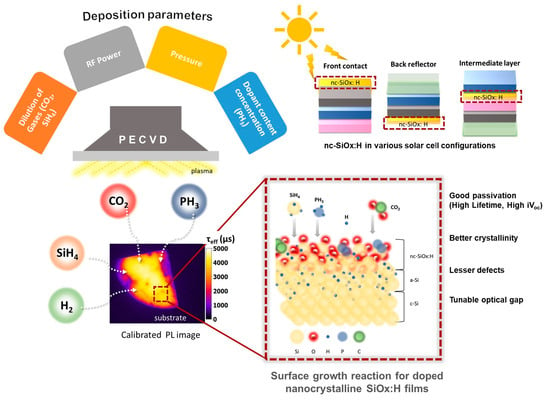
Development of n-Type, Passivating Nanocrystalline Silicon Oxide Films via Plasma-Enhanced Chemical Vapor Deposition
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Optimization of Oxygen Content (CO2 Series)
3.2. Optimization of SiH4 Series
3.3. Optimization of RF Power
3.4. Optimization of Total Pressure
3.5. Optimization of Phosphine Flow Rate (PH3 Series)
- Fine-tune the ratio to achieve the desired balance between passivation quality and crystallinity, considering the specific requirements of the solar cell application.
- Controlling the dilution rate (R) during deposition allows for tuning of the hydrogen content in the film for effective passivation.
- Tailor the to achieve the desired doping level for specific solar cell designs, ensuring optimal passivation properties without introducing excessive defects.
- Choose an appropriate plasma property (power and pressure) based on the desired balance between crystallinity and passivation.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mazzarella, L.; Morales-Vilches, A.; Korte, L.; Schlatmann, R.; Stannowski, B. Versatility of Nanocrystalline Silicon Films: From Thin-Film to Perovskite/c-Si Tandem Solar Cell Applications. Coatings 2020, 10, 759. [Google Scholar] [CrossRef]
- Cuony, P.; Marending, M.; Alexander, D.T.L.; Boccard, M.; Bugnon, G.; Despeisse, M.; Ballif, C. Mixed-phase p-type silicon oxide containing silicon nanocrystals and its role in thin-film silicon solar cells. Appl. Phys. Lett. 2010, 97, 213502. [Google Scholar] [CrossRef]
- Cuony, P.; Alexander, D.T.L.; Perez-Wurfl, I.; Despeisse, M.; Bugnon, G.; Boccard, M.; Söderström, T.; Hessler-Wyser, A.; Hébert, C.; Ballif, C. Silicon Filaments in Silicon Oxide for Next-Generation Photovoltaics. Adv. Mater. 2012, 24, 1182–1186. [Google Scholar] [CrossRef]
- Ding, K.; Aeberhard, U.; Smirnov, V.; Holländer, B.; Finger, F.; Rau, U. Wide Gap Microcrystalline Silicon Oxide Emitter for a-SiOx:H/c-Si Heterojunction Solar Cells. Jpn. J. Appl. Phys. 2013, 52, 122304. [Google Scholar] [CrossRef]
- Ding, K.; Aeberhard, U.; Lambertz, A.; Smirnov, V.; Holländer, B.; Finger, F.; Rau, U. Impact of doped microcrystalline silicon oxide layers on crystalline silicon surface passivation. Can. J. Phys. 2014, 92, 758–762. [Google Scholar] [CrossRef]
- Lambertz, A.; Grundler, T.; Finger, F. Hydrogenated amorphous silicon oxide containing a microcrystalline silicon phase and usage as an intermediate reflector in thin-film silicon solar cells. J. Appl. Phys. 2011, 109, 113109. [Google Scholar] [CrossRef]
- Lambertz, A. Development of Doped Microcrystalline Silicon Oxide andits Application to Thin-Film Silicon Solar Cells, University Utrecht. 2015. Available online: https://juser.fz-juelich.de/record/190115/export/he?ln=en (accessed on 9 June 2023).
- Richter, A.; Smirnov, V.; Lambertz, A.; Nomoto, K.; Welter, K.; Ding, K. Versatility of doped nanocrystalline silicon oxide for applications in silicon thin-film and heterojunction solar cells. Sol. Energy Mater. Sol. Cells 2018, 174, 196–201. [Google Scholar] [CrossRef]
- Smirnov, V.; Böttler, W.; Lambertz, A.; Wang, H.; Carius, R.; Finger, F. Microcrystalline silicon n-i-p solar cells prepared with microcrystalline silicon oxide (μc-SiOx:H) n-layer. Phys. Status Solidi C 2010, 7, 1053–1056. [Google Scholar] [CrossRef]
- Smirnov, V.; Lambertz, A.; Grootoonk, B.; Carius, R.; Finger, F. Microcrystalline silicon oxide (μc-SiOx:H) alloys: A versatile material for application in thin film silicon single and tandem junction solar cells. J. Non-Cryst. Solids 2012, 358, 1954–1957. [Google Scholar] [CrossRef]
- Smirnov, V.; Boettler, W.; Lambertz, A.; Astakhov, O.; Carius, R.; Finger, F. N-type Microcrystalline Silicon Oxide (μc-SiOx:H) Window Layers with Combined Anti-reflection Effects for n-i-p Thin Film Silicon Solar Cells. MRS Proc. 2010, 1245, 2102. [Google Scholar] [CrossRef]
- Das, G.; Mandal, S.; Dhar, S.; Bose, S.; Mukhopadhyay, S.; Banerjee, C.; Barua, A.K. Development of n-type microcrystalline SiOx:H films and its application by innovative way to improve the performance of single junction µc-Si:H solar cell. J. Mater. Sci. Mater. Electron. 2017, 28, 5746–5753. [Google Scholar] [CrossRef]
- Mazzarella, L.; Morales-Vilches, A.B.; Hendrichs, M.; Kirner, S.; Korte, L.; Schlatmann, R.; Stannowski, B. Nanocrystalline n-Type Silicon Oxide Front Contacts for Silicon Heterojunction Solar Cells: Photocurrent Enhancement on Planar and Textured Substrates. IEEE J. Photovolt. 2018, 8, 70–78. [Google Scholar] [CrossRef]
- Olivares, A.J.; Seif, J.P.; Repecaud, P.-A.; Longeaud, C.; Morales-Masis, M.; Bivour, M.; Roca i Cabarrocas, P. Highly conductive p-type nc-SiOX:H thin films deposited at 130 °C via efficient incorporation of plasma synthesized silicon nanocrystals and their application in SHJ solar cells. Sol. Energy Mater. Sol. Cells 2024, 266, 112675. [Google Scholar] [CrossRef]
- Mazzarella, L.; Morales-Vilches, A.B.; Korte, L.; Schlatmann, R.; Stannowski, B. Ultra-thin nanocrystalline n-type silicon oxide front contact layers for rear-emitter silicon heterojunction solar cells. Sol. Energy Mater. Sol. Cells 2018, 179, 386–391. [Google Scholar] [CrossRef]
- Qiu, D.; Duan, W.; Lambertz, A.; Bittkau, K.; Steuter, P.; Liu, Y.; Gad, A.; Pomaska, M.; Rau, U.; Ding, K. Front contact optimization for rear-junction SHJ solar cells with ultra-thin n-type nanocrystalline silicon oxide. Sol. Energy Mater. Sol. Cells 2020, 209, 110471. [Google Scholar] [CrossRef]
- Smirnov, V.; Lambertz, A.; Tillmanns, S.; Finger, F. p- and n-type microcrystalline silicon oxide (μc-SiOx:H) for applications in thin film silicon tandem solar cells. Can. J. Phys. 2014, 92, 932–935. [Google Scholar] [CrossRef]
- Khokhar, M.Q.; Chowdhury, S.; Pham, D.P.; Hussain, S.Q.; Cho, E.-C.; Yi, J. Improving passivation properties using a nano-crystalline silicon oxide layer for high-efficiency TOPCon cells. Infrared Phys. Technol. 2021, 115, 103723. [Google Scholar] [CrossRef]
- Kaur, G.; Xin, Z.; Dutta, T.; Sridharan, R.; Stangl, R.; Danner, A. Improved silicon oxide/polysilicon passivated contacts for high efficiency solar cells via optimized tunnel layer annealing. Sol. Energy Mater. Sol. Cells 2020, 217, 110720. [Google Scholar] [CrossRef]
- Mazzarella, L.; Werth, M.; Jäger, K.; Jošt, M.; Korte, L.; Albrecht, S.; Schlatmann, R.; Stannowski, B. Infrared photocurrent management in monolithic perovskite/silicon heterojunction tandem solar cells by using a nanocrystalline silicon oxide interlayer. Opt. Express 2018, 26, A487–A497. [Google Scholar] [CrossRef] [PubMed]
- Jäger, K.; Sutter, J.; Hammerschmidt, M.; Schneider, P.-I.; Becker, C. Prospects of light management in perovskite/silicon tandem solar cells. Nanophotonics 2020, 10, 1991–2000. [Google Scholar] [CrossRef]
- Sinton, R.A.; Swanson, R.M. Recombination in highly injected silicon. IEEE Trans. Electron Devices 1987, 34, 1380–1389. [Google Scholar] [CrossRef]
- Sinton, R.A.; Cuevas, A. Contactless determination of current–voltage characteristics and minority-carrier lifetimes in semiconductors from quasi-steady-state photoconductance data. Appl. Phys. Lett. 1996, 69, 2510–2512. [Google Scholar] [CrossRef]
- Richter, A.; Glunz, S.W.; Werner, F.; Schmidt, J.; Cuevas, A. Improved quantitative description of Auger recombination in crystalline silicon. Phys. Rev. B 2012, 86, 165202. [Google Scholar] [CrossRef]
- Shin, C.; Iftiquar, S.M.; Park, J.; Kim, Y.; Kim, S.; Jung, J.; Yi, J. Development of highly conducting n-type micro-crystalline silicon oxide thin film and its application in high efficiency amorphous silicon solar cell. Mater. Sci. Semicond. Process. 2017, 66, 223–231. [Google Scholar] [CrossRef]
- Saha, S.C.; Ray, S. Development of highly conductive n-type μc-Si:H films at low power for device applications. J. Appl. Phys. 1995, 78, 5713–5720. [Google Scholar] [CrossRef]
- Iftiquar, S.M. The roles of deposition pressure and rf power in opto-electronic properties of a-SiO:H films. J. Phys. Appl. Phys. 1998, 31, 1630–1641. [Google Scholar] [CrossRef]
- Smirnov, V.; Lambertz, A.; Finger, F. Electronic and Structural Properties of N-Type Microcrystalline Silicon Oxide (Mc Siox:H) Films for Applications in Thin Film Silicon Solar Cells. Energy Procedia 2015, 84, 71–77. [Google Scholar] [CrossRef]
- Bruneau, B.; Cariou, R.; Dornstetter, J.-C.; Lepecq, M.; Maurice, J.-L.; Roca i Cabarrocas, P.; Johnson, E.V. Ion Energy Threshold in Low-Temperature Silicon Epitaxy for Thin-Film Crystalline Photovoltaics. IEEE J. Photovolt. 2014, 4, 1361–1367. [Google Scholar] [CrossRef]
- Juneja, S.; Kumar, S. Effect of Power on Crystallinity and Opto-Electronic Properties of Silicon Thin Films Grown Using VHF PECVD Process. Silicon 2021, 13, 3927–3940. [Google Scholar] [CrossRef]
- Layadi, N.; Roca i Cabarrocas, P.; Drévillon, B.; Solomon, I. Real-time spectroscopic ellipsometry study of the growth of amorphous and microcrystalline silicon thin films prepared by alternating silicon deposition and hydrogen plasma treatment. Phys. Rev. B 1995, 52, 5136–5143. [Google Scholar] [CrossRef]
- Zhou, H.P.; Xu, M.; Xu, S.; Feng, Y.Y.; Xu, L.X.; Wei, D.Y.; Xiao, S.Q. A comparative study on the direct deposition of μc-Si:H and plasma-induced recrystallization of a-Si:H: Insight into Si crystallization in a high-density plasma. Appl. Surf. Sci. 2018, 433, 285–291. [Google Scholar] [CrossRef]
- Olivares, A.J.; Kaur, G.; Poplawski, M.; Desthieux, A.; Roca i Cabarrocas, P. Optimization of the Conductivity and Crystalline Fraction of p-Type μc-SiOx:H Films for Silicon Heterojunction Solar Cells. In Optimization of the Conductivity and Crystalline Fraction of p-Type μc-SiOx:H Films for Silicon Heterojunction Solar Cells; EUPVSEC Proceedings: Vienna, Austria, 2021; pp. 212–216. [Google Scholar] [CrossRef]
- Gabriel, O.; Kirner, S.; Klingsporn, M.; Friedrich, F.; Stannowski, B.; Schlatmann, R. On the Plasma Chemistry During Plasma Enhanced Chemical Vapor Deposition of Microcrystalline Silicon Oxides. Plasma Process. Polym. 2015, 12, 82–91. [Google Scholar] [CrossRef]
- Janev, R.K.; Langer, W.D.; Post, D.E.; Evans, K. Collision Processes and Reaction of H2+Ions. In Elementary Processes in Hydrogen-Helium Plasmas: Cross Sections and Reaction Rate Coefficients; Janev, R.K., Langer, W.D., Post, D.E., Evans, K., Eds.; Springer Series on Atoms+Plasmas; Springer: Berlin, Heidelberg, 1987; pp. 167–179. [Google Scholar] [CrossRef]
- Coltrin, M.E.; Kee, R.J.; Miller, J.A. A Mathematical Model of Silicon Chemical Vapor Deposition: Further Refinements and the Effects of Thermal Diffusion. J. Electrochem. Soc. 1986, 133, 1206. [Google Scholar] [CrossRef]
- Landheer, K.; Goedheer, W.J.; Poulios, I.; Schropp, R.E.I.; Rath, J.K. Chemical sputtering by H2+ and H3+ ions during silicon deposition. J. Appl. Phys. 2016, 120, 053304. [Google Scholar] [CrossRef]
- Zhang, T.; Orlac’h, J.-M.; Ghosh, M.; Giovangigli, V.; Roca i Cabarrocas, P.; Novikova, T. Role of H3+ ions in deposition of silicon thin films from SiH4/H2 discharges: Modeling and experiments. Plasma Sources Sci. Technol. 2021, 30, 075024. [Google Scholar] [CrossRef]














Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, G.; Olivares, A.J.; Roca i Cabarrocas, P. Development of n-Type, Passivating Nanocrystalline Silicon Oxide Films via Plasma-Enhanced Chemical Vapor Deposition. Solar 2024, 4, 162-178. https://doi.org/10.3390/solar4010007
Kaur G, Olivares AJ, Roca i Cabarrocas P. Development of n-Type, Passivating Nanocrystalline Silicon Oxide Films via Plasma-Enhanced Chemical Vapor Deposition. Solar. 2024; 4(1):162-178. https://doi.org/10.3390/solar4010007
Chicago/Turabian StyleKaur, Gurleen, Antonio J. Olivares, and Pere Roca i Cabarrocas. 2024. "Development of n-Type, Passivating Nanocrystalline Silicon Oxide Films via Plasma-Enhanced Chemical Vapor Deposition" Solar 4, no. 1: 162-178. https://doi.org/10.3390/solar4010007