Multiple Myeloma and Cellular Therapies
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Cellular Pathology".
Viewed by 22321
Share This Topical Collection
Editor
 Dr. Francesca Patriarca
Dr. Francesca Patriarca
 Dr. Francesca Patriarca
Dr. Francesca Patriarca
E-Mail
Website
Collection Editor
1. Clinica Ematologica e Unità di Terapie Cellulari, Azienda Sanitaria Universitaria Friuli Centrale, 33100 Udine, Italy
2. Dipartimento di Area Medica, Università di Udine, 33100 Udine, Italy
Interests: hematology-oncology; multiple myeloma; hematopoietic stem cell transplantation; cellular therapies
Topical Collection Information
Dear Colleagues,
Although the introduction of innovative drug therapies has led to the improvement of median overall survival from 3 to 6–7 years in the last 2 decades, multiple myeloma (MM) remains an incurable disease. The most recent clinical research includes monoclonal antibodies and cellular therapies. In this Topical Collection, we will focus on the following items: (1) high dose therapy followed by the reinfusion of autologous stem cells is the “gold standard” for newly diagnosed MM; however, the eligibility of elderly patients, new conditioning regimens, and novel drugs before and after autologous transplant are areas of current active investigation; (2) allogeneic stem cell transplantation can be reserved to fit patients with very high-risk MM, but transplant indications and strategies to reduce transplant-related mortality and the relapse risk are controversial; (3) chimeric antigen receptor (CAR) T cell therapy directed to B cell maturation antigen (BCMA) is the most innovative treatment for relapsed and refractory disease with several phase II and III studies recruiting worldwide, and with ongoing basic research aiming to improve T cell engineering and persistence.
We look forward to your contributions.
Dr. Francesca Patriarca
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- autologous stem cell transplantation
- allogeneic stem cell transplantation
- CAR-T cells
- donor lymphocyte infusions
Published Papers (5 papers)
Open AccessReview
Autologous Stem Cell Transplantation in Multiple Myeloma: Where Are We and Where Do We Want to Go?
by
Sonia Morè, Laura Corvatta, Valentina Maria Manieri, Francesco Saraceni, Ilaria Scortechini, Giorgia Mancini, Alessandro Fiorentini, Attilio Olivieri and Massimo Offidani
Cited by 10 | Viewed by 4361
Abstract
The introduction of high-dose therapy in the 1990s as well as the development of drugs such as thalidomide, lenalidomide, and bortezomib in the 2000s led to an impressive improvement in outcome of patients with multiple myeloma (MM) eligible for autologous stem cell transplantation
[...] Read more.
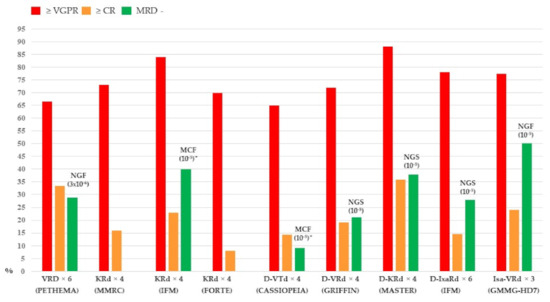
The introduction of high-dose therapy in the 1990s as well as the development of drugs such as thalidomide, lenalidomide, and bortezomib in the 2000s led to an impressive improvement in outcome of patients with multiple myeloma (MM) eligible for autologous stem cell transplantation (ASCT). Clinical trials conducted in the first ten years of the twenty-first century established as standard therapy for these patients a therapeutic approach including induction, single or double ASCT, consolidation, and maintenance therapy. More recently, incorporating second-generation proteasome inhibitors carfilzomib and monoclonal antibody daratumumab into each phase of treatment significantly improved the efficacy of ASCT in terms of measurable residual disease (MRD) negativity, Progression Free Survival (PFS), and Overall Survival (OS). The availability of techniques such as multiparameter flow cytometry (MFC) and next-generation sequencing (NGS) for MRD assessment allowed the design of MRD-based response-adjusted trials that will define, in particular, the role of consolidation and maintenance therapies. In this review, we will provide an overview of the most recent evidence and the future prospects of ASCT in MM patients.
Full article
►▼
Show Figures
Open AccessReview
Adoptive Cellular Therapy for Multiple Myeloma Using CAR- and TCR-Transgenic T Cells: Response and Resistance
by
Franziska Füchsl and Angela M. Krackhardt
Cited by 10 | Viewed by 5058
Abstract
Despite the substantial improvement of therapeutic approaches, multiple myeloma (MM) remains mostly incurable. However, immunotherapeutic and especially T cell-based approaches pioneered the therapeutic landscape for relapsed and refractory disease recently. Targeting B-cell maturation antigen (BCMA) on myeloma cells has been demonstrated to be
[...] Read more.
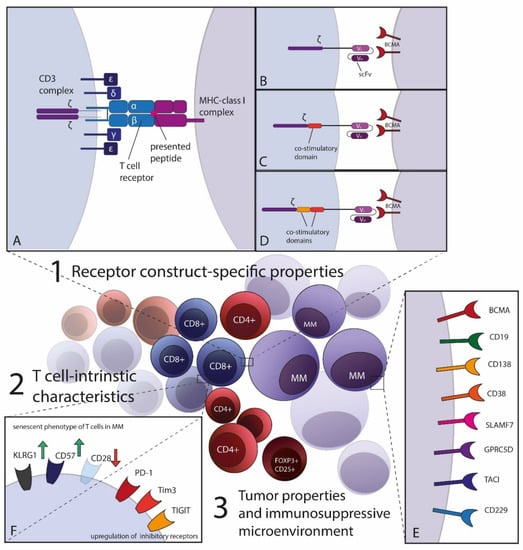
Despite the substantial improvement of therapeutic approaches, multiple myeloma (MM) remains mostly incurable. However, immunotherapeutic and especially T cell-based approaches pioneered the therapeutic landscape for relapsed and refractory disease recently. Targeting B-cell maturation antigen (BCMA) on myeloma cells has been demonstrated to be highly effective not only by antibody-derived constructs but also by adoptive cellular therapies. Chimeric antigen receptor (CAR)-transgenic T cells lead to deep, albeit mostly not durable responses with manageable side-effects in intensively pretreated patients. The spectrum of adoptive T cell-transfer covers synthetic CARs with diverse specificities as well as currently less well-established T cell receptor (TCR)-based personalized strategies. In this review, we want to focus on treatment characteristics including efficacy and safety of CAR- and TCR-transgenic T cells in MM as well as the future potential these novel therapies may have. ACT with transgenic T cells has only entered clinical trials and various engineering strategies for optimization of T cell responses are necessary to overcome therapy resistance mechanisms. We want to outline the current success in engineering CAR- and TCR-T cells, but also discuss challenges including resistance mechanisms of MM for evading T cell therapy and point out possible novel strategies.
Full article
►▼
Show Figures
Open AccessReview
Harnessing the Potential of NK Cell-Based Immunotherapies against Multiple Myeloma
by
Chantal Reina-Ortiz, David Giraldos, Gemma Azaceta, Luis Palomera, Isabel Marzo, Javier Naval, Martín Villalba and Alberto Anel
Cited by 8 | Viewed by 4526
Abstract
Natural killer (NK) cell-based therapies have emerged as promising anticancer treatments due to their potency as cytolytic effectors and synergy with concurrent treatments. Multiple myeloma (MM) is an aggressive B-cell malignancy that, despite development of novel therapeutic agents, remains incurable with a high
[...] Read more.
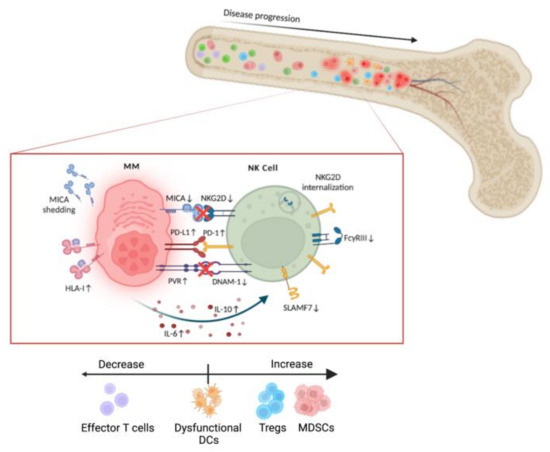
Natural killer (NK) cell-based therapies have emerged as promising anticancer treatments due to their potency as cytolytic effectors and synergy with concurrent treatments. Multiple myeloma (MM) is an aggressive B-cell malignancy that, despite development of novel therapeutic agents, remains incurable with a high rate of relapse. In MM, the inhospitable tumor microenvironment prevents host NK cells from exerting their cytolytic function. The development of NK cell immunotherapy works to overcome this altered immune landscape and can be classified in two major groups based on the origin of the cell: autologous or allogeneic. In this review, we compare the treatments in each group, such as autologous chimeric antigen receptor (CAR) NKs and allogeneic off-the-shelf NK cell infusions, and their combinatorial effect with existing MM therapies including monoclonal antibodies and proteasome inhibitors. We also discuss their placement in clinical treatment regimens based on the immune profile of each patient. Through this examination, we would like to discover precisely when each NK cell-based treatment will produce the maximum benefit to the MM patient.
Full article
►▼
Show Figures
Open AccessReview
Risk Stratification and Treatment in Smoldering Multiple Myeloma
by
Tyler Lussier, Natalie Schoebe and Sabine Mai
Cited by 4 | Viewed by 2669
Abstract
Smoldering multiple myeloma is a heterogeneous asymptomatic precursor to multiple myeloma. Since its identification in 1980, risk stratification models have been developed using two main stratification methods: clinical measurement-based and genetics-based. Clinical measurement models can be subdivided in three types: baseline measurements (performed
[...] Read more.
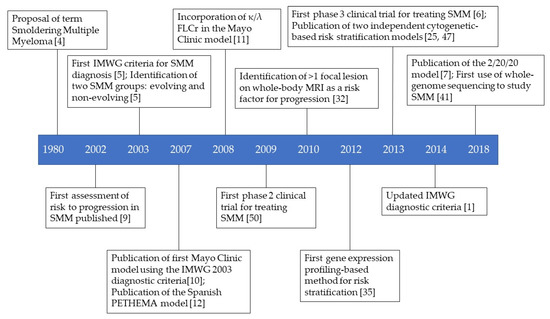
Smoldering multiple myeloma is a heterogeneous asymptomatic precursor to multiple myeloma. Since its identification in 1980, risk stratification models have been developed using two main stratification methods: clinical measurement-based and genetics-based. Clinical measurement models can be subdivided in three types: baseline measurements (performed at diagnosis), evolving measurements (performed over time during follow-up appointments), and imaging (for example, magnetic resonance imaging). Genetic approaches include gene expression profiling, DNA/RNA sequencing, and cytogenetics. It is important to accurately distinguish patients with indolent disease from those with aggressive disease, as clinical trials have shown that patients designated as “high-risk of progression” have improved outcomes when treated early. The risk stratification models, and clinical trials are discussed in this review.
Full article
►▼
Show Figures
Open AccessArticle
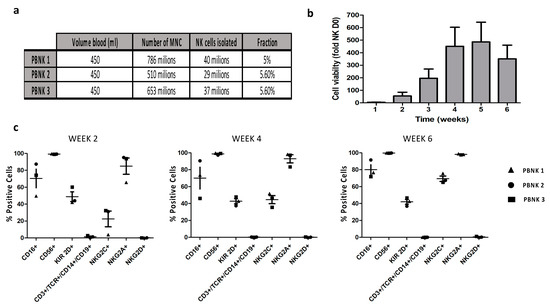
Selection, Expansion, and Unique Pretreatment of Allogeneic Human Natural Killer Cells with Anti-CD38 Monoclonal Antibody for Efficient Multiple Myeloma Treatment
by
Benjamin Motais, Sandra Charvátová, Zuzana Walek, Matouš Hrdinka, Ryszard Smolarczyk, Tomasz Cichoń, Justyna Czapla, Sebastian Giebel, Michal Šimíček, Tomáš Jelínek, Tereza Ševčíková, Jiří Sobotka, Zdeněk Kořístek, Roman Hájek and Juli R. Bagó
Cited by 9 | Viewed by 4862
Abstract
Cellular immunotherapy is becoming a new pillar in cancer treatment after recent striking results in different clinical trials with chimeric antigen receptor T cells. However, this innovative therapy is not exempt from challenges such as off-tumor toxicity, tumor recurrence in heterogeneous tumors, and
[...] Read more.
Cellular immunotherapy is becoming a new pillar in cancer treatment after recent striking results in different clinical trials with chimeric antigen receptor T cells. However, this innovative therapy is not exempt from challenges such as off-tumor toxicity, tumor recurrence in heterogeneous tumors, and affordability. To surpass these limitations, we exploit the unique anti-tumor characteristics of natural killer (NK) cells. In this study, we aimed to obtain a clinically relevant number of allogeneic NK cells derived from peripheral blood (median of 14,050 million cells from a single donor) to target a broad spectrum of solid and liquid tumor types. To boost their anti-tumor activity, we combined allogeneic NK cells with the approved anti-cluster of differentiation 38 (CD-38) monoclonal antibody Daratumumab to obtain a synergistic therapeutic effect against incurable multiple myeloma. The combination therapy was refined with CD16 polymorphism donor selection and uncomplicated novel in vitro pretreatment to avoid undesired fratricide, increasing the in vitro therapeutic effect against the CD-38 positive multiple myeloma cell line by more than 20%. Time-lapse imaging of mice with established human multiple myeloma xenografts revealed that combination therapy of selected and pretreated NK cells with Daratumumab presented tumor volumes 43-fold smaller than control ones. Combination therapy with an allogeneic source of fully functional NK cells could be beneficial in future clinical settings to circumvent monoclonal antibodies’ low therapeutic efficiency due to NK cell dysfunctionality in MM patients.
Full article
►▼
Show Figures