,
Chronic inflammation plays crucial roles in various kinds of diseases, including autoimmune diseases, cancer, arteriosclerosis, obesity, and Alzheimer's disease. Chronic inflammation is usually accompanied by reactive oxygen species (ROS), which are overproduced by activated immune cells and local fibroblasts in the tissue. When ROS overwhelm the antioxidant system, oxidative stress occurs. Oxidative stress injures cellular compounds (including DNA, proteins, and lipids), resulting in apoptotic and necrotic cell death. This process induces the secretion of proinflammatory cytokines in cells and consequently worsens the inflammation. As a result, cells and tissues eventually become functionally depleted.
Adult stem cells (also known as somatic stem cells or tissue stem cells) are undifferentiated cells (that replace damaged functional cells) in the tissue. During chronic inflammation, adult stem cells repair oxidative-stress-induced injuries to the tissue to maintain tissue homeostasis. However, oxidative stress and inflammatory stimuli can also cause stem cell senescence or mutation that leads to the abovementioned diseases.
This Topical Collection welcomes manuscripts (including original research articles and comprehensive reviews) providing insight into aspects of the relationship between chronic inflammation, oxidative stress, and adult stem cells. We are interested in a wide range of work, including experimental, preclinical, and clinical studies.
We look forward to your contributions.
Dr. Li Xiao
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- chronic inflammation
- adult stem cells
- oxidative stress
- reactive oxygen species (ROS)
- regenerative medicine
- tissue engineering
- cell culture
- DNA damage
- aging-associated disease
- SASP (senescence-associated secretory phenotype)
Published Papers (7 papers)
Open AccessReview
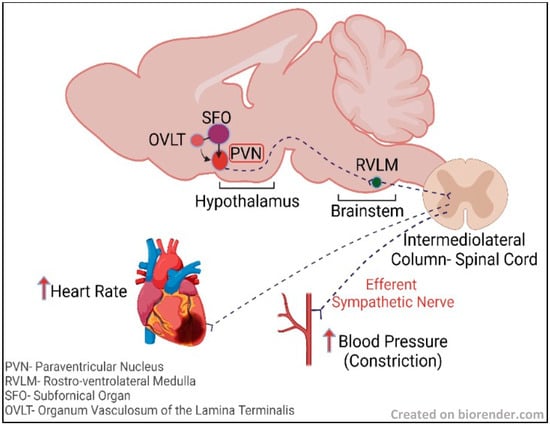
Implications of Hypothalamic Neural Stem Cells on Aging and Obesity-Associated Cardiovascular Diseases
by
Bhuvana Plakkot, Ashley Di Agostino and Madhan Subramanian
Cited by 4 | Viewed by 2108
Abstract
The hypothalamus, one of the major regulatory centers in the brain, controls various homeostatic processes, and hypothalamic neural stem cells (htNSCs) have been observed to interfere with hypothalamic mechanisms regulating aging. NSCs play a pivotal role in the repair and regeneration of brain
[...] Read more.
The hypothalamus, one of the major regulatory centers in the brain, controls various homeostatic processes, and hypothalamic neural stem cells (htNSCs) have been observed to interfere with hypothalamic mechanisms regulating aging. NSCs play a pivotal role in the repair and regeneration of brain cells during neurodegenerative diseases and rejuvenate the brain tissue microenvironment. The hypothalamus was recently observed to be involved in neuroinflammation mediated by cellular senescence. Cellular senescence, or systemic aging, is characterized by a progressive irreversible state of cell cycle arrest that causes physiological dysregulation in the body and it is evident in many neuroinflammatory conditions, including obesity. Upregulation of neuroinflammation and oxidative stress due to senescence has the potential to alter the functioning of NSCs. Various studies have substantiated the chances of obesity inducing accelerated aging. Therefore, it is essential to explore the potential effects of htNSC dysregulation in obesity and underlying pathways to develop strategies to address obesity-induced comorbidities associated with brain aging. This review will summarize hypothalamic neurogenesis associated with obesity and prospective NSC-based regenerative therapy for the treatment of obesity-induced cardiovascular conditions.
Full article
►▼
Show Figures
Open AccessArticle
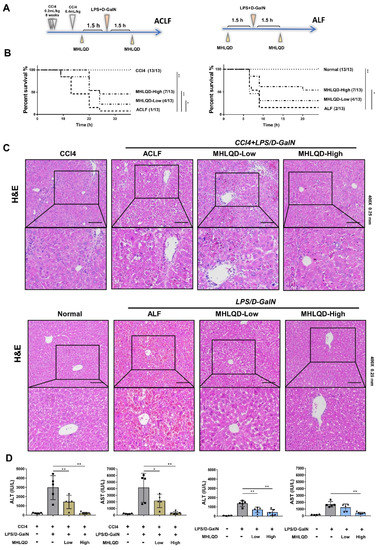
Ancient Herbal Formula Mahuang Lianqiao Chixiaodou Decoction Protects Acute and Acute-on-Chronic Liver Failure via Inhibiting von Willebrand Factor Signaling
by
Jiacheng Lin, Qihua Ling, Liang Yan, Bowu Chen, Fang Wang, Yihan Qian, Yueqiu Gao, Qian Wang, Hailong Wu, Xuehua Sun, Yanjun Shi and Xiaoni Kong
Cited by 4 | Viewed by 1805
Abstract
Background: Acute liver failure (ALF) and acute-on-chronic liver failure (ACLF) are characterized by systemic inflammation and high mortality, but there is no effective clinical treatment. As a classic traditional Chinese medicine (TCM) formula, MaHuang-LianQiao-ChiXiaoDou decoction (MHLQD) has been used clinically for centuries to
[...] Read more.
Background: Acute liver failure (ALF) and acute-on-chronic liver failure (ACLF) are characterized by systemic inflammation and high mortality, but there is no effective clinical treatment. As a classic traditional Chinese medicine (TCM) formula, MaHuang-LianQiao-ChiXiaoDou decoction (MHLQD) has been used clinically for centuries to treat liver diseases. Methods: The LPS/D−GalN-induced ALF mice model and the CCl
4+LPS/D−GalN-induced ACLF mice model were used to observe the therapeutic effects of MHLQD on mice mortality, hepatocytes death, liver injury, and immune responses. Results: MHLQD treatment significantly improved mice mortality. Liver injury and systemic and hepatic immune responses were also ameliorated after MHLQD treatment. Mechanistically, proteomic changes in MHLQD-treated liver tissues were analyzed and the result showed that the thrombogenic von Willebrand factor (VWF) was significantly inhibited in MHLQD-treated ALF and ACLF models. Histological staining and western blotting confirmed that VWF/RAP1B/ITGB3 signaling was suppressed in MHLQD-treated ALF and ACLF models. Furthermore, mice treated with the VWF inhibitor ADAMTS13 showed a reduced therapeutic effect from MHLQD treatment. Conclusions: Our study indicated that MHLQD is an effective herbal formula for the treatment of ALF and ACLF, which might be attributed to the protection of hepatocytes from death via VWF/RAP1B/ITGB3 signaling.
Full article
►▼
Show Figures
Open AccessArticle
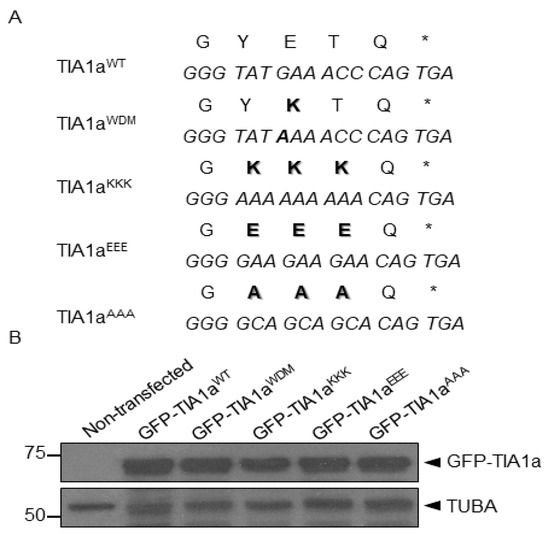
Dynamics of T-Cell Intracellular Antigen 1-Dependent Stress Granules in Proteostasis and Welander Distal Myopathy under Oxidative Stress
by
Andrea Fernández-Gómez, Beatriz Ramos Velasco and José M. Izquierdo
Viewed by 2498
Abstract
T-cell intracellular antigen 1 (TIA1) is an RNA-binding protein that is primarily involved in the post-transcriptional regulation of cellular RNAs. Furthermore, it is a key component of stress granules (SGs), RNA, and protein aggregates that are formed in response to stressful stimuli to
[...] Read more.
T-cell intracellular antigen 1 (TIA1) is an RNA-binding protein that is primarily involved in the post-transcriptional regulation of cellular RNAs. Furthermore, it is a key component of stress granules (SGs), RNA, and protein aggregates that are formed in response to stressful stimuli to reduce cellular activity as a survival mechanism. TIA1 p.E384K mutation is the genetic cause of Welander distal myopathy (WDM), a late-onset muscular dystrophy whose pathogenesis has been related to modifying SG dynamics. In this study, we present the results obtained by analyzing two specific aspects: (i) SGs properties and dynamics depending on the amino acid at position 384 of TIA1; and (ii) the formation/disassembly time-course of TIA1
WT/WDM-dependent SGs under oxidative stress. The generation of TIA1 variants—in which the amino acid mutated in WDM and the adjacent ones were replaced by lysines, glutamic acids, or alanines—allowed us to verify that the inclusion of a single lysine is necessary and sufficient to alter SGs dynamics. Moreover, time-lapse microscopy analysis allowed us to establish in vivo the dynamics of TIA1
WT/WDM-dependent SG formation and disassembly, after the elimination of the oxidizing agent, for 1 and 3 h, respectively. Our observations show distinct dynamics between the formation and disassembly of TIA1
WT/WDM-dependent SGs. Taken together, this study has allowed us to expand the existing knowledge on the role of TIA1 and the WDM mutation in SG formation.
Full article
►▼
Show Figures
Open AccessArticle
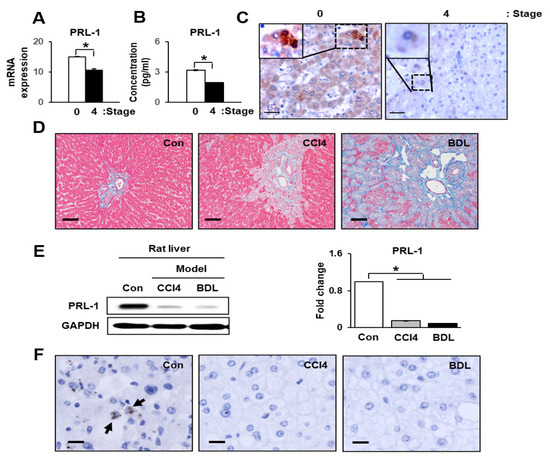
Increased Phosphatase of Regenerating Liver-1 by Placental Stem Cells Promotes Hepatic Regeneration in a Bile-Duct-Ligated Rat Model
by
Jong Ho Choi, Sohae Park, Gi Dae Kim, Jae Yeon Kim, Ji Hye Jun, Si Hyun Bae, Soon Koo Baik, Seong-Gyu Hwang and Gi Jin Kim
Cited by 2 | Viewed by 2257
Abstract
Phosphatase of regenerating liver-1 (PRL-1) controls various cellular processes and liver regeneration. However, the roles of PRL-1 in liver regeneration induced by chorionic-plate-derived mesenchymal stem cells (CP-MSCs) transplantation remain unknown. Here, we found that increased PRL-1 expression by CP-MSC transplantation enhanced liver regeneration
[...] Read more.
Phosphatase of regenerating liver-1 (PRL-1) controls various cellular processes and liver regeneration. However, the roles of PRL-1 in liver regeneration induced by chorionic-plate-derived mesenchymal stem cells (CP-MSCs) transplantation remain unknown. Here, we found that increased PRL-1 expression by CP-MSC transplantation enhanced liver regeneration in a bile duct ligation (BDL) rat model by promoting the migration and proliferation of hepatocytes. Engrafted CP-MSCs promoted liver function via enhanced hepatocyte proliferation through increased PRL-1 expression in vivo and in vitro. Moreover, higher increased expression of PRL-1 regulated CP-MSC migration into BDL-injured rat liver through enhancement of migration-related signals by increasing Rho family proteins. The dual effects of PRL-1 on proliferation of hepatocytes and migration of CP-MSCs were substantially reduced when PRL-1 was silenced with siRNA-PRL-1 treatment. These findings suggest that PRL-1 may serve as a multifunctional enhancer for therapeutic applications of CP-MSC transplantation.
Full article
►▼
Show Figures
Open AccessArticle
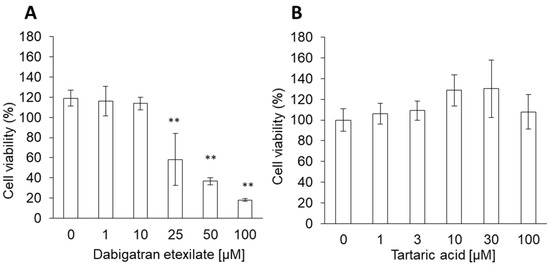
Dabigatran Etexilate Induces Cytotoxicity in Rat Gastric Epithelial Cell Line via Mitochondrial Reactive Oxygen Species Production
by
Hiromi Kurokawa, Atsushi Taninaka, Hidemi Shigekawa and Hirofumi Matsui
Cited by 5 | Viewed by 2159
Abstract
Dabigatran is a novel oral anticoagulant that directly inhibits free and fibrin-bound thrombins and exerts rapid and predictable anticoagulant effects. While the use of this reagent has been associated with an increased risk of gastrointestinal bleeding, the reason why dabigatran use increases gastrointestinal
[...] Read more.
Dabigatran is a novel oral anticoagulant that directly inhibits free and fibrin-bound thrombins and exerts rapid and predictable anticoagulant effects. While the use of this reagent has been associated with an increased risk of gastrointestinal bleeding, the reason why dabigatran use increases gastrointestinal bleeding risk remains unknown. We investigated the cytotoxicity of dabigatran etexilate and tartaric acid, the two primary components of dabigatran. The cytotoxicity of dabigatran etexilate and tartaric acid was measured in a cell viability assay. Intracellular mitochondrial reactive oxygen species (mitROS) production and lipid peroxidation were measured using fluorescence dyes. Cell membrane viscosity was measured using atomic force microscopy. The potential of ascorbic acid as an inhibitor of dabigatran cytotoxicity was also evaluated. The cytotoxicity of dabigatran etexilate was higher than that of tartaric acid. Dabigatran etexilate induced mitROS production and lipid peroxidation and altered the cell membrane viscosity. Ascorbic acid inhibited the cytotoxicity and mitROS production induced by dabigatran etexilate. Therefore, we attributed the cytotoxicity of dabigatran to dabigatran etexilate, and proposed that the cytotoxic effects of dabigatran etexilate are mediated via mitROS production. Additionally, we demonstrated that dabigatran cytotoxicity can be prevented via antioxidant treatment.
Full article
►▼
Show Figures
Open AccessArticle
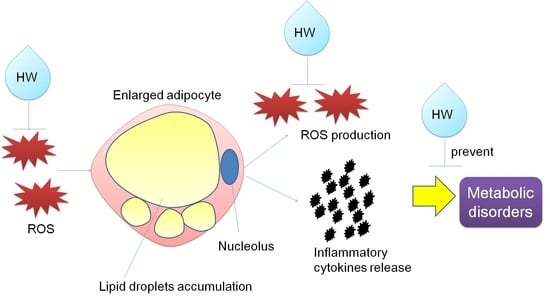
Hydrogen Nano-Bubble Water Suppresses ROS Generation, Adipogenesis, and Interleukin-6 Secretion in Hydrogen-Peroxide- or PMA-Stimulated Adipocytes and Three-Dimensional Subcutaneous Adipose Equivalents
by
Li Xiao and Nobuhiko Miwa
Cited by 16 | Viewed by 4394
Abstract
Reactive oxygen species (ROS)-induced oxidative stress in adipose tissue is associated with inflammation and the development of obesity-related metabolic disorders. The aim of this study is to investigate the effects of hydrogen nano-bubble water (HW) on ROS generation, adipogenesis, and interleukin-6 (IL-6) secretion
[...] Read more.
Reactive oxygen species (ROS)-induced oxidative stress in adipose tissue is associated with inflammation and the development of obesity-related metabolic disorders. The aim of this study is to investigate the effects of hydrogen nano-bubble water (HW) on ROS generation, adipogenesis, and interleukin-6 (IL-6) secretion in hydrogen peroxide (H
2O
2) or phorbol 12-myristate 13-acetate (PMA)-stimulated OP9 adipocytes, and three-dimensional (3D) subcutaneous adipose equivalents. Nanoparticle tracking analysis showed that fresh HW contains 1.17 × 10
8/mL of nano-sized hydrogen bubbles. Even after 8 to 13 months of storage, approximately half of the bubbles still remained in the water. CellROX
® staining showed that HW could diminish H
2O
2- or PMA-induced intracellular ROS generation in human keratinocytes HaCaT and OP9 cells. We discovered that PMA could markedly increase lipid accumulation to 180% and IL-6 secretion 2.7-fold in OP9 adipocytes. Similarly, H
2O
2 (5 µM) also significantly stimulated lipid accumulation in OP9 cells and the 3D adipose equivalents. HW treatment significantly repressed H
2O
2- or PMA-induced lipid accumulation and IL-6 secretion in OP9 adipocytes and the 3D adipose equivalents. In conclusion, HW showed a possibility of repressing oxidative stress, inflammatory response, and adipogenesis at cellular/tissue levels. It can be used for preventing the development of metabolic disorders amongst obese people.
Full article
►▼
Show Figures
Open AccessArticle
Senescence-Associated Secretory Phenotype Suppression Mediated by Small-Sized Mesenchymal Stem Cells Delays Cellular Senescence through TLR2 and TLR5 Signaling
by
Ji Hye Kwon, Miyeon Kim, Soyoun Um, Hyang Ju Lee, Yun Kyung Bae, Soo Jin Choi, Hyun Ho Hwang, Wonil Oh and Hye Jin Jin
Cited by 12 | Viewed by 2857
Abstract
In order to provide a sufficient number of cells for clinical use, mesenchymal stem cells (MSCs) must be cultured for long-term expansion, which inevitably triggers cellular senescence. Although the small size of MSCs is known as a critical determinant of their fate, the
[...] Read more.
In order to provide a sufficient number of cells for clinical use, mesenchymal stem cells (MSCs) must be cultured for long-term expansion, which inevitably triggers cellular senescence. Although the small size of MSCs is known as a critical determinant of their fate, the main regulators of stem cell senescence and the underlying signaling have not been addressed. Umbilical cord blood-derived MSCs (UCB-MSCs) were obtained using size-isolation methods and then cultured with control or small cells to investigate the major factors that modulate MSC senescence. Cytokine array data suggested that the secretion of interukin-8 (IL-8) or growth-regulated oncogene-alpha (GROa) by senescent cells was markedly inhibited during incubation of small cells along with suppression of cognate receptor (C-X-C motif chemokine receptor2, CXCR2) via blockade of the autocrine/paracrine positive loop. Moreover, signaling via toll-like receptor 2 (TLR2) and TLR5, both pattern recognition receptors, drove cellular senescence of MSCs, but was inhibited in small cells. The activation of TLRs (2 and 5) through ligand treatment induced a senescent phenotype in small cells. Collectively, our data suggest that small cell from UCB-MSCs exhibit delayed cellular senescence by inhibiting the process of TLR signaling-mediated senescence-associated secretory phenotype (SASP) activation.
Full article
►▼
Show Figures
Planned Papers
The below list represents only planned manuscripts. Some of these
manuscripts have not been received by the Editorial Office yet. Papers
submitted to MDPI journals are subject to peer-review.
Title: Therapeutic potential of mesenchymal stem cell-derived microRNAs in the treatment of inflammatory diseases
Authors: Carl Randall Harrell, Valentin Djonov, Vladislav Volarevic; C. Randall Harrell, M.D.
Affiliation: /
Abstract: Mesenchymal stem cells (MSC) are adult stem cells which produce immunomodulatory molecules (proteins, lipids and nucleic acids (messenger RNA, long non-coding RNA, microRNA (miRNA), and circular RNA)) that alter phenotype and function of immune cells. MSC efficiently suppress generation of inflammatory macrophages, dendritic cells and T lymphocytes and promote expansion of immunosuppressive immune cells, leading to the attenuation of on-going inflammation. MSC-sourced exosomes (MSC-Exos) are MSC-derived extracellular vesicles that contain all immunoregulatory molecules as their parental cells. Due to their nano-sized dimension, MSC-Exos easily penetrate through the tissue and deliver their content directly in the target immune cells. A large number of experimental studies demonstrated that MSC-sourced miRNAs played crucially important role in the MSC-Exo-based immunosuppression. In this review article, we summarized current knowledge about molecular mechanisms responsible for immunoregulatory properties of MSC-derived miRNAs, emphasizing their therapeutic potential in the treatment of inflammatory diseases.