The Rhodamine–Perylene Compact Electron Donor–Acceptor Dyad: Spin-Orbit Charge-Transfer Intersystem Crossing and the Energy Balance of the Triplet Excited States
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Methods
2.2. Synthesis of Compound RB–Pery
2.3. Singlet Oxygen Quantum Yield Measurements
2.4. Nanosecond Time-Resolved Transient Absorption Spectroscopy
2.5. Electrochemical Measurements
2.6. DFT Calculations
3. Results and Discussion
3.1. Molecular Structure Design and Synthesis
3.2. Density Functional Theory (DFT) Calculations
3.3. UV–Vis Absorption and Fluorescence Emission Spectra
3.4. Cyclic Voltammograms of RB-Ph and RB–Pery
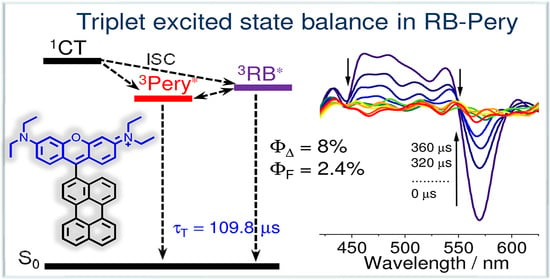
3.5. Nanosecond Transient Absorption (ns-TA) Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khramov, D.M.; Boydston, A.J.; Bielawski, C.W. Synthesis and Study of Janus Bis(carbene)s and Their Transition-Metal Complexes. Angew. Chem. Int. Ed. 2006, 45, 6186–6189. [Google Scholar] [CrossRef]
- Wang, X.; Goeb, S.; Ji, Z.; Pogulaichenko, N.A.; Castellano, F.N. Homogeneous Photocatalytic Hydrogen Production Using π-Conjugated Platinum(II) Arylacetylide Sensitizers. Inorg. Chem. 2011, 50, 705–707. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, W.; Sun, J.; Guo, S. Triplet photosensitizers: From molecular design to applications. Chem. Soc. Rev. 2013, 42, 5323–5351. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, K.; Yang, W.; Wang, Z.; Zhong, F. The triplet excited state of Bodipy: Formation, modulation and application. Chem. Soc. Rev. 2015, 44, 8904–8939. [Google Scholar] [CrossRef]
- Zhou, G.-J.; Wong, W.-Y. Organometallic acetylides of PtII, AuI and HgII as new generation optical power limiting materials. Chem. Soc. Rev. 2011, 40, 2541–2566. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, F.; Huang, C. Phosphorescent chemosensors based on heavy-metal complexes. Chem. Soc. Rev. 2010, 39, 3007–3030. [Google Scholar] [CrossRef]
- Ji, S.; Wu, W.; Wu, W.; Guo, H.; Zhao, J. Ruthenium(II) Polyimine Complexes with a Long-Lived 3IL Excited State or a 3MLCT/3IL Equilibrium: Efficient Triplet Sensitizers for Low-Power Upconversion. Angew. Chem. Int. Ed. 2011, 50, 1626–1629. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, J.; Barbon, A.; Toffoletti, A.; Liu, Y.; An, Y.; Xu, L.; Karatay, A.; Yaglioglu, H.G.; Yildiz, E.A.; et al. Radical-Enhanced Intersystem Crossing in New Bodipy Derivatives and Application for Efficient Triplet–Triplet Annihilation Upconversion. J. Am. Chem. Soc. 2017, 139, 7831–7842. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Chou, P.-T. Contemporary progresses on neutral, highly emissive Os(II) and Ru(II) complexes. Chem. Soc. Rev. 2007, 36, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Moreira, V.; Thorp-Greenwood, F.L.; Coogan, M.P. Application of d6 transition metal complexes in fluorescence cell imaging. Chem. Commun. 2010, 46, 186–202. [Google Scholar] [CrossRef] [PubMed]
- Herberger, J.; Winter, R.F. Platinum emitters with dye-based σ-aryl ligands. Coord. Chem. Rev. 2019, 400, 213048. [Google Scholar] [CrossRef]
- Ziessel, R.; Allen, B.D.; Rewinska, D.B.; Harriman, A. Selective Triplet-State Formation during Charge Recombination in a Fullerene/Bodipy Molecular Dyad (Bodipy=Borondipyrromethene). Chem.—Eur. J. 2009, 15, 7382–7393. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.N.; El-Khouly, M.E.; Subbaiyan, N.K.; Zandler, M.E.; Fukuzumi, S.; D’Souza, F. A novel BF2-chelated azadipyrromethene–fullerene dyad: Synthesis, electrochemistry and photodynamics. Chem. Commun. 2012, 48, 206–208. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yu, X.; Wu, W.; Zhao, J. Styryl Bodipy-C60 Dyads as Efficient Heavy-Atom-Free Organic Triplet Photosensitizers. Org. Lett. 2012, 14, 2594–2597. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, K.; Liu, H.; Zhang, Y.; Zhang, H.; Yao, J.; Fu, H. Impact of Intermolecular Distance on Singlet Fission in a Series of TIPS Pentacene Compounds. J. Phys. Chem. Lett. 2014, 5, 3451–3455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-D.; Wu, Y.; Xu, Y.; Wang, Q.; Liu, K.; Chen, J.-W.; Cao, J.-J.; Zhang, C.; Fu, H.; Zhang, H.-L. Excessive Exoergicity Reduces Singlet Exciton Fission Efficiency of Heteroacenes in Solutions. J. Am. Chem. Soc. 2016, 138, 6739–6745. [Google Scholar] [CrossRef]
- Bröring, M.; Krüger, R.; Link, S.; Kleeberg, C.; Köhler, S.; Xie, X.; Ventura, B.; Flamigni, L. Bis(BF2)-2,2′-Bidipyrrins (BisBODIPYs): Highly Fluorescent BODIPY Dimers with Large Stokes Shifts. Chem.—Eur. J. 2008, 14, 2976–2983. [Google Scholar] [CrossRef]
- Hussain, M.; Taddei, M.; Bussotti, L.; Foggi, P.; Zhao, J.; Liu, Q.; Di Donato, M. Intersystem Crossing in Naphthalenediimide–Oxoverdazyl Dyads: Synthesis and Study of the Photophysical Properties. Chem.—Eur. J. 2019, 25, 15615–15627. [Google Scholar] [CrossRef]
- Dance, Z.E.X.; Mi, Q.; McCamant, D.W.; Ahrens, M.J.; Ratner, M.A.; Wasielewski, M.R. Time-Resolved EPR Studies of Photogenerated Radical Ion Pairs Separated by p-Phenylene Oligomers and of Triplet States Resulting from Charge Recombination. J. Phys. Chem. B 2006, 110, 25163–25173. [Google Scholar] [CrossRef]
- Colvin, M.T.; Ricks, A.B.; Scott, A.M.; Smeigh, A.L.; Carmieli, R.; Miura, T.; Wasielewski, M.R. Magnetic Field-Induced Switching of the Radical-Pair Intersystem Crossing Mechanism in a Donor−Bridge−Acceptor Molecule for Artificial Photosynthesis. J. Am. Chem. Soc. 2011, 133, 1240–1243. [Google Scholar] [CrossRef]
- Kasha, M.; Rawls, H.R.; El-Bayoumi, M.A. The Exciton Model in Molecular Spectroscopy. Pure Appl. Chem. 1965, 11, 371–392. [Google Scholar] [CrossRef]
- Weiss, E.A.; Ahrens, M.J.; Sinks, L.E.; Ratner, M.A.; Wasielewski, M.R. Solvent Control of Spin-Dependent Charge Recombination Mechanisms within Donor−Conjugated Bridge−Acceptor Molecules. J. Am. Chem. Soc. 2004, 126, 9510–9511. [Google Scholar] [CrossRef] [PubMed]
- Buck, J.T.; Boudreau, A.M.; DeCarmine, A.; Wilson, R.W.; Hampsey, J.; Mani, T. Spin-Allowed Transitions Control the Formation of Triplet Excited States in Orthogonal Donor-Acceptor Dyads. Chem 2019, 5, 138–155. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, J. Bodipy–Anthracene Dyads as Triplet Photosensitizers: Effect of Chromophore Orientation on Triplet-State Formation Efficiency and Application in Triplet–Triplet Annihilation Upconversion. Org. Lett. 2017, 19, 4492–4495. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Biskup, T.; Rein, S.; Wang, Z.; Bussotti, L.; Russo, N.; Foggi, P.; Zhao, J.; Di Donato, M.; Mazzone, G.; et al. Spin–Orbit Charge Recombination Intersystem Crossing in Phenothiazine–Anthracene Compact Dyads: Effect of Molecular Conformation on Electronic Coupling, Electronic Transitions, and Electron Spin Polarizations of the Triplet States. J. Phys. Chem. C 2018, 122, 27850–27865. [Google Scholar] [CrossRef]
- Chen, K.; Yang, W.; Wang, Z.; Iagatti, A.; Bussotti, L.; Foggi, P.; Ji, W.; Zhao, J.; Di Donato, M. Triplet Excited State of BODIPY Accessed by Charge Recombination and Its Application in Triplet–Triplet Annihilation Upconversion. J. Phys. Chem. A 2017, 121, 7550–7564. [Google Scholar] [CrossRef]
- Wang, Z.; Ivanov, M.; Gao, Y.; Bussotti, L.; Foggi, P.; Zhang, H.; Russo, N.; Dick, B.; Zhao, J.; Di Donato, M.; et al. Spin–Orbit Charge-Transfer Intersystem Crossing (ISC) in Compact Electron Donor–Acceptor Dyads: ISC Mechanism and Application as Novel and Potent Photodynamic Therapy Reagents. Chem.—Eur. J. 2020, 26, 1091–1102. [Google Scholar] [CrossRef]
- Imran, M.; Sukhanov, A.A.; Wang, Z.; Karatay, A.; Zhao, J.; Mahmood, Z.; Elmali, A.; Voronkova, V.K.; Hayvali, M.; Xing, Y.H.; et al. Electronic Coupling and Spin–Orbit Charge-Transfer Intersystem Crossing in Phenothiazine–Perylene Compact Electron Donor/Acceptor Dyads. J. Phys. Chem. C 2019, 123, 7010–7024. [Google Scholar] [CrossRef]
- Zhao, Y.; Duan, R.; Zhao, J.; Li, C. Spin–Orbit Charge Transfer Intersystem Crossing in Perylenemonoimide–Phenothiazine Compact Electron Donor–Acceptor Dyads. Chem. Commun. 2018, 54, 12329–12332. [Google Scholar] [CrossRef]
- Zhang, X.; Elmali, A.; Duan, R.; Liu, Q.; Ji, W.; Zhao, J.; Li, C.; Karatay, A. Charge Separation, Recombination and Intersystem Crossing of Directly Connected Perylenemonoimide–Carbazole Electron Donor/Acceptor Dyads. Phys. Chem. Chem. Phys. 2020, 22, 6376–6390. [Google Scholar] [CrossRef]
- Imran, M.; El-Zohry, A.M.; Matt, C.; Taddei, M.; Doria, S.; Bussotti, L.; Foggi, P.; Zhao, J.; Di Donato, M.; Mohammed, O.F.; et al. Intersystem Crossing via Charge Recombination in a Perylene–Naphthalimide Compact Electron Donor/Acceptor Dyad. J. Mater. Chem. C 2020, 8, 8305–8319. [Google Scholar] [CrossRef]
- Filatov, M.A. Heavy-atom-free BODIPY Photosensitizers with Intersystem Crossing Mediated by Intramolecular Photoinduced Electron Transfer. Org. Biomol. Chem. 2020, 18, 10–27. [Google Scholar] [CrossRef]
- Gibbons, D.J.; Farawar, A.; Mazzella, P.; Leroy-Lhez, S.; Williams, R.M. Making Triplets from Photo-Generated Charges: Observations, Mechanisms and Theory. Photochem. Photobiol. Sci. 2020, 19, 136–158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Z.; Hou, Y.; Yan, Y.; Zhao, J.; Dick, B. Recent Development of Heavy-Atom-Free Triplet Photosensitizers: Molecular Structure Design, Photophysics and Application. J. Mater. Chem. C 2021, 9, 11944–11973. [Google Scholar] [CrossRef]
- He, G.; Guo, D.; He, C.; Zhang, X.; Zhao, X.; Duan, C. A Color-Tunable Europium Complex Emitting Three Primary Colors and White Light. Angew. Chem. Int. Ed. 2009, 48, 6132–6135. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Yuan, L.; Cao, Z.; Feng, Y.; Song, J. Through-Bond Energy Transfer Cassettes with Minimal Spectral Overlap between the Donor Emission and Acceptor Absorption: Coumarin–Rhodamine Dyads with Large Pseudo-Stokes Shifts and Emission Shifts. Angew. Chem. Int. Ed. 2010, 49, 375–379. [Google Scholar] [CrossRef]
- Belov, V.N.; Wurm, C.A.; Boyarskiy, V.P.; Jakobs, S.; Hell, S.W. Rhodamines NN: A Novel Class of Caged Fluorescent Dyes. Angew. Chem. Int. Ed. 2010, 49, 3520–3523. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, K.; Begley, T.P. Reagent for the Detection of Protein Thiocarboxylates in the Bacterial Proteome: Lissamine Rhodamine B Sulfonyl Azide. J. Am. Chem. Soc. 2010, 132, 11608–11612. [Google Scholar] [CrossRef]
- Koide, Y.; Urano, Y.; Hanaoka, K.; Terai, T.; Nagano, T. Development of an Si-Rhodamine-Based Far-Red to Near-Infrared Fluorescence Probe Selective for Hypochlorous Acid and Its Applications for Biological Imaging. J. Am. Chem. Soc. 2011, 133, 5680–5682. [Google Scholar] [CrossRef]
- Hu, L.; Pei, C.; Li, Z.; Wang, C.; Yang, G.; Sun, W. Synthesis and Photophysics of a Broadband Absorbing Texaphyrin Derivative Bearing a Rhodamine 6G Motif. Org. Chem. Front. 2014, 1, 506–514. [Google Scholar] [CrossRef]
- Majumdar, P.; Cui, X.; Xu, K.; Zhao, J. Switching of the Photophysical Properties of Bodipy-Derived trans Bis(tributylphosphine) Pt(II) Bisacetylide Complexes with Rhodamine as the Acid-Activatable Unit. Dalton Trans. 2015, 44, 4032–4045. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Li, L.; Wei, F.; Wong, K.M.-C. Alkynylplatinum(II) Terpyridine System Coupled with Rhodamine Derivative: Interplay of Aggregation, Deaggregation, and Ring-Opening Processes for Ratiometric Luminescence Sensing. Inorg. Chem. 2018, 57, 6439–6446. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zeng, L.; Guo, H.; Wu, W.; Wu, W.; Ji, S.; Zhao, J. Room-Temperature Long-Lived 3IL Excited State of Rhodamine in an NN PtII Bis(acetylide) Complex with Intense Visible-Light Absorption. Eur. J. Inorg. Chem. 2011, 2011, 4527–4533. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, L.; Wei, F.; Li, L.; Zhao, S.; Gong, P.; Cai, L.; Wong, K.M.-C. Versatile Strategy To Generate a Rhodamine Triplet State as Mitochondria-Targeting Visible-Light Photosensitizers for Efficient Photodynamic Therapy. ACS Appl. Mater. Interfaces 2019, 11, 8797–8806. [Google Scholar] [CrossRef]
- Yang, H.; Han, C.; Zhu, X.; Liu, Y.; Zhang, K.Y.; Liu, S.; Zhao, Q.; Li, F.; Huang, W. Upconversion Luminescent Chemodosimeter Based on NIR Organic Dye for Monitoring Methylmercury In Vivo. Adv. Funct. Mater. 2016, 26, 1945–1953. [Google Scholar] [CrossRef]
- Tian, R.; Sun, W.; Li, M.; Long, S.; Li, M.; Fan, J.; Guo, L.; Peng, X. Development of a Novel Anti-tumor Theranostic Platform: A Near-Infrared Molecular Upconversion Sensitizer for Deep-Seated Cancer Photodynamic Therapy. Chem. Sci. 2019, 10, 10106–10112. [Google Scholar] [CrossRef]
- Wan, W.; Li, A.D.Q. Discovery of a New Light–Molecule Interaction: Supracence Reveals What Is Missing in Fluorescence Imaging. Angew. Chem. Int. Ed. 2019, 58, 13739–13743. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09W, Revision E.01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Liu, D.; El-Zohry, A.M.; Taddei, M.; Matt, C.; Bussotti, L.; Wang, Z.; Zhao, J.; Mohammed, O.F.; Di Donato, M.; Weber, S. Long-Lived Charge-Transfer State Induced by Spin-Orbit Charge Transfer Intersystem Crossing (SOCT–ISC) in a Compact Spiro Electron Donor/Acceptor Dyad. Angew. Chem. Int. Ed. 2020, 59, 11591–11599. [Google Scholar] [CrossRef]
- Hu, M.; Sukhanov, A.A.; Zhang, X.; Elmali, A.; Zhao, J.; Ji, S.; Karatay, A.; Voronkova, V.K. Spiro Rhodamine-Perylene Compact Electron Donor–Acceptor Dyads: Conformation Restriction, Charge Separation, and Spin–Orbit Charge Transfer Intersystem Crossing. J. Phys. Chem. B 2021, 125, 4187–4203. [Google Scholar] [CrossRef]
- Filatov, M.A.; Karuthedath, S.; Polestshuk, P.M.; Callaghan, S.; Flanagan, K.J.; Wiesner, T.; Laquai, F.; Senge, M.O. BODIPY-Pyrene and Perylene Dyads as Heavy-Atom-Free Singlet Oxygen Sensitizers. ChemPhotoChem 2018, 2, 606–615. [Google Scholar] [CrossRef]
- Dance, Z.E.X.; Mickley, S.M.; Wilson, T.M.; Ricks, A.B.; Scott, A.M.; Ratner, M.A.; Wasielewski, M.R. Intersystem Crossing Mediated by Photoinduced Intramolecular Charge Transfer: Julolidine−Anthracene Molecules with Perpendicular π Systems. J. Phys. Chem. A 2008, 112, 4194–4201. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Feng, N. Photoinduced Electron Transfer-based Halogen-free Photosensitizers: Covalent meso-Aryl (Phenyl, Naphthyl, Anthryl, and Pyrenyl) as Electron Donors to Effectively Induce the Formation of the Excited Triplet State and Singlet Oxygen for BODIPY Compounds. Chem.—Asian J. 2017, 12, 2447–2456. [Google Scholar] [CrossRef]
- Wang, Z.; Sukhanov, A.A.; Toffoletti, A.; Sadiq, F.; Zhao, J.; Barbon, A.; Voronkova, V.K.; Dick, B. Insights into the Efficient Intersystem Crossing of Bodipy-Anthracene Compact Dyads with Steady-State and Time-Resolved Optical/Magnetic Spectroscopies and Observation of the Delayed Fluorescence. J. Phys. Chem. C 2019, 123, 265–274. [Google Scholar] [CrossRef]
- Ganesan, P.; Baggerman, J.; Zhang, H.; Sudhölter, E.J.R.; Zuilhof, H. Femtosecond Time-Resolved Photophysics of 1,4,5,8-Naphthalene Diimides. J. Phys. Chem. A 2007, 111, 6151–6156. [Google Scholar] [CrossRef]
- Arbeloa, F.L.; Arbeloa, T.L.; Bartolomé, P.H.; Estévez, M.J.T.; Arbeloa, I.L. TICT and ULM models for the Radiationless Deactivation of Rhodamines. Proc. Indian Acad. Sci. (Chem. Sci.) 1992, 104, 165–171. [Google Scholar] [CrossRef]
- Liu, X.; Qiao, Q.; Tian, W.; Liu, W.; Chen, J.; Lang, M.J.; Xu, Z. Aziridinyl Fluorophores Demonstrate Bright Fluorescence and Superior Photostability by Effectively Inhibiting Twisted Intramolecular Charge Transfer. J. Am. Chem. Soc. 2016, 138, 6960–6963. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Gao, C.; Han, T.; Shi, H.; Guo, W. Improving the Quantum Yields of Fluorophores by Inhibiting Twisted Intramolecular Charge Transfer Using Electron-Withdrawing Group-Functionalized Piperidine Auxochromes. Chem. Commun. 2020, 56, 715–718. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Zhao, J.; Moore, E.G. Covalently Bonded Perylene–DiiodoBodipy Dyads for Thiol-Activatable Triplet–Triplet Annihilation Upconversion. J. Phys. Chem. C 2017, 121, 22665–22679. [Google Scholar] [CrossRef]
- Li, G.; Mark, M.F.; Lv, H.; McCamant, D.W.; Eisenberg, R. Rhodamine-Platinum Diimine Dithiolate Complex Dyads as Efficient and Robust Photosensitizers for Light-Driven Aqueous Proton Reduction to Hydrogen. J. Am. Chem. Soc. 2018, 140, 2575–2586. [Google Scholar] [CrossRef]
- Cui, X.; El-Zohry, A.M.; Wang, Z.; Zhao, J.; Mohammed, O.F. Homo- or Hetero-Triplet–Triplet Annihilation? A Case Study with Perylene-BODIPY Dyads/Triads. J. Phys. Chem. C 2017, 121, 16182–16192. [Google Scholar] [CrossRef]
- Beaumont, P.C.; Johnson, D.G.; Parsons, B.J. Excited State and Free Radical Properties of Rhodamine Dyes in Aqueous Solution: A Laser Flash Photolysis and Pulse Radiolysis Study. J. Photochem. Photobiol. A 1997, 107, 175–183. [Google Scholar] [CrossRef]
- van de Linde, S.; Krstić, I.; Prisner, T.; Doose, S.; Heilemann, M.; Sauer, M. Photoinduced Formation of Reversible Dye Radicals and Their Impact on Super-Resolution Imaging. Photochem. Photobiol. Sci. 2011, 10, 499–506. [Google Scholar] [CrossRef]
- Slanina, T.; Oberschmid, T. Rhodamine 6G Radical: A Spectro (Fluoro) Electrochemical and Transient Spectroscopic Study. ChemCatChem 2018, 10, 4182–4190. [Google Scholar] [CrossRef]
- Kawai, K.; Yamamoto, N.; Tsubomura, H. Simultaneous Formation of Perylene Cation and Anion by Flash Excitation of Perylene in Solutions. Bull. Chem. Soc. Jpn. 1970, 43, 2266–2268. [Google Scholar] [CrossRef]
- Kabe, R.; Adachi, C. Organic long persistent luminescence. Nature 2017, 550, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, M.W.; Beaumont, P.C.; Jones, S.E.; Navaratnam, S.; Parsons, B.J. Excited state and free radical properties of Rhodamine 123: A laser flash photolysis and radiolysis study. Phys. Chem. Chem. Phys. 1999, 1, 261–268. [Google Scholar] [CrossRef]
- Navaratnam, S.; Parsons, B.J. Kinetic and spectral properties of rhodamine 6G free radicals: A pulse radiolysis study. J. Photochem. Photobiol. A. 2002, 153, 153–162. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, P.; Murakami, T.N.; Zakeeruddin, S.M.; Grätzel, M. The 2,2,6,6-Tetramethyl-1-piperidinyloxy Radical: An Efficient, Iodine-Free Redox Mediator for Dye-Sensitized Solar Cells. Adv. Funct. Mater. 2008, 18, 341–346. [Google Scholar] [CrossRef]
- Heilemann, M.; van de Linde, S.; Mukherjee, A.; Sauer, M. Super-Resolution Imaging with Small Organic Fluorophores. Angew. Chem. Int. Ed. 2009, 48, 6903–6908. [Google Scholar] [CrossRef] [PubMed]
- van de Linde, S.; Löschberger, A.; Klein, T.; Heidbreder, M.; Wolter, S.; Heilemann, M.; Sauer, M. Direct stochastic optical reconstruction microscopy with standard fluorescent probes. Nat. Protoc. 2011, 6, 991–1009. [Google Scholar] [CrossRef]
- Frisenda, R.; Gaudenzi, R.; Franco, C.; Mas-Torrent, M.; Rovira, C.; Veciana, J.; Alcon, I.; Bromley, S.T.; Burzurí, E.; van der Zant, H.S.J. Kondo Effect in a Neutral and Stable All Organic Radical Single Molecule Break Junction. Nano Lett. 2015, 15, 3109–3114. [Google Scholar] [CrossRef]
- Peng, Q.; Obolda, A.; Zhang, M.; Li, F. Organic Light-Emitting Diodes Using a Neutral π Radical as Emitter: The Emission from a Doublet. Angew. Chem. Int. Ed. 2015, 54, 7091–7095. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, I.; König, B. Chromoselective Photocatalysis: Controlled Bond Activation through Light-Color Regulation of Redox Potentials. Angew. Chem. Int. Ed. 2016, 55, 7676–7679. [Google Scholar] [CrossRef] [PubMed]
- Romero, N.A.; Nicewicz, D.A. Organic Photoredox Catalysis. Chem. Rev. 2016, 116, 10075–10166. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhao, Y.; Wang, Z.; Xu, K.; Zhao, J. Exploiting the Benefit of S0 → T1 Excitation in Triplet–Triplet Annihilation Upconversion to Attain Large Anti-Stokes Shifts: Tuning the Triplet State Lifetime of a Tris(2,2′-bipyridine) Osmium(II) Complex. Dalton Trans. 2018, 47, 8619–8628. [Google Scholar] [CrossRef]
- Lei, Y.; Chen, K.; Tang, G.; Zhao, J.; Gurzadyan, G.G. Bodipy-Phenylethynyl Anthracene Dyad: Spin-Orbit Charge Transfer Intersystem Crossing and Triplet Excited-State Equilibrium. J. Photochem. Photobiol. A 2020, 398, 112573. [Google Scholar] [CrossRef]










| Compounds | λabsb (nm) | ε c | λemd (nm) | ΦΔ e (%) | ΦF f (%) | τF g (ns) | τT h (μs) |
|---|---|---|---|---|---|---|---|
| Pery | 438 | 4.2 | 443 | 4.8 ± 0.5 | 77.7 | 3.9 ± 0.5 | 596 ± 5 i |
| RB-Ph | 567 | 7.0 | 590 | – j | 14.2 | 1.3 ± 0.5 (97%) 8.5 ± 0.5 (3%) | – j |
| RB–Pery | 445, 575 | 2.2, 6.3 | 453, 598 | 8.0 ± 1 | 2.4 (RB), 6.0 (Pery) | 3.3 (96%) 16.7 (4%)± 0.2 | 109.8 ± 5 |
| Compounds | EOX (V) | ERED (V) |
|---|---|---|
| Pery | +0.61 b | – c |
| RB-Ph | +0.89 | −1.32 |
| RB–Pery | +0.58, +0.87 | −1.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imran, M.; Liu, D.; Ye, K.; Zhang, X.; Zhao, J. The Rhodamine–Perylene Compact Electron Donor–Acceptor Dyad: Spin-Orbit Charge-Transfer Intersystem Crossing and the Energy Balance of the Triplet Excited States. Photochem 2024, 4, 40-56. https://doi.org/10.3390/photochem4010004
Imran M, Liu D, Ye K, Zhang X, Zhao J. The Rhodamine–Perylene Compact Electron Donor–Acceptor Dyad: Spin-Orbit Charge-Transfer Intersystem Crossing and the Energy Balance of the Triplet Excited States. Photochem. 2024; 4(1):40-56. https://doi.org/10.3390/photochem4010004
Chicago/Turabian StyleImran, Muhammad, Dongyi Liu, Kaiyue Ye, Xue Zhang, and Jianzhang Zhao. 2024. "The Rhodamine–Perylene Compact Electron Donor–Acceptor Dyad: Spin-Orbit Charge-Transfer Intersystem Crossing and the Energy Balance of the Triplet Excited States" Photochem 4, no. 1: 40-56. https://doi.org/10.3390/photochem4010004