Carbon-Based Materials for Hydrogen Production, Storage and Conversion
Share This Topical Collection
Editors
 Dr. Nikolaos Kostoglou
Dr. Nikolaos Kostoglou
 Dr. Nikolaos Kostoglou
Dr. Nikolaos Kostoglou
E-Mail
Website
Guest Editor
Department of Materials Science, Montanuniversität Leoben, Leoben, Austria
Interests: nanomaterials; nanoporous materials; functionalized carbons; metal-decorated carbons; nanocomposites; nanoparticles; carbon nanostructures; few-layer graphene; carbon nanotubes; graphene oxide foams; activated carbons; boron nitride nanostructures; plasma treatment; gas adsorption; hydrogen storage; selective gas separation; electrochemical energy storage; supercapacitors; water splitting; water purification; detection of chemical and biological substances; surface enhanced Raman spectroscopy
Special Issues, Collections and Topics in MDPI journals
 Dr. Claus Rebholz
Dr. Claus Rebholz
 Dr. Claus Rebholz
Dr. Claus Rebholz
E-Mail
Website
Guest Editor
Department of Mechanical and Manufacturing Engineering, University of Cyprus, Nicosia, Cyprus
Interests: thin films and coatings; surface engineering technologies; nanostructured materials; nanoscale manufacturing technologies; thermal manufacturing and joining processes; carbon materials and energy; engineering design
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
The aim of this Special Issue is to present the state-of-the-art research progress in the emerging field of carbon-based nanoporous materials for hydrogen (H2) production, storage, and conversion purposes. H2 is considered to be the ideal carbon-free energy carrier for stationary, mobile, and portable applications, in addition to being the most promising alternative to fossil fuel combustion. Nanoporous carbons and novel composites thereof could play a key role in the development of H2 technologies and provide practical solutions for open issues and on-going challenges. Carbon-based materials (e.g., activated carbons, fullerenes, carbon nanotubes, fibers, foams, and ordered mesoporous carbons) have attracted significant attention in the past four decades due to their large surface areas and pore volumes, lightweight nature, plethora of macroscopic forms and nanostructures, mass-scale availability, excellent recyclability, and low manufacturing cost. Even more attractive and promising carbonaceous materials have emerged in recent years, including 0D (e.g., carbon dots), 1D (e.g., carbon nanowires), 2D (e.g., graphene flakes), and 3D (e.g., graphene/graphene oxide foams) nanostructures and novel nanocomposites (e.g., carbons decorated with nanoparticles, doped with heteroatoms, etc.). This Special Issue will highlight the implementation of different carbons and composite structures produced in various forms (e.g., powders, monoliths, cloths, membranes, electrodes, thin films, coatings, etc.) for advanced applications related to H2 generation (e.g., electrolyzers), solid-state H2 storage (e.g., physical or chemical adsorption), and H2 conversion (e.g., fuel cells). Emphasis will be given to the effect of the porosity-related properties (e.g., surface area, pore volume, pore size distribution, average pore size, etc.) and surface chemistry characteristics (e.g., functionalities, dopants, etc.) on the respective performance. Both experimental and theoretical studies are welcome for submission in this Special Issue.
Dr. Nikolaos Kostoglou
Dr. Claus Rebholz
Guest Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. C is an international peer-reviewed open access quarterly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 1600 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- nanoporous carbons
- novel composites
- hydrogen production
- hydrogen storage
- physical adsorption
- chemical adsorption
- hydrogen conversion
- fuel cells
Published Papers (17 papers)
Open AccessArticle
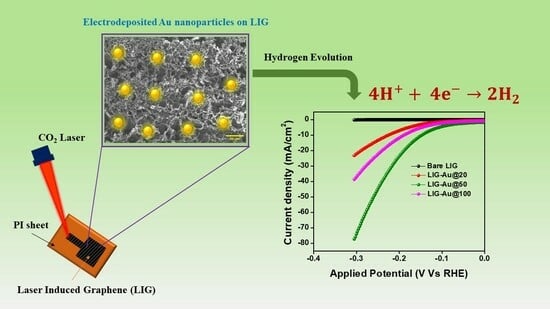
Fabrication of Gold Nanoparticles Embedded Laser-Induced Graphene (LIG) Electrode for Hydrogen Evolution Reaction
by
Deepak Deepak, Vennela Vuruputuri, Gourav Bhattacharya, James A. McLaughlin and Susanta Sinha Roy
Viewed by 1708
Abstract
The advancement of renewable energy technologies like water electrolysis and hydrogen fuel cells relies on the fabrication of effective and reliable catalysts for the hydrogen evolution process (HER). In this regard, we report gold nanoparticles embedded in laser-induced graphene electrodes for regulation of
[...] Read more.
The advancement of renewable energy technologies like water electrolysis and hydrogen fuel cells relies on the fabrication of effective and reliable catalysts for the hydrogen evolution process (HER). In this regard, we report gold nanoparticles embedded in laser-induced graphene electrodes for regulation of overpotential and electrocatalytic performance of hydrogen evolution reaction. Gold nanoparticles were deposited onto the LIG surface using electrode deposition via cyclic voltammetry (CV) at different cycle lengths. The catalyst fabrication technique enables the manipulation of many electrochemical parameters, such as overpotential value, charge transfer resistance, electrochemical active surface area, and tafel slope, through the adjustment of cyclic voltammetry (CV) cycles. The LIG-Au@50 sample demonstrates remarkable electrocatalytic characteristics, as evidenced by its low overpotential of 141 mV at a current density of 10 mA/cm
2 and reduced tafel slope of 131 mV/decade in an acidic environment. Furthermore, the presence of an augmented electrochemical active surface area, a mass activity of 8.80 A/g, and a high turnover frequency of 0.0091 s
−1 suggest elevated and significant accessibility to plentiful active sites. A significant decrease in charge transfer resistance resulted in an enhanced rate of the water-splitting reaction.
Full article
►▼
Show Figures
Open AccessArticle
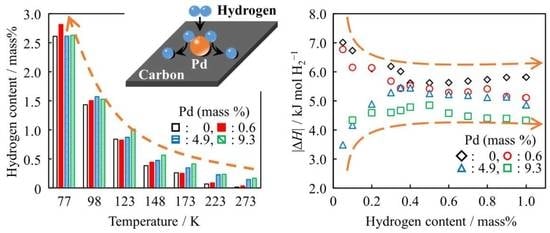
Temperature Dependence of Hydrogen Adsorption on Pd-Modified Carbon Blacks and Their Enthalpy-Entropy Changes
by
Takehiro Kaneko, Takeshi Toyama, Yoshiyuki Kojima and Nobuyuki Nishimiya
Viewed by 2775
Abstract
Metal-carbon composites have recently gained attention as potential hydrogen storage materials. In the present investigation, carbon blacks (CBs) with 0.6 mass %, 4.9 mass %, and 9.3 mass % of Pd were prepared to investigate the cooperative effect together with Pd and CB
[...] Read more.
Metal-carbon composites have recently gained attention as potential hydrogen storage materials. In the present investigation, carbon blacks (CBs) with 0.6 mass %, 4.9 mass %, and 9.3 mass % of Pd were prepared to investigate the cooperative effect together with Pd and CB for hydrogen storage. The hydrogen adsorption isotherms were measured at 77 K, 98 K, 123 K, 148 K, 173 K, 223 K, and 273 K under mild pressures below 1 MPa. The lower temperature gave the higher hydrogen content. Almost all the hydrogen contents of Pd-modified CBs exceeded the sum of the adsorption contents of CB and the occluded amounts of the assumed hydride, PdH
0.6. The highest hydrogen content was recorded for Pd 0.6 mass %-modified CB at 77 K. At temperatures above 77 K, CBs with the higher Pd contents adsorbed more hydrogen than Pd 0.6 mass %-modified CB, and they indicated an increase in the absolute values of adsorption enthalpy with the progress of adsorption. Pd was thought to be at first blocking deep potential sites, with accessibility to hydrogen acceptable sites gradually increasing as adsorption progressed.
Full article
►▼
Show Figures
Open AccessArticle
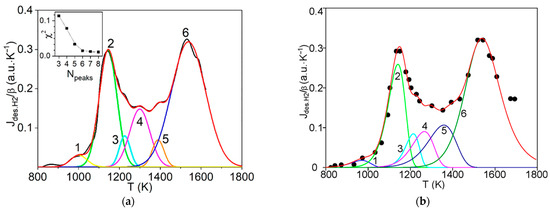
Revealing Hydrogen States in Carbon Structures by Analyzing the Thermal Desorption Spectra
by
Yury S. Nechaev, Evgeny A. Denisov, Nadezhda A. Shurygina, Alisa O. Cheretaeva, Ekaterina K. Kostikova, Sergei Yu. Davydov and Andreas Öchsner
Cited by 4 | Viewed by 2398
Abstract
An effective methodology for the detailed analysis of thermal desorption spectra (TDS) of hydrogen in carbon structures at micro- and nanoscale was further developed and applied for a number of TDS data of one heating rate, in particular, for graphite materials irradiated with
[...] Read more.
An effective methodology for the detailed analysis of thermal desorption spectra (TDS) of hydrogen in carbon structures at micro- and nanoscale was further developed and applied for a number of TDS data of one heating rate, in particular, for graphite materials irradiated with atomic hydrogen. The technique is based on a preliminary description of hydrogen desorption spectra by symmetric Gaussians with their special processing in the approximation of the first- and the second-order reactions. As a result, the activation energies and the pre-exponential factors of the rate constants of the hydrogen desorption processes are determined, analyzed and interpreted. Some final verification of the results was completed using methods of numerical simulation of thermal desorption peaks (non-Gaussians) corresponding to the first- and the second-order reactions. The main research finding of this work is a further refinement and/or disclosure of poorly studied characteristics and physics of various states of hydrogen in microscale graphite structures after irradiation with atomic hydrogen, and comparison with the related results for nanoscale carbon structures. This is important for understanding the behavior and relationship of hydrogen in a number of cases of high energy carbon-based materials and nanomaterials.
Full article
►▼
Show Figures
Open AccessArticle
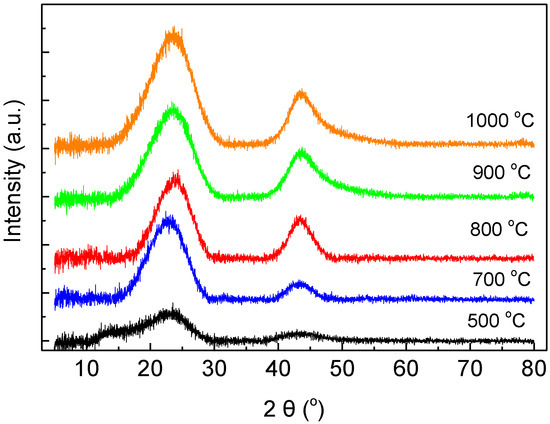
Carbon Membranes Prepared from Poly (Furfuryl Alcohol–Furfural) Precursors: Effect of FeCl3 Additive
by
Areti Zaharopoulou, Spyros N. Yannopoulos and Theophilos Ioannides
Cited by 5 | Viewed by 2713
Abstract
Thermosetting resins, such as poly (furfuryl alcohol), are efficient precursors for preparation of carbon membranes with molecular sieving properties. Polymerization of furfuryl alcohol is catalyzed by Bronsted or Lewis acids. FeCl
3, showing Lewis-acid behavior, is an interesting polymerization catalyst, because it
[...] Read more.
Thermosetting resins, such as poly (furfuryl alcohol), are efficient precursors for preparation of carbon membranes with molecular sieving properties. Polymerization of furfuryl alcohol is catalyzed by Bronsted or Lewis acids. FeCl
3, showing Lewis-acid behavior, is an interesting polymerization catalyst, because it gets reduced into metallic iron during pyrolysis of the resin, promoting transformation of amorphous carbon into graphitic domains. The goal of the present work was to examine whether use of FeCl
3 as a polymerization catalyst of furfuryl alcohol–furfural mixtures could lead to preparation of carbon membranes with improved gas separation performance compared to those prepared with use of p-toluenesulfonic acid. The resins were deposited onto tubular porous ceramic supports and pyrolyzed at temperatures in the range of 500–1000 °C. Material characterization was carried out by X-Ray Diffraction, N
2 physisorption, Raman spectroscopy and Scanning Electron Microscopy. The membrane performance was examined using H
2, CO
2 and CH
4 as probe molecules. It was found that the membranes operate mainly via the molecular sieving mechanism and the use of FeCl
3 instead of p-toluenesulfonic acid does not lead to an improvement in the permeation characteristics of the respective membranes.
Full article
►▼
Show Figures
Open AccessFeature PaperReview
Characterization of Carbon Materials for Hydrogen Storage and Compression
by
Giuseppe Sdanghi, Rafael L. S. Canevesi, Alain Celzard, Matthias Thommes and Vanessa Fierro
Cited by 38 | Viewed by 5975
Abstract
Carbon materials have proven to be a suitable choice for hydrogen storage and, recently, for hydrogen compression. Their developed textural properties, such as large surface area and high microporosity, are essential features for hydrogen adsorption. In this work, we first review recent advances
[...] Read more.
Carbon materials have proven to be a suitable choice for hydrogen storage and, recently, for hydrogen compression. Their developed textural properties, such as large surface area and high microporosity, are essential features for hydrogen adsorption. In this work, we first review recent advances in the physisorption characterization of nanoporous carbon materials. Among them, approaches based on the density functional theory are considered now standard methods for obtaining a reliable assessment of the pore size distribution (PSD) over the whole range from narrow micropores to mesopores. Both a high surface area and ultramicropores (pore width < 0.7 nm) are needed to achieve significant hydrogen adsorption at pressures below 1 MPa and 77 K. However, due to the wide PSD typical of activated carbons, it follows from an extensive literature review that pressures above 3 MP are needed to reach maximum excess uptakes in the range of ca. 7 wt.%. Finally, we present the adsorption–desorption compression technology, allowing hydrogen to be compressed at 70 MPa by cooling/heating cycles between 77 and 298 K, and being an alternative to mechanical compressors. The cyclic, thermally driven hydrogen compression might open a new scenario within the vast field of hydrogen applications.
Full article
►▼
Show Figures
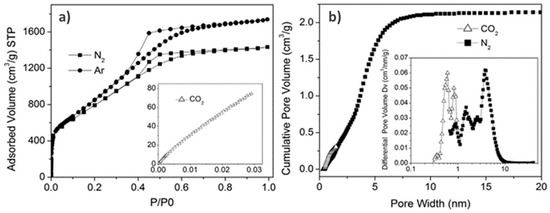
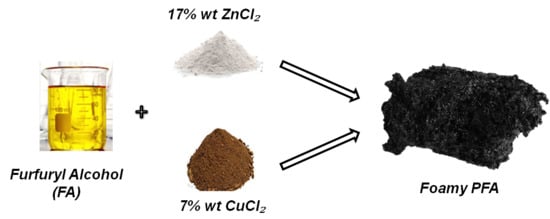
Open AccessArticle
Synthesis and Characterization of Activated Carbon Foam from Polymerization of Furfuryl Alcohol Activated by Zinc and Copper Chlorides
by
Elisabetta M. Cepollaro, Domenico Caputo, Stefano Cimino, Nicola Gargiulo and Luciana Lisi
Cited by 7 | Viewed by 3030
Abstract
Polymerization of furfuryl alcohol carried out using ZnCl
2 or CuCl
2 as Lewis acid activators was investigated by exploring various synthesis parameters in order to produce activated carbons with different porosity and metal load. The temperature of polymerization was changed according to
[...] Read more.
Polymerization of furfuryl alcohol carried out using ZnCl
2 or CuCl
2 as Lewis acid activators was investigated by exploring various synthesis parameters in order to produce activated carbons with different porosity and metal load. The temperature of polymerization was changed according to Lewis acidity strength of the two metal chlorides: 0 °C for CuCl
2 and 80 °C for ZnCl
2. The polymer obtained was pyrolyzed under pure He flow or under 1000 ppm O
2/He flow at 600 or 850 °C in order to produce activated carbons with specific textural features. The load and nature of the residual metal after pyrolysis were determined by ICP and XRD analyses, respectively. Copper was mostly preserved even at high pyrolysis temperature in contrast to zinc, which was almost totally lost at 850 °C. A foamy structure was detected by SEM analysis for all samples. Textural properties were determined by both N
2 and CO
2 physisorption; surface areas and pore size distributions were evaluated according to BET, DFT and DR models. The polymerization activated by ZnCl
2 produced carbons with larger surface areas were also related to the presence of some mesopores, whereas CuCl
2 promoted the prevailing formation of narrow micropores, making these materials particularly suited to H
2 storage applications.
Full article
►▼
Show Figures
Open AccessArticle
In Situ Formation of Metal Hydrides Inside Carbon Aerogel Frameworks for Hydrogen Storage Applications
by
Mohammad Reza Ghaani, Mahdi Alam, Michele Catti and Niall J. English
Cited by 3 | Viewed by 3005
Abstract
Nano-confined chemical reactions bear great promise for a wide range of important applications in the near-to-medium term, e.g., within the emerging area of chemical storage of renewable energy. To explore this important trend, in the present work, resorcinol-/formaldehyde-based carbon aerogels were prepared by
[...] Read more.
Nano-confined chemical reactions bear great promise for a wide range of important applications in the near-to-medium term, e.g., within the emerging area of chemical storage of renewable energy. To explore this important trend, in the present work, resorcinol-/formaldehyde-based carbon aerogels were prepared by sol-gel polymerisation of resorcinol, with furfural catalysed by a sodium-carbonate solution using ambient-pressure drying. These aerogels were further carbonised in nitrogen to obtain their corresponding carbon aerogels. Through this study, the synthesis parameters were selected in a way to obtain minimum shrinkage during the drying step. The microstructure of the product was observed using Scanning Electron Microscopy (SEM) and Field Emission Scanning Electron Microscopy (FESEM) imaging techniques. The optimised carbon aerogels were found to have pore sizes of ~21 nm with a specific accessible surface area equal to 854.0 m
2/g. Physical activation of the carbon aerogel with CO
2 generates activated carbon aerogels with a surface area of 1756 m
2/g and a total porosity volume up to 3.23 cm
3/g. The product was then used as a scaffold for magnesium/cobalt-hydride formation. At first, cobalt nanoparticles were formed inside the scaffold, by reducing the confined cobalt oxide, then MgH
2 was synthesised as the second required component in the scaffold, by infiltrating the solution of dibutyl magnesium (MgBu
2) precursor, followed by a hydrogenation reaction. Further hydrogenation at higher temperature leads to the formation of Mg
2CoH
5. In situ synchrotron X-ray diffraction was employed to study the mechanism of hydride formation during the heating process.
Full article
►▼
Show Figures
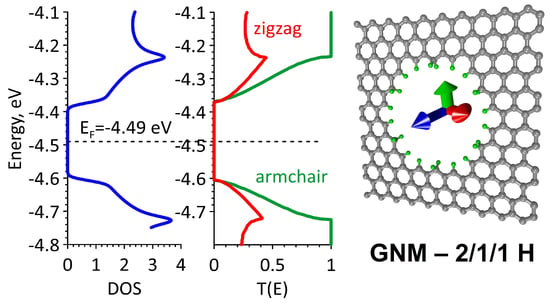
Open AccessArticle
The Effect of Hydrogen on the Electrical Properties of the Graphene Nanomeshes
by
Pavel V. Barkov, Michael M. Slepchenkov and Olga E. Glukhova
Viewed by 2195
Abstract
This paper is devoted to the in silico study of the electronic properties and electrical conductivity of hydrogenated graphene nanomesh (GNM). It is found that the conductivity of GNM can be controlled by varying the type of hydrogenation. Due to the hydrogenation of
[...] Read more.
This paper is devoted to the in silico study of the electronic properties and electrical conductivity of hydrogenated graphene nanomesh (GNM). It is found that the conductivity of GNM can be controlled by varying the type of hydrogenation. Due to the hydrogenation of the nanohole edges by one or two hydrogen atoms, the energy gap can be changed, the anisotropy of the electrical conductivity can be enhanced, and the electron work function can be controlled. By varying the type of hydrogenation, it is possible to form conductive and insulating paths on 2D GNM. Thus, a certain combination of the sp
2- and sp
3-topologies of the GNM edge atoms allows one to fully “turn off” the electronic conductivity in all directions or, conversely, “turn on” the desired direction for current transfer.
Full article
►▼
Show Figures
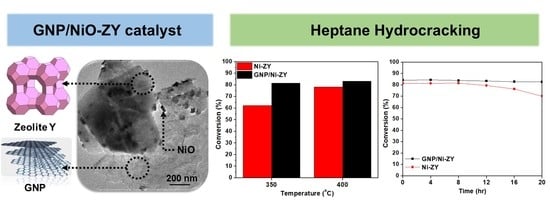
Open AccessArticle
Graphene Nanoplatelets-Based Ni-Zeolite Composite Catalysts for Heptane Hydrocracking
by
Roba Saab, Kyriaki Polychronopoulou, Nikolaos Charisiou, Maria A. Goula and Andreas Schiffer
Cited by 6 | Viewed by 2420
Abstract
This paper examines the effect of incorporating graphene nanoplatelets (GNPs) in an Ni-based/Zeolite-Y catalyst on the hydrocracking of heptane fuel at two temperatures, 350 and 400 °C. Specifically, reduced GNP/NiO-ZY and NiO-ZY catalysts, each with a 5 wt. % Ni loading, were compared
[...] Read more.
This paper examines the effect of incorporating graphene nanoplatelets (GNPs) in an Ni-based/Zeolite-Y catalyst on the hydrocracking of heptane fuel at two temperatures, 350 and 400 °C. Specifically, reduced GNP/NiO-ZY and NiO-ZY catalysts, each with a 5 wt. % Ni loading, were compared in this study. The results show that the reduced GNP/NiO-ZY enhanced the conversion percentage by 31% at 350 °C and by 6% at 400 °C as compared with the reduced NiO-ZY, and the GNP/NiO-ZY also showed superior stability, reporting a less than 2% drop in conversion over 20 h of time-on-stream. The enhancement in performance is linked to the surface and texture characteristics of both catalysts. Although the calcined GNP/NiO-ZY possessed a lower Brunauer–Emmett–Teller (BET) surface area of 458 m
2/g compared with 536 m
2/g for the calcined NiO-ZY, it showed a more hydrophobic nature, as deduced from the water adsorption profiles, which corroborates the hypothesis that the increased affinity between the catalyst surface and heptane molecules during the reaction leads to an improved catalytic activity.
Full article
►▼
Show Figures
Open AccessReview
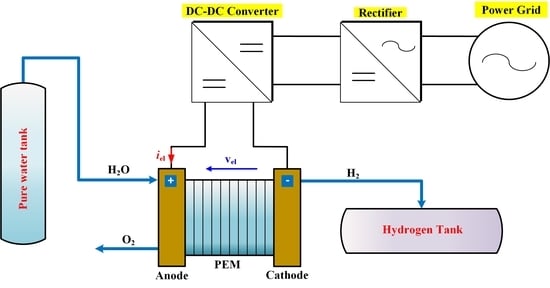
Proton Exchange Membrane Electrolyzer Modeling for Power Electronics Control: A Short Review
by
Burin Yodwong, Damien Guilbert, Matheepot Phattanasak, Wattana Kaewmanee, Melika Hinaje and Gianpaolo Vitale
Cited by 30 | Viewed by 9927
Abstract
The main purpose of this article is to provide a short review of proton exchange membrane electrolyzer (PEMEL) modeling used for power electronics control. So far, three types of PEMEL modeling have been adopted in the literature: resistive load, static load (including an
[...] Read more.
The main purpose of this article is to provide a short review of proton exchange membrane electrolyzer (PEMEL) modeling used for power electronics control. So far, three types of PEMEL modeling have been adopted in the literature: resistive load, static load (including an equivalent resistance series-connected with a DC voltage generator representing the reversible voltage), and dynamic load (taking into consideration the dynamics both at the anode and the cathode). The modeling of the load is crucial for control purposes since it may have an impact on the performance of the system. This article aims at providing essential information and comparing the different load modeling.
Full article
►▼
Show Figures
Open AccessReview
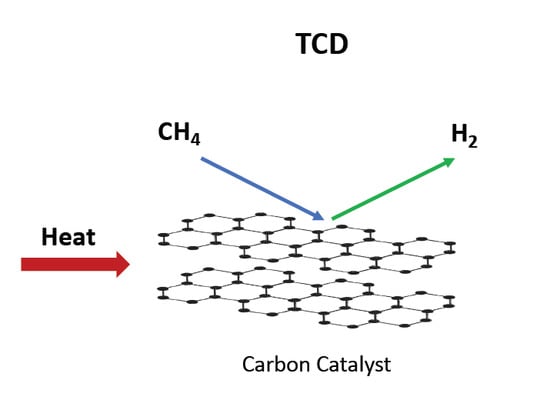
Carbons as Catalysts in Thermo-Catalytic Hydrocarbon Decomposition: A Review
by
Randy Vander Wal and Mpila Makiesse Nkiawete
Cited by 8 | Viewed by 3698
Abstract
Thermo-catalytic decomposition is well-suited for the generation of hydrogen from natural gas. In a decarbonization process for fossil fuel—pre-combustion—solid carbon is produced, with potential commercial uses including energy storage. Metal catalysts have the disadvantages of coking and deactivation, whereas carbon materials as catalysts
[...] Read more.
Thermo-catalytic decomposition is well-suited for the generation of hydrogen from natural gas. In a decarbonization process for fossil fuel—pre-combustion—solid carbon is produced, with potential commercial uses including energy storage. Metal catalysts have the disadvantages of coking and deactivation, whereas carbon materials as catalysts offer resistance to deactivation and poisoning. Many forms of carbon have been tested with varied characterization techniques providing insights into the catalyzed carbon deposition. The breadth of studies testing carbon materials motivated this review. Thermocatalytic decomposition (TCD) rates and active duration vary widely across carbons tested. Regeneration remains rarely investigated but does appear necessary in a cyclic TCD–partial oxidation sequence. Presently, studies making fundamental connections between active sites and deposit nanostructures are few.
Full article
►▼
Show Figures
Open AccessArticle
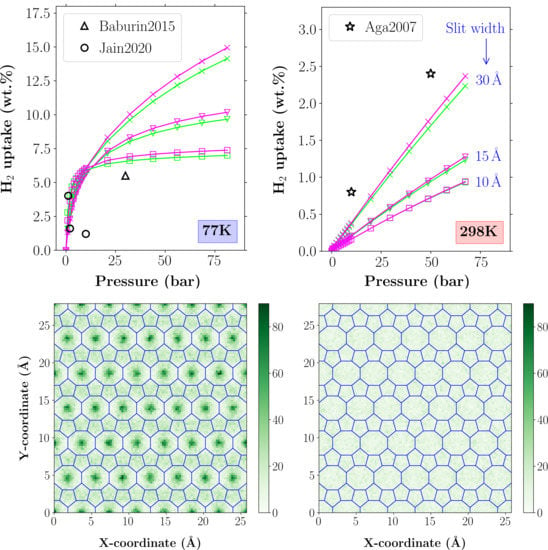
Adsorption of H2 on Penta-Octa-Penta Graphene: Grand Canonical Monte Carlo Study
by
Maxim N. Popov, Thomas Dengg, Dominik Gehringer and David Holec
Cited by 1 | Viewed by 2486
Abstract
In this paper, we report the results of hydrogen adsorption properties of a new 2D carbon-based material, consisting of pentagons and octagons (Penta-Octa-Penta-graphene or POP-graphene), based on the Grand-Canonical Monte Carlo simulations. The new material exhibits a moderately higher gravimetric uptake at cryogenic
[...] Read more.
In this paper, we report the results of hydrogen adsorption properties of a new 2D carbon-based material, consisting of pentagons and octagons (Penta-Octa-Penta-graphene or POP-graphene), based on the Grand-Canonical Monte Carlo simulations. The new material exhibits a moderately higher gravimetric uptake at cryogenic temperatures (77 K), as compared to the regular graphene. We discuss the origin of the enhanced uptake of POP-graphene and offer a consistent explanation.
Full article
►▼
Show Figures
Open AccessFeature PaperArticle
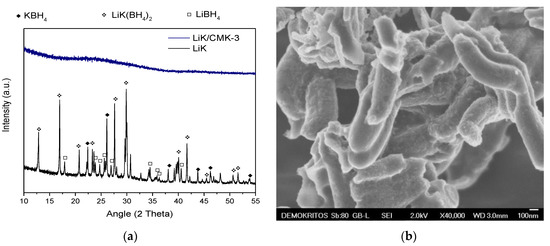
Hydrogen Sorption and Reversibility of the LiBH4-KBH4 Eutectic System Confined in a CMK-3 Type Carbon via Melt Infiltration
by
Filippo Peru, SeyedHosein Payandeh, Georgia Charalambopoulou, Torben R. Jensen and Theodore Steriotis
Cited by 8 | Viewed by 2551
Abstract
Metal borohydrides have very high hydrogen densities but their poor thermodynamic and kinetic properties hinder their use as solid hydrogen stores. An interesting approach to improve their functionality is nano-sizing by confinement in mesoporous materials. In this respect, we used the 0.725 LiBH
[...] Read more.
Metal borohydrides have very high hydrogen densities but their poor thermodynamic and kinetic properties hinder their use as solid hydrogen stores. An interesting approach to improve their functionality is nano-sizing by confinement in mesoporous materials. In this respect, we used the 0.725 LiBH
4–0.275 KBH
4 eutectic mixture, and by exploiting its very low melting temperature (378 K) it was possible to successfully melt infiltrate the borohydrides in a mesoporous CMK-3 type carbon (pore diameter ~5 nm). The obtained carbon–borohydride composite appears to partially alleviate the irreversibility of the dehydrogenation reaction when compared with the bulk LiBH
4-KBH
4, and shows a constant hydrogen uptake of 2.5 wt%–3 wt% for at least five absorption–desorption cycles. Moreover, pore infiltration resulted in a drastic decrease of the decomposition temperature (more than 100 K) compared to the bulk eutectic mixture. The increased reversibility and the improved kinetics may be a combined result of several phenomena such as the catalytic action of the carbon surface, the nano-sizing of the borohydride particles or the reduction of irreversible side-reactions.
Full article
►▼
Show Figures
Open AccessArticle
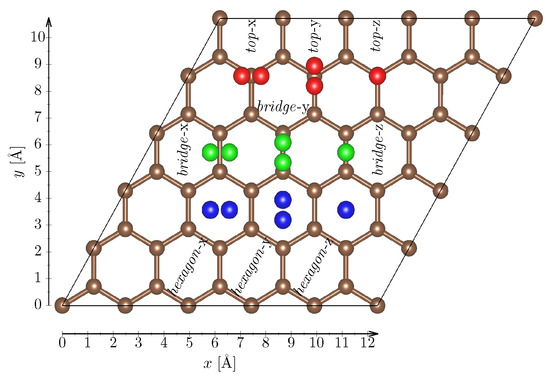
Interactions between a H2 Molecule and Carbon Nanostructures: A DFT Study
by
Dominik Gehringer, Thomas Dengg, Maxim N. Popov and David Holec
Cited by 9 | Viewed by 3554
Abstract
On a long path of finding appropriate materials to store hydrogen, graphene and carbon nanotubes have drawn a lot of attention as potential storage materials. Their advantages lie at hand since those materials provide a large surface area (which can be used for
[...] Read more.
On a long path of finding appropriate materials to store hydrogen, graphene and carbon nanotubes have drawn a lot of attention as potential storage materials. Their advantages lie at hand since those materials provide a large surface area (which can be used for physisorption), are cheap compared to metal hydrides, are abundant nearly everywhere, and most importantly, can increase safety to existing storage solutions. Therefore, a great variety of theoretical studies were employed to study those materials. After a benchmark study of different van-der-Waals corrections to Generalized Gradient Approximation (GGA), the present Density Functional Theory (DFT) study employs Tkatchenko–Scheffler (TS) correction to study the influence of vacancy and Stone–Wales defects in graphene on the physisorption of the hydrogen molecule. Furthermore, we investigate a large-angle (1,0) grain boundary as well as the adsorption behaviour of Penta-Octa-Penta (POP)-graphene.
Full article
►▼
Show Figures
Open AccessArticle
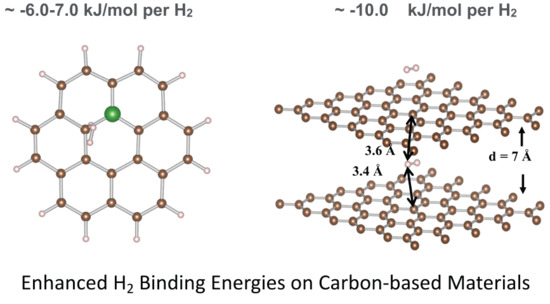
Physi-Sorption of H2 on Pure and Boron–Doped Graphene Monolayers: A Dispersion–Corrected DFT Study
by
Iffat Nayyar, Bojana Ginovska, Abhijeet Karkamkar, Thomas Gennett and Thomas Autrey
Cited by 18 | Viewed by 3329
Abstract
High-surface-area carbons are of interest as potential candidates to store H
2 for fuel–cell power applications. Earlier work has been ambiguous and inconclusive on the effect of boron doping on H
2 binding energy. Here, we describe a systematic dispersion–corrected density functional theory
[...] Read more.
High-surface-area carbons are of interest as potential candidates to store H
2 for fuel–cell power applications. Earlier work has been ambiguous and inconclusive on the effect of boron doping on H
2 binding energy. Here, we describe a systematic dispersion–corrected density functional theory study to evaluate the effect of boron doping. We observe some enhancement in H
2 binding, due to the presence of a defect, such as terminal hydrogen or distortion from planarity, introduced by the inclusion of boron into a graphene ring, which creates hydrogen adsorption sites with slightly increased binding energy. The increase is from −5 kJ/mol H
2 for the pure carbon matrix to −7 kJ/mol H
2 for the boron–doped system with the boron content of ~7%. The H
2 binding sites have little direct interaction with boron. However, the largest enhancement in physi-sorption energy is seen for systems, where H
2 is confined between layers at a distance of about 7 Å, where the H
2 binding nearly doubles to −11 kJ/mol H
2. These findings suggest that interplanar nanoconfinement might be more effective in enhancing H
2 binding. Smaller coronene model is shown to be beneficial for understanding the dependence of interaction energy on the structural configurations and preferential H
2 binding sites.
Full article
►▼
Show Figures
Open AccessFeature PaperArticle
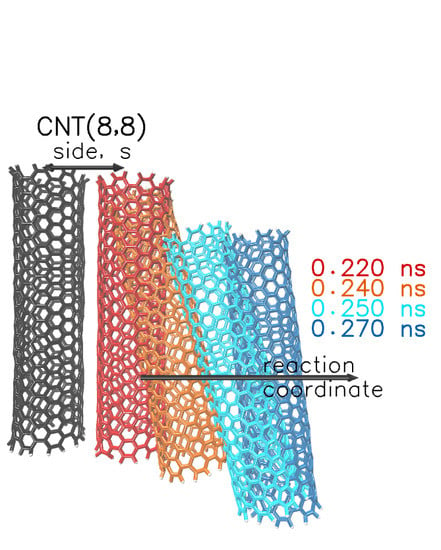
Pulling Simulations and Hydrogen Sorption Modelling on Carbon Nanotube Bundles
by
Anastasios Gotzias and Andreas Sapalidis
Cited by 8 | Viewed by 3180
Abstract
Recent progress in molecular simulation technology has developed an interest in modernizing the usual computational methods and approaches. For instance, most of the theoretical work on hydrogen adsorption on carbon nanotubes was conducted a decade ago. It should be insightful to reinvestigate the
[...] Read more.
Recent progress in molecular simulation technology has developed an interest in modernizing the usual computational methods and approaches. For instance, most of the theoretical work on hydrogen adsorption on carbon nanotubes was conducted a decade ago. It should be insightful to reinvestigate the field and take advantage of code improvements and features implemented in contemporary software. One example of such features is the pulling simulation modules now available in many molecular dynamics programs. We conduct pulling simulations on pairs of carbon nanotubes and measure the inter-tube distance before they dissociate in water. We use this distance to set the interval size between adjacent nanotubes as we arrange them in bundle configurations. We consider bundles with triangular, intermediate and honeycomb patterns, and armchair nanotubes with a chiral index from n = 5 to n = 10. Then, we simulate low pressure hydrogen adsorption isotherms at 77 K, using the grand canonical Monte Carlo method. The different bundle configurations adsorb great hydrogen amounts that may exceed 2% wt at ambient pressures. The computed hydrogen capacities are considered large for physisorption on carbon nanostructures and attributed to the ultra-microporous network and extraordinary high surface area of the configured models.
Full article
►▼
Show Figures
Open AccessFeature PaperArticle
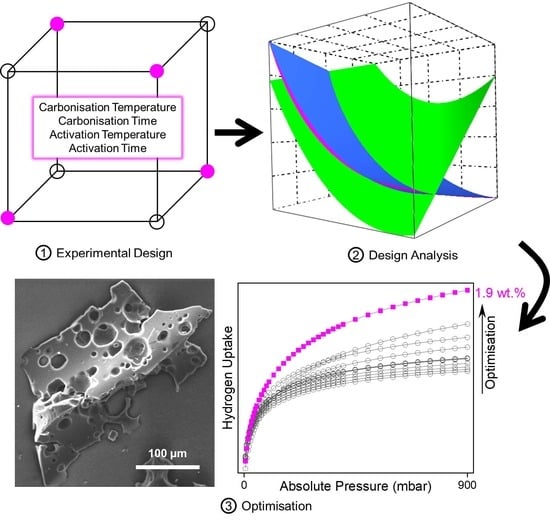
Application of Experimental Design to Hydrogen Storage: Optimisation of Lignin-Derived Carbons
by
Jemma L. Rowlandson, James Coombs OBrien, Karen J. Edler, Mi Tian and Valeska P. Ting
Cited by 5 | Viewed by 3875
Abstract
Lignin is a significant by-product of the paper pulping and biofuel industries. Upgrading lignin to a high-value product is essential for the economic viability of biorefineries for bioethanol production and environmentally benign pulping processes. In this work, the feasibility of lignin-derived activated carbons
[...] Read more.
Lignin is a significant by-product of the paper pulping and biofuel industries. Upgrading lignin to a high-value product is essential for the economic viability of biorefineries for bioethanol production and environmentally benign pulping processes. In this work, the feasibility of lignin-derived activated carbons for hydrogen storage was studied using a Design of Experiments methodology, for a time and cost-efficient exploration of the synthesis process. Four factors (carbonisation temperature, activation temperature, carbonisation time, and activation time) were investigated simultaneously. Development of a mathematical model allowed the factors with the greatest impact to be identified using regression analysis for three responses: surface area, average pore size, and hydrogen uptake at 77 K and 1 bar. Maximising the surface area required activation conditions using the highest settings, however, a low carbonisation temperature was also revealed to be integral to prevent detrimental and excessive pore widening. A small pore size, vital for efficient hydrogen uptake, could be achieved by using low carbonisation temperature but also low activation temperatures. An optimum was achieved using the lowest carbonisation conditions (350 °C for 30 min) to retain a smaller pore size, followed by activation under the severest conditions (1000 °C for 60 min) to maximise surface area and hydrogen uptake. These conditions yielded a material with a high surface area of 1400 m
2 g
−1 and hydrogen uptake of 1.9 wt.% at 77 K and 1 bar.
Full article
►▼
Show Figures
Planned Papers
The below list represents only planned manuscripts. Some of these
manuscripts have not been received by the Editorial Office yet. Papers
submitted to MDPI journals are subject to peer-review.
Title: Application of Experimental Design to Hydrogen Storage: Optimisation of Lignin-Derived Carbons
Authors: Jemma L Rowlandson; James Coombs O'Brien; Karen J Edler; Mi Tian; Valeska P Ting
Abstract: Lignin is a significant by-product of the paper pulping and biofuel industries. Upgrading lignin to a high-value product is essential for the economic viability of biorefineries for bioethanol production and environmentally benign pulping processes. In this work, the feasibility of lignin-derived activated carbons for hydrogen storage was studied using a Design of Experiments methodology; for a time and cost-efficient exploration of the synthesis process. Four factors (carbonisation temperature, activation temperature, carbonisation time, and activation time) were investigated simultaneously. Development of a mathematical model allowed the factors with the greatest impact to be identified using regression analysis for three responses: surface area, average pore size, and hydrogen uptake at 77 K and 1 bar. Maximising the surface area required activation conditions using the highest settings; however, a low carbonisation temperature was also revealed to be integral to prevent detrimental and excessive pore widening. A small pore size, vital for efficient hydrogen uptake, could be achieved by using low carbonisation temperature but also low activation temperatures. An optimum was achieved using the lowest carbonisation conditions (350 °C for 30 min) to retain a smaller pore size, followed by activation under the severest conditions (1000 °C for 60 min) to maximise surface area and hydrogen uptake. These conditions yielded a material with a high surface area of 1400 m2 g-1 and hydrogen uptake of 1.9 wt.% at 77 K and 1 bar.
Title: In-situ formation of metal hydrates inside carbon aerogel framework for hydrogen storage application
Authors: Mohammad Reza Ghaani,1,* Mehdi Alam2, Michele Catti3 and Niall J. English1
Affiliation:
1 School of Chemical and Bioprocess Engineering, University College Dublin, Belfield, Dublin 4, Ireland.
2 Department of Civil and Environmental Engineering, K.N. Toosi University of Technology, Vali Asr Street, Tehran, Iran.
3 Dipartimento di Scienza Dei Materiali, Universita di Milano Bicocca, Via Cozzi 55, 20125, Milano, Italy.
Abstract: Nano-confined chemical reactions bear great promise for a wide range of important applications in the near- to medium-term, e.g., within the emerging area of chemical storage of renewable energy. To explore this important trend, in the present work, resorcinol-/formaldehyde-based carbon aerogels were prepared by sol-gel polymerisation of resorcinol, with furfural catalysed by a sodium-carbonate solution using ambient-pressure drying. These aerogels were further carbonised in nitrogen to obtain their corresponding carbon aerogels. Through this study, the synthesis parameters ware selected in a way to obtain minimum shrinkage during the drying step. The microstructure of the product was observed using FESEM-imaging techniques. The optimised carbon aerogels were found to have pore sizes of ~21 nm with a specific accessible surface area equal to 854.0 m2/g. Physical activation of the carbon aerogel with CO2 generates activated carbon aerogels with a surface area of 1756 m2/g and a total porosity volume up to 3.23 cm3/g. The product was then used as a scaffold for magnesium-hydride formation. MgH2 was synthesised in the nanometre-sized aerogel scaffold by infiltrating the aerogel with a solution of dibutyl magnesium (MgBu2) precursor, and then hydrogenating the incorporated MgBu2 to MgH2. In-situ synchrotron X-ray diffraction was employed to study the mechanism of hydride formation and dissociation during the heating process.