Assessing the Effect of Glyphosate Toxicity on Lemna minor in Different Temperature Regimes
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
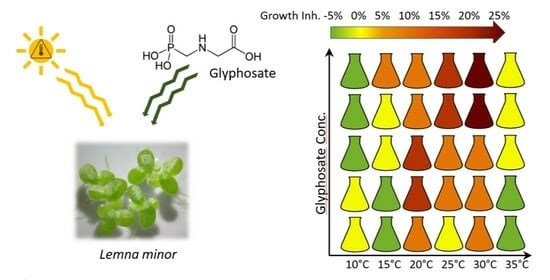
3.1. Growth Inhibition
3.2. Peroxidase Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Öğlü, B.; Möls, T.; Kaart, T.; Cremona, F.; Kangur, K. Parameterization of surface water temperature and long-term trends in Europe’s fourth largest lake shows recent and rapid warming in winter. Limnologica 2020, 82, 125777. [Google Scholar] [CrossRef]
- Jeppesen, E.; Mehner, T.; Winfield, I.J.; Kangur, K.; Sarvala, J.; Gerdeaux, D.; Rask, M.; Malmquist, H.J.; Holmgren, K.; Volta, P.; et al. Impacts of climate warming on the long-term dynamics of key fish species in 24 European lakes. Hydrobiologia 2012, 694, 1–39. [Google Scholar] [CrossRef]
- Noyes, P.D.; McElwee, M.K.; Miller, H.D.; Clark, B.W.; Van Tiem, L.A.; Walcott, K.C.; Erwin, K.N.; Levin, E.D. The toxicology of climate change: Environmental contaminants in a warming world. Environ. Int. 2009, 35, 971–986. [Google Scholar] [CrossRef] [PubMed]
- Daam, M.A.; Van den Brink, P.J. Implications of differences between temperate and tropical freshwater ecosystems for the ecological risk assessment of pesticides. Ecotoxicology 2010, 19, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Howe, G.E.; Marking, L.L.; Bills, T.D.; Rach, J.J.; Mayer, F.L., Jr. Effects of water temperature and pH on toxicity of terbufos, trichlorfon, 4-nitrophenol and 2, 4-dinitrophenol to the amphipod Gammarus pseudolimnaeus and rainbow trout (Oncorhynchus mykiss). Environ. Toxicol. Chem. 1994, 13, 51–66. [Google Scholar] [CrossRef]
- Dyer, S.D.; Belanger, S.E.; Carr, G.J. An initial evaluation of the use of Euro/North American fish species for tropical effects assessments. Chemosphere 1997, 35, 2767–2781. [Google Scholar] [CrossRef]
- Heugens, E.H.W.; Tokkie, L.T.B.; Kraak, M.H.S.; Hendriks, A.J.; Van Straalen, N.M.; Admiraal, W. Population growth of Daphnia magna under multiple stress conditions: Joint effects of temperature, food, and cadmium. Environ. Toxicol. Chem. 2006, 25, 1399–1407. [Google Scholar] [CrossRef]
- Viswanathan, P.N.; Krishna Murti, C.R. Effects of temperature and humidity on ecotoxicology of chemicals. In Ecotoxicology and Climate: With Special Reference to Hot and Cold Climates; Bourdeau, P., Haines, J.A., Klein, W., Krishna Murti, C.R., Eds.; Wiley: Chichester, UK, 1989; pp. 139–154. [Google Scholar]
- Bérard, A.; Leboulanger, C.; Pelte, T. Tolerance of Oscillatoria limnetica Lemmermann to atrazine in natural phytoplankton populations and in pure culture: Influence of season and temperature. Arch. Environ. Contam. Toxicol. 1999, 37, 472–479. [Google Scholar] [CrossRef]
- Benbrook, C.M. Trends in glyphosate herbicide use in the United States and globally. Environ. Sci. Eur. 2016, 28, 3. [Google Scholar] [CrossRef]
- Meftaul, I.M.; Venkateswarlu, K.; Dharmarajan, R.; Annamalai, P.; Asaduzzaman, M.; Parven, A.; Megharaj, M. Controversies over human health and ecological impacts of glyphosate: Is it to be banned in modern agriculture? Environ. Pollut. 2020, 263, 114372. [Google Scholar] [CrossRef]
- Torstensson, L. Behaviour of glyphosate in soils and its degradation. In The Herbicide Glyphosate; Grossbard, E., Atkinson, D., Eds.; Butterworths: London, UK, 1985; pp. 137–150. [Google Scholar]
- Radwan, D.E.M.; Fayez, K.A. Photosynthesis, antioxidant status and gas-exchange are altered by glyphosate application in peanut leaves. Photosynthetica 2016, 54, 307–316. [Google Scholar] [CrossRef]
- Székács, A.; Mörtl, M.; Darvas, B. Monitoring Pesticide Residues in Surface and Ground Water in Hungary: Surveys in 1990–2015. J. Chem. 2015, 2015, 717948. [Google Scholar] [CrossRef]
- Maloschik, E.; Ernst, A.; Hegedűs, G.; Darvas, B.; Székács, A. Monitoring water-polluting pesticides in Hungary. Microchem. J. 2007, 85, 88–97. [Google Scholar] [CrossRef]
- OECD. Test No. 221: Lemna sp. Growth Inhibition Test. In OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2006. [Google Scholar] [CrossRef]
- Kostopoulou, S.; Ntatsi, G.; Arapis, G.; Aliferis, K.A. Assessment of the effects of metribuzin, glyphosate, and their mixtures on the metabolism of the model plant Lemna minor L. applying metabolomics. Chemosphere 2020, 239, 124582. [Google Scholar] [CrossRef] [PubMed]
- Vera, M.S.; Lagomarsino, L.; Sylvester, M.; Pérez, G.L.; Rodríguez, P.; Mugni, H.; Sinistro, R.; Ferraro, M.; Bonetto, C.; Zagarese, H.; et al. New evidences of Roundup (glyphosate formulation) impact on the periphyton community and the water quality of freshwater ecosystems. Ecotoxicology 2010, 19, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Sikorski, Ł.; Baciak, M.; Bęś, A.; Adomas, B. The effects of glyphosate-based herbicide formulations on Lemna minor, a non-target species. Aquat. Toxicol. 2019, 209, 70–80. [Google Scholar] [CrossRef]
- Lewis, M.; Thursby, G. Aquatic plants: Test species sensitivity and minimum data requirement evaluations for chemical risk assessments and aquatic life criteria development for the USA. Environ. Pollut. 2018, 238, 270–280. [Google Scholar] [CrossRef]
- Piccolroaz, S.; Zhu, S.; Ptak, M.; Sojka, M.; Du, X. Warming of lowland Polish lakes under future climate change scenarios and consequences for ice cover and mixing dynamics. J. Hydrol. Reg. Stud. 2021, 34, 100780. [Google Scholar] [CrossRef]
- Blanchoud, H.; Moreau-Guigno, E.; Farrugia, F.; Chevreuil, M.; Mouchel, J.M. Contribution by urban and agricultural pesticide uses to water contamination at the scale of the Marne watershed. Sci. Total Environ. 2007, 375, 168–179. [Google Scholar] [CrossRef]
- Camp, A.A.; Buchwalter, D.B. Can’t take the heat: Temperature-enhanced toxicity in the mayfly Isonychia bicolor exposed to the neonicotinoid insecticide imidacloprid. Aquat. Toxicol. 2016, 178, 49–57. [Google Scholar] [CrossRef]
- Passardi, F.; Cosio, C.; Penel, C.; Dunand, C. Peroxidases have more functions than a Swiss army knife. Plant Cell Rep. 2005, 24, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Koce, J.D. Effects of exposure to nano and bulk sized TiO2 and CuO in Lemna minor. Plant Physiol. Biochem. 2017, 119, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.C.M.; Daam, M.A.; Gusmao, F. Acclimation alters glyphosate temperature-dependent toxicity: Implications for risk assessment under climate change. J. Hazard. Mater. 2020, 385, 121512. [Google Scholar] [CrossRef] [PubMed]
- Di Guardo, A.; Finizio, A. A new methodology to identify surface water bodies at risk by using pesticide monitoring data: The glyphosate case study in Lombardy Region (Italy). Sci. Total Environ. 2018, 610–611, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Sobrero, M.C.; Rimoldi, F.; Ronco, A.E. Effects of the Glyphosate Active Ingredient and a Formulation on Lemna gibba L. at Different Exposure Levels and Assessment End-Points. Bull. Environ. Contam. Toxicol. 2007, 79, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Imberty, A.; Goldberg, R.; Catesson, A.M. Tetramethylbenzidine and p-phenylenediamine-pyrocatechol for peroxidase histochemistry and biochemistry: Two new, non-carcinogenic chromogens for investigating lignification process. Plant Sci. Lett. 1984, 35, 103–108. [Google Scholar] [CrossRef]
- Basiglini, E.; Pintore, M.; Forni, C. Effects of treated industrial wastewaters and temperatures on growth and enzymatic activities of duckweed (Lemna minor L.). Ecotoxicol. Environ. Saf. 2018, 153, 54–59. [Google Scholar] [CrossRef]
- Scherr, C.; Simon, M.; Spranger, J.; Baumgartner, S. Test system stability and natural variability of a Lemna gibba L. bioassay. PLoS ONE 2008, 3, e3133. [Google Scholar] [CrossRef]
- Van Der Heide, T.; Roijackers, R.M.M.; Peeters, E.T.H.M.; Van Nes, E.H. Experiments with duckweed–moth systems suggest that global warming may reduce rather than promote herbivory. Freshw. Biol. 2006, 51, 110–116. [Google Scholar] [CrossRef]
- Rosenkrantz, R.T.; Cedergreen, N.; Baun, A.; Kusk, K.O. Influence of pH, light cycle, and temperature on ecotoxicity of four sulfonylurea herbicides towards Lemna gibba. Ecotoxicology 2013, 22, 33–41. [Google Scholar] [CrossRef]
- Niu, S.; Li, Z.; Xia, J.; Han, Y.; Wu, M.; Wan, S. Climatic warming changes plant photosynthesis and its temperature dependence in a temperate steppe of northern China. Environ. Exp. Bot. 2008, 63, 91–101. [Google Scholar] [CrossRef]
- Zezulka, S.; Kummerová, M.; Babula, P.; Vánová, L. Lemna minor exposed to fluoranthene: Growth, biochemical, physiological and histochemical changes. Aquat. Toxicol. 2013, 140–141, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Naranjo, E.; Perez-Martin, A. Effects of sub-lethal glyphosate concentrations on growth and photosynthetic performance of non-target species Bolboschoenus maritimus. Chemosphere 2013, 93, 2631–2638. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.P.; da Silva Cruz, F.V.; Bicalho, E.M.; Borges, F.V.; Fonseca, M.B. Effects of glyphosate acid and the glyphosate-commercial formulation (Roundup) on Dimorphandra wilsonii seed germination: Interference of seed respiratory metabolism. Environ. Pollut. 2017, 220, 452–459. [Google Scholar] [CrossRef] [PubMed]
- da Silva Santos, J.; da Silva Pontes, M.; Grillo, R.; Fiorucci, A.R.; de Arruda, G.J.; Santiago, E.F. Physiological mechanisms and phytoremediation potential of the macrophyte Salvinia biloba towards a commercial formulation and an analytical standard of glyphosate. Chemosphere 2020, 259, 127417. [Google Scholar] [CrossRef] [PubMed]
- Paolacci, S.; Harrison, S.; Jansen, M.A.K. Are alien species necessarily stress sensitive? A case study on Lemna minuta and Lemna minor. Flora 2018, 249, 31–39. [Google Scholar] [CrossRef]
- Sváb, E. Sekélyvizű Tavak Vízminıség-Vizsgálata, Állapotfelmérése Műholdas Távérzékelés Segítségével. Ph.D. Thesis, Eötvös Loránd University, Budapest, Hungary, 2008. (In Hungarian). [Google Scholar]
- Brovini, E.M.; de Deus, B.C.T.; Vilas-Boas, J.A.; Quadra, G.R.; Carvalho, L.; Mendonça, R.F.; Pereira, R.d.O.; Cardoso, S.J. Three-bestseller pesticides in Brazil: Freshwater concentrations and potential environmental risks. Sci. Total Environ. 2021, 771, 144754. [Google Scholar] [CrossRef]
- Hubai, K.; Kováts, N.; Sainnokhoi, T.A.; Eck-Varanka BHoffer, A.; Tóth, Á.; Teke, G. Phytotoxicity of particulate matter from controlled burning of different plastic waste types. Bull. Environ. Contam. Toxicol. 2022, 109, 852–858. [Google Scholar] [CrossRef]
- Alla, M.M.N.; Hassan, N.M. Changes of antioxidants levels in two maize lines following atrazine treatments. Plant Physiol. Biochem. 2006, 44, 202–210. [Google Scholar] [CrossRef]
- Mitrovic, S.M.; Pflugmacher, S.; James, K.J.; Furey, A. Anatoxin-a elicits an increase in peroxidase and glutathione S-transferase activity in aquatic plants. Aquat. Toxicol. 2004, 68, 185–192. [Google Scholar] [CrossRef]
- Huang, L.; Lu, Y.; Gao, X.; Du, G.; Ma, X.; Liu, M.; Guo, J.; Chen, Y. Ammonium-induced oxidative stress on plant growth and antioxidative response of duckweed (Lemna minor L.). Ecol. Eng. 2013, 58, 355–362. [Google Scholar] [CrossRef]
- Liu, H.; Weisman, D.; Ye, Y.-B.; Cui, B.; Huang, Y.-H.; Colón-Carmona, A.; Wang, Z.-H. An oxidative stress response to polycyclic aromatic hydrocarbon exposure is rapid and complex in Arabidopsis thaliana. Plant Sci. 2009, 176, 375–382. [Google Scholar] [CrossRef]
- Wang, Q.; Que, X.; Zheng, R.; Pang, Z.; Li, C.; Xiao, B. Phytotoxicity assessment of atrazine on growth and physiology of three emergent plants. Environ. Sci. Pollut. Res. 2015, 22, 9646–9657. [Google Scholar] [CrossRef] [PubMed]
- Teisseire, H.; Vernet, G. Is the “Diuron Effect” Due to a Herbicide Strengthening of Antioxidative Defenses of Lemna minor? Pestic. Biochem. Physiol. 2000, 66, 153–160. [Google Scholar] [CrossRef]
- Hu, C.; Liu, L.; Li, X.; Xu, Y.; Ge, Z.; Zhao, Y. Effect of graphene oxide on copper stress in Lemna minor L.: Evaluating growth, biochemical responses, and nutrient uptake. J. Hazard. Mater. 2018, 341, 168–176. [Google Scholar] [CrossRef]
- Pouresmaeil, M.; Sabzi-Nojadeh, M.; Movafeghi, A.; Aghbash, B.N.; Kosari-Nasab, M.; Zengin, G.; Maggi, F. Phytotoxic activity of Moldavian dragonhead (Dracocephalum moldavica L.) essential oil and its possible use as bio-herbicide. Process. Biochem. 2022, 114, 86–92. [Google Scholar] [CrossRef]
- Gomes, M.P.; Juneau, P. Temperature and light modulation of herbicide toxicity on algal and cyanobacterial physiology. Front. Environ. Sci. 2017, 5, 50. [Google Scholar] [CrossRef]
- Smedbol, E.; Gomes, M.P.; Paquet, S.; Labrecque, M.; Lepage, L.; Lucotte, M.; Juneau, P. Effects of low concentrations of glyphosate-based herbicide factor 540® on an agricultural stream freshwater phytoplankton community. Chemosphere 2018, 192, 133–141. [Google Scholar] [CrossRef]
- Geoffroy, L.; Frankart, C.; Eullaffroy, P. Comparison of different physiological parameter responses in Lemna minor and Scenedesmus obliquus exposed to herbicide flumioxazin. Environ. Pollut. 2004, 131, 233–241. [Google Scholar] [CrossRef]
- Morais, H.; Arenas, F.; Cruzeiro, C.; Galante-Oliveira, S.; Cardoso, P.G. Combined effects of climate change and environmentally relevant mixtures of endocrine disrupting compounds on the fitness and gonads’ maturation dynamics of Nucella lapillus (Gastropoda). Mar. Pollut. Bull. 2023, 190, 114841. [Google Scholar] [CrossRef]
- Hermann, M.; Peeters, E.T.H.M.; Van den Brink, P.J. Heatwaves, elevated temperatures, and a pesticide cause interactive effects on multi-trophic levels of a freshwater ecosystem. Environ. Pollut. 2023, 327, 121498. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Megías, C.; Mentzel, S.; Fuentes-Edfuf, Y.; Moe, S.J.; Rico, A. Influence of climate change and pesticide use practices on the ecological risks of pesticides in a protected Mediterranean wetland: A Bayesian network approach. Sci. Total Environ. 2023, 878, 163018. [Google Scholar] [CrossRef] [PubMed]






| Concentration | 10 °C | 15 °C | 20 °C | 25 °C | 30 °C | 35 °C |
|---|---|---|---|---|---|---|
| 25 µg/L | 1.51 | −4.36 | 14.23 | 0.26 | 10.34 | −3.95 |
| 50 µg/L | 1.51 | −4.84 | 18.69 | 13.24 | 10.19 | −1.92 |
| 100 µg/L | −0.25 | 0.55 | 16.87 | 10.69 | 14.03 | 0.25 |
| 200 µg/L | −2.38 | 0.00 | 11.80 | 15.16 | 21.74 | 1.77 |
| 400 µg/L | −4.60 | 11.25 | 12.44 | 18.42 | 23.64 | 4.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eck-Varanka, B.; Kováts, N.; Hubai, K.; Sainnokhoi, T.-A. Assessing the Effect of Glyphosate Toxicity on Lemna minor in Different Temperature Regimes. Pollutants 2023, 3, 451-460. https://doi.org/10.3390/pollutants3040031
Eck-Varanka B, Kováts N, Hubai K, Sainnokhoi T-A. Assessing the Effect of Glyphosate Toxicity on Lemna minor in Different Temperature Regimes. Pollutants. 2023; 3(4):451-460. https://doi.org/10.3390/pollutants3040031
Chicago/Turabian StyleEck-Varanka, Bettina, Nóra Kováts, Katalin Hubai, and Tsend-Ayush Sainnokhoi. 2023. "Assessing the Effect of Glyphosate Toxicity on Lemna minor in Different Temperature Regimes" Pollutants 3, no. 4: 451-460. https://doi.org/10.3390/pollutants3040031