Mitochondrial Dysfunction in Kidney Diseases
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Autophagy".
Viewed by 3643Editor
Interests: mitophagy; mitochondrial dysfunction; autophagy; immune cells; macrophages; kidney diseases; kidney fibrosis
Topical Collection Information
Dear Colleagues,
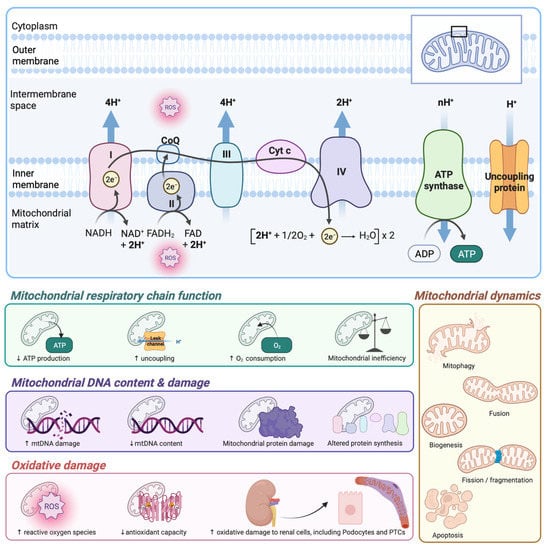
Mitochondria satisfy the high metabolic needs of the kidney and efficiently combat kidney injury-induced stresses. The kidney has the highest number of mitochondria after the heart. Mitochondrial structural and functional aberrations are widely reported during both acute kidney injury (AKI) and chronic kidney disease (CKD). Mitochondrial dysfunction is an early event during kidney injury, and exerts a critical role in exaggerating inflammation in various forms of AKI, including cisplatin, sepsis, or ischemia-reperfusion-mediated kidney injuries. Defects in mitochondrial quality control and bioenergetics are also known to promote inflammatory and fibrotic responses and progression of tubulointerstitial fibrosis in different forms of CKD, including diabetic, membranous, and IgA nephropathies and polycystic kidney disease.
Both the production of new mitochondrial networks and recycling of dysfunctional mitochondria via mitophagy in the kidney are crucial, and help maintain the overall metabolic status, sensing and responding to different triggers and oxidative stress. The balance between mitochondrial fusion and fission processes also influences mitochondrial structure and functions. Hyperfused mitochondria produce higher energy, while fragmented mitochondria with impaired membrane potentials are recycled through mitophagy. The therapeutic approaches that help in regulating mitochondrial health have the potential to attenuate cell death related to kidney injury-induced inflammation, tissue disruption, failure of tissue repair, and resultant tubulointerstitial fibrosis and progression of CKD to end-stage kidney disease.
In this Special Issue, we invite submissions focused on investigating mitochondrial functions, associated molecular pathways including mitophagy, approaches to study mitochondrial dynamics (fusion/fission) during AKI and CKD using different experimental models of kidney diseases, and mitochondria-targeted potential therapeutic strategies.
Dr. Divya Bhatia
Guest Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- mitochondrial dynamics
- mitophagy
- mitochondrial fusion
- mitochondrial fission
- oxidative stress
- acute kidney injury
- chronic kidney disease
- inflammation
- kidney fibrosis