N-b-Hydroxyethyl Oleyl Imidazole as Synergist to Enhance the Corrosion Protection Effect of Natural Cocoyl Sarcosine on Steel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Metal Specimens and Weight Loss
2.2. Inhibitors
2.3. Electrochemical Measurements
2.4. Spray Corrosion Test
3. Results and Discussion
3.1. Weight Loss (WL)
3.2. Potentiodynamic Polarization Measurement
3.3. Electrochemical Impedance Spectroscopy Measurement
3.4. Surface Characterization
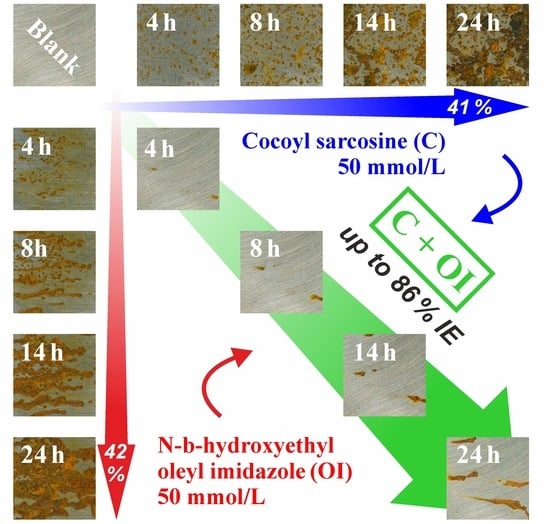
3.5. Effect of Increasing Immersion Time
3.6. Spray Corrosion Chamber Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Popov, B.N. Corrosion Engineering Principles and Solved Problems; Elsevier B.V.: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Eliaz, N.; Gileadi, E. Physical Electrochemistry: Fundamentals, Techniques and Applications; Wiley-VCH Verlag GmbH & Co., KGaA: Weinheim, Germany, 2018. [Google Scholar]
- Pathan, S.; Ahmad, S. Synergistic effect of linseed oil based waterborne alkyd and 3-isocynatoropyl triethoxysilane: Highly transparent, mechanically robust, thermally stable, hydrophobic, anticorrosive coatings. ACS Sustain. Chem. Eng. 2016, 4, 3062–3075. [Google Scholar] [CrossRef]
- Dariva, C.G.; Galio, A. Corrosion Inhibitors—Principles, Mechanisms and Applications, Ch. 16. In Developments in Corrosion Protection; Aliofhazraei, M., Ed.; IntechOpen: London, UK, 2014. [Google Scholar] [CrossRef]
- Agarwal, P.; Landolt, D. Corrosion Inhibitors, Part 5; Application Note COR05; Metrohm Autolab B.V.: Herisau, Switzerland, 2011. [Google Scholar]
- Kaskah, S.E.; Pfeiffer, M.; Klock, H.; Bergen, H.; Ehrenhaft, G.; Ferreira, P.; Gollnick, J.; Fischer, C.B. Surface protection of low carbon steel with N-acyl sarcosine derivatives as green corrosion inhibitors. Surf. Interfaces 2017, 9, 70–78. [Google Scholar] [CrossRef]
- Bommersbach, P.; Alemany-Dumont, C.; Millet, J.P.; Normand, B. Formation and behaviour study of an environment-friendly corrosion inhibitor by electrochemical methods. Electrochim. Acta 2005, 51, 1076–1084. [Google Scholar] [CrossRef]
- Bentiss, F.; Bouanis, M.; Mernari, B.; Traisnel, M.; Vezin, H.; Lagrenée, M. Understanding the adsorption of 4H-1,2,4-triazole derivatives on mild steel surface in molar hydrochloric acid. Appl. Surf. Sci. 2007, 253, 3696–3704. [Google Scholar] [CrossRef]
- Sharma, S.K. Green Corrosion Chemistry and Engineering, Opportunities and Challenges; Wiley-VCH Verlag GmbH & Co., KGaA: Weinheim, Germany, 2011. [Google Scholar]
- Papavinsam, S. Corrosion inhibitors, Ch. 59. In Uhlig’s Corrosion Handbook, 2nd ed.; Revie, R.W., Ed.; John Wiley: Hoboken, NJ, USA, 2000. [Google Scholar]
- Rahim, A.A.; Kassim, J. Recent development of vegetal tannins in corrosion protection of iron and steel. Recent Pat. Mater. Sci. 2008, 1, 223–231. [Google Scholar] [CrossRef]
- Zhao, L.; Teng, H.K.; Yang, Y.S.; Tan, X. Corrosion inhibition approach of oil production systems in offshore oilfields. Mater. Corros. 2004, 55, 684–688. [Google Scholar] [CrossRef]
- Adamu, M.; Umoru, L.E.; Ige, O.O. Effect of toluene and dioctylphthalate on the rebar corrosion of medium carbon steel in seawater and cassava fluid. J. Miner. Mater. Charact. Eng. 2014, 2, 1–7. [Google Scholar] [CrossRef]
- Yadav, M.; Debasis, B.; Sinha, R.R.; Yadav, P.N. Experimental and quantum studies on adsorption and corrosion inhibition effect on mild steel in hydrochloric acid by thiophene derivatives. Acta Metall. Sin. 2014, 27, 37–46. [Google Scholar] [CrossRef]
- Chong, A.L.; Mardel, J.I.; MacFarlane, D.R.; Forsyth, M.; Somers, A.E. Synergistic corrosion inhibition of mild steel in aqueous chloride solution by imidazolinium carboxylate salt. ACS Sustain. Chem. Eng. 2016, 4, 1746–1755. [Google Scholar] [CrossRef]
- Ostovari, A.; Hoseinieh, S.M.; Peikari, M.; Shadizadeh, S.R.; Hashemi, S.J. Corrosion inhibition of mild steel in 1 M HCl solution by henna extract: A comparative study of the inhibition by henna and its constituents (Lawsone, Gallic acid, α-d-Glucose and Tannic acid). Corros. Sci. 2009, 51, 1935–1949. [Google Scholar] [CrossRef]
- Salensky, G.A.; Cobb, M.G.; Everhart, D.S. Corrosion-Inhibitor Orientation on Steel. Ind. Eng. Chem. Prod. Res. Dev. 1986, 25, 133–140. [Google Scholar] [CrossRef]
- Rodriguez-Clemente, E.; Gonzalez-Rodriguez, J.G.; Valladares-Cisneros, M.G. Allium sativum as corrosion inhibitor for carbon steel in sulfuric acid. Int. J. Electrochem. Sci. 2014, 9, 5924–5936. [Google Scholar]
- Ibrahim, T.H.; Zour, M.A. Corrosion inhibition of mild steel using fig leaves extract in hydrochloric acid solution. Int. J. Electrochem. Sci. 2011, 6, 6442–6455. [Google Scholar]
- Lanigan, R.S. Final report on the safety assessment of Cocoyl sarcosine, Lauroyl sarcosine, Myristoyl sarcosine, Oleoyl sarcosine, Stearoyl Sarcosine, Sodium Cocoyl sarcosinate, Sodium Lauroyl sarcosinate, Sodium Myristoyl sarcosinate, Ammonium Cocoyl sarcosinate, and Ammonium Lauroyl sarcosinate. Int. J. Toxicol. 2001, 20 (Suppl. 1), 1–14. [Google Scholar] [PubMed]
- Rocca, E.; Steinmetz, J. Inhibition of lead corrosion with saturated linear aliphatic chain monocarboxylates of sodium. Corros. Sci. 2001, 43, 891–902. [Google Scholar] [CrossRef]
- Boisier, G.; Lamure, A.; Pébère, N.; Portail, N.; Villatte, M. Corrosion protection of A2024 sealed anodic layers using the hydrophobic properties of carboxylic acids. Surf. Coat. Technol. 2009, 203, 3420–3426. [Google Scholar] [CrossRef]
- Hefter, G.T.; North, N.A.; Tan, S.H. Organic corrosion inhibitors in neutral solutions; Part 1—Inhibition of steel, copper, and aluminum by straight chain carboxylates. NACE Int. Corros. 1997, 53, 657–667. [Google Scholar] [CrossRef]
- Lahem, D.; Poelman, M.; Atmani, F.; Olivier, M.-G. Synergistic improvement of inhibitive activity of dicarboxylates in preventing mild steel corrosion in neutral aqueous solution. Corros. Eng. Sci. Technol. 2012, 47, 463–471. [Google Scholar] [CrossRef]
- Abboud, Y.; Tanane, O.; El Bouari, A.; Salghi, R.; Hammouti, B.; Chetouani, A.; Jodeh, S. Corrosion inhibition of carbon steel in hydrochloric acid solution using pomegranate leave extract. Corros. Eng. Sci. Technol. 2016, 51, 557–565. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Velardi, U.V.; Brenna, A.; Mele, A.; Pedeferri, M.; Ormellese, M. Compatibility of imidazolium-based ionic liquids for CO2 capture with steel alloys: A corrosion prespective. Electrochim. Acta 2016, 192, 414–421. [Google Scholar] [CrossRef]
- Kálmán, E.; Kármán, F.H.; Telegdi, J.; Várhegyi, B.; Balla, J.; Kiss, T. Inhibition efficiency of N-containing carboxylic and carboxy-phosphonic acids. Corros. Sci. 1993, 35, 1477–1482. [Google Scholar] [CrossRef]
- Sastri, V.S. Challenges in Corrosion: Cost, Causes, Consequences, and Control; John Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Lgaz, H.; Anejjar, A.; Salghi, R.; Jodeh, S.; Zougagh, M.; Warad, I.; Larouj, M.; Sims, P. Characterization of corrosion products formed on carbon steel in hydrochloric acid medium by 4-(dimethlamino)-1-(6-methoxy-6-oxohexyl) pyridinium bromide. Int. J. Corros. Scale Inhib. 2016, 5, 209–231. [Google Scholar]
- Heydari, M.; Javidi, M. Corrosion inhibition and adsorption behaviour of an amido-imidazoline derivative on API 5L X52 steel in CO2-saturated solution and synergistic effect of iodide ions. Corros. Sci. 2012, 61, 148–155. [Google Scholar] [CrossRef]
- Ochoa, N.; Moran, F.; Pébère, N.; Tribollet, B. Influence of flow on the corrosion inhibition of carbon steel by fatty amines in association with phosphonocarboxylic acid salts. Corros. Sci. 2005, 47, 593–604. [Google Scholar] [CrossRef]
- Ochoa, N.; Moran, F.; Pébère, N. The synergistic effect between phosphonocarboxylic acid salts and fatty amines for the corrosion protection of a carbon steel. J. Appl. Electrochem. 2004, 34, 487–493. [Google Scholar] [CrossRef]
- Ochoa, N.; Baril, G.; Moran, F.; Pébère, N. Study of the properties of a multi-component inhibitor used for water treatment in cooling circuits. J. Appl. Electrochem. 2002, 32, 497–504. [Google Scholar] [CrossRef]
- Kaskah, S.E.; Ehrenhaft, G.; Gollnick, J.; Fischer, C.B. Concentration and coating time effects of N-acyl sarcosine derivatives for corrosion protection of low-carbon steel CR4 in salt water—Defining the window of application. Corros. Eng. Sci. Technol. 2019, 3, 216–224. [Google Scholar] [CrossRef]
- Edwards, A.; Osborns, C.; Webster, S.; Klenerman, D.; Joseph, M.; Ostovar, P.; Doyle, M. Mechanistic studies of the corrosion inhibitor oleic imidazoline. Corros. Sci. 1994, 36, 315–325. [Google Scholar] [CrossRef]
- Wegst, C.; Wegst, M. Stahlschlüssel, 22; Auflage, Verlag Stahlschlüssel Wegst GmbH: Marbach, Germany, 2010. [Google Scholar]
- DIN EN ISO 9227:2006; Corrosion Tests in Artificial Atmospheres—Salt Spray Tests. European Committee for Standardization: Brussels, Belgium, 2006.
- DIN EN ISO 1514:2005-02; Paints and Varnishes—Standard Panels for Testing. European Committee for Standardization: Brussels, Belgium, 2004.
- Tawfik, S.M.; Negm, N.A. Vanillin-derived non-ionic surfactants as green corrosion inhibitors for carbon steel in acidic environments. Res. Chem. Intermed. 2016, 42, 3579–3607. [Google Scholar] [CrossRef]
- Duarte, T.; Meyer, Y.A.; Osório, W.R. The Holes of Zn Phosphate and Hot Dip Galvanizing on Electrochemical Behaviors of Multi-Coatings on Steel Substrates. Metals 2022, 12, 863. [Google Scholar] [CrossRef]
- Zhang, X.L.; Jiang, Z.H.; Yao, Z.P.; Song, Y.; Wu, Z.D. Effects of scan rate on the potentiodynamic polarization curve obtained to determine the Tafel slopes and corrosion current density. Corros. Sci. 2009, 51, 581–587. [Google Scholar] [CrossRef]
- McCafferty, E. Validation of corrosion rates measured by Tafel extrapolation method. Corros. Sci. 2005, 47, 3202–3215. [Google Scholar] [CrossRef]
- ImageJ Homepage. Available online: https://imagej.nih.gov/ij/index.html (accessed on 16 July 2022).
- Origin 8.1 SR2, Home Page. Available online: http://www.originlab.com (accessed on 16 July 2022).
- Ma, Y.; Han, F.; Li, Z.; Xia, C. Corrosion behavior of metallic materials in acidic-functionalized ionic liquids. ACS Sustain. Chem. Eng. 2016, 4, 633–639. [Google Scholar] [CrossRef]
- Mounir, F.; Salghi, R.; Jodeh, S.; El Issami, S.; Bazzi, L. Argania spinosa (L.) as a source of new and efficient green corrosion inhibitor for copper in acidic medium: A comparative study of three green compounds. Int. J. Corros. Scale Inhib. 2016, 5, 159–171. [Google Scholar] [CrossRef]
- Vashisht, H.; Kumar, S.; Bahadur, I.; Singh, G. Evaluation of (2-hydroxyethyl) triphenyl phosphonium bromide as corrosion inhibitor for mild steel in sulphuric acid. Int. J. Electrochem. Sci. 2013, 8, 684–699. [Google Scholar]
- Jacobsen, T.; West, K. Diffusion impedance in planar, cylindrical and spherical symmetry. Electrochim. Acta 1995, 40, 255–262. [Google Scholar] [CrossRef]
- Zia, A.I.; Mukhopadhyay, S.C. Impedance Spectroscopy and Experimental Setup, Ch 2. In Electrochemical Sensing: Carcinogens in Beverages; Springer Nature: Berlin/Heidelberg, Germany, 2016. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.A.; Asghari, E.; Ejbari, P. Electrochemical studies of adsorption and inhibitive performance of basic yellow 28 dye on mild steel corrosion in acid solutions. Acta Chim. Slov. 2011, 58, 270–277. [Google Scholar]
- Trabanelli, G.; Carassiti, V. Mechanism and phenomenology of organic inhibitors. In Advances in Corrosion Science and Technology; Fontana, M.G., Staehle, R.W., Eds.; Springer: Boston, MA, USA, 1970; Volume I, pp. 147–228. [Google Scholar]








| Element | C | W | Mn | P | S | Cu | Al | Cr | Mo | Ni | Co | W |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Norm [36] | max. 0.120 | n.a. | max. 0.600 | max. 0.045 | max. 0.045 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Present | 0.063 | 0.013 | 0.260 | 0.017 | 0.008 | 0.000 | 0.033 | 0.003 | 0.006 | 0.000 | 0.000 | 0.006 |
| Concentration (mmol/L) | WL (mg) × 10−3 | CR (mg⋅cm−2⋅h−1) × 10−5 | Surface Coverage (θ) | IE (%) | |
|---|---|---|---|---|---|
| Blank | 0 | 5.70 ± 0.86 | 1.900 | - | - |
| Inhibitor | |||||
| C | 25 | 3.40 ± 0.30 | 1.133 | 0.404 | 40.4 |
| 50 | 3.25 ± 0.21 | 1.083 | 0.430 | 43.0 | |
| 75 | 3.23 ± 0.65 | 1.077 | 0.433 | 43.3 | |
| 100 | 3.13 ± 0.91 | 1.041 | 0.452 | 45.2 | |
| OI | 25 | 3.85 ± 0.78 | 1.283 | 0.325 | 32.5 |
| 50 | 3.37 ± 1.29 | 1.122 | 0.409 | 40.9 | |
| 75 | 3.30 ± 0.28 | 1.100 | 0.421 | 42.1 | |
| 100 | 3.25 ± 2.47 | 1.083 | 0.430 | 43.0 | |
| C + OI | 25 | 1.50 ± 0.90 | 0.500 | 0.737 | 73.7 |
| 50 | 1.24 ± 0.92 | 0.413 | 0.782 | 78.2 | |
| 75 | 1.03 ± 1.56 | 0.343 | 0.819 | 81.9 | |
| 100 | 0.98 ± 1.55 | 0.328 | 0.827 | 82.7 |
| Cinh (mmol/L) | icorr (µA/cm2) | CR (mm/y) | βa (mV/dec) | βc (mV/dec) | Ecorr (mV) | Surface Coverage (θ) | IE (%) | |
|---|---|---|---|---|---|---|---|---|
| Blank [34] | 0 | 61.93 ± 7.47 | 1.339 | 16.87 | −2.98 | −324 | - | - |
| Inhibitor | ||||||||
| C | 25 | 49.89 ± 9.01 | 1.082 | 31.00 | −3.54 | −321 | 0.195 | 19.5 |
| 50 | 40.34 ± 4.93 | 0.875 | 30.03 | −3.91 | −300 | 0.349 | 34.9 | |
| 75 | 34.59 ± 4.50 | 0.750 | 21.80 | −4.38 | −293 | 0.442 | 44.2 | |
| 100 | 32.78 ± 1.29 | 0.711 | 22.23 | −4.06 | −288 | 0.471 | 47.1 | |
| OI | 25 | 45.55 ± 7.56 | 0.988 | 20.70 | −3.44 | −413 | 0.265 | 26.5 |
| 50 | 41.76 ± 10.79 | 0.906 | 27.84 | −3.62 | −395 | 0.326 | 32.6 | |
| 75 | 36.73 ± 2.30 | 0.797 | 23.65 | −3.98 | −411 | 0.407 | 40.7 | |
| 100 | 33.89 ± 6.06 | 0.735 | 30.58 | −3.04 | −417 | 0.453 | 45.3 | |
| C + OI | 25 | 15.77 ± 0.45 | 0.342 | 29.60 | −4.26 | −249 | 0.745 | 74.5 |
| 50 | 8.52 ± 1.60 | 0.184 | 29.28 | −3.46 | −260 | 0.862 | 86.2 | |
| 75 | 5.86 ± 2.20 | 0.126 | 36.08 | −4.07 | −232 | 0.905 | 90.5 | |
| 100 | 5.31 ± 0.52 | 0.115 | 25.90 | −3.64 | −253 | 0.914 | 91.4 |
| Cinh (mmol/L) | Rs (Ω cm2) | Rct (Ω cm2) | Cdl (μF/cm2) | Surface Coverage (θ) | IE (%) | |
|---|---|---|---|---|---|---|
| Blank | 0 | 64.38 | 178.86 | 2.6425 | - | - |
| Inhibitor | ||||||
| OI | 50 | 90.95 | 331.33 | 2.1919 | 0.460 | 46.0 |
| C | 25 | 79.50 | 570.12 | 1.6087 | 0.686 | 68.6 |
| 50 | 80.34 | 687.26 | 1.5994 | 0.739 | 73.9 | |
| 75 | 81.40 | 350.28 | 1.5242 | 0.489 | 48.9 | |
| 100 | 82.17 | 301.33 | 1.8155 | 0.406 | 40.6 | |
| C + OI | 25 | 204.15 | 779.33 | 2.2667 | 0.770 | 77.0 |
| 50 | 267.67 | 977.13 | 2.1470 | 0.816 | 81.6 | |
| 75 | 96.6 | 1077.27 | 2.1034 | 0.817 | 81.7 | |
| 100 | 229.56 | 1158.68 | 2.0878 | 0.845 | 84.5 |
| Inhibitor | Coating Time (min) | icorr (µA/cm2) | CR (mm/y) | βa (mV/dec) | βc (mV/dec) | Ecorr (mV) | Surface Coverage (θ) | IE (%) |
|---|---|---|---|---|---|---|---|---|
| Blank [34] | 0 | 61.93 ± 7.47 | 1.339 | 16.87 | −2.98 | −324 | - | - |
| C | 1 | 58.62 ± 19.66 | 1.268 | 18.18 | −2.98 | −307 | 0.0534 | 5.34 |
| 2.5 | 51.66 ± 7.76 | 1.117 | 15.69 | −4.11 | −307 | 0.1658 | 16.6 | |
| 5 | 36.85 ± 1.88 | 0.797 | 14.59 | −4.28 | −304 | 0.4049 | 40.5 | |
| 10 | 36.68 ± 4.93 | 0.793 | 30.03 | −3.91 | −300 | 0.4077 | 40.8 | |
| 30 | 47.21 ± 7.27 | 1.021 | 11.04 | −3.55 | −319 | 0.2376 | 23.8 | |
| OI | 1 | 46.06 ± 6.74 | 0.996 | 20.53 | −2.88 | −395 | 0.2562 | 25.6 |
| 2.5 | 43.31 ± 10.05 | 0.936 | 22.50 | −3.72 | −280 | 0.3006 | 30.1 | |
| 5 | 41.52 ± 2.01 | 0.898 | 17.42 | −3.68 | −406 | 0.3295 | 33.0 | |
| 10 | 35.67 ± 0.45 | 0.771 | 27.84 | −3.62 | −395 | 0.4240 | 42.4 | |
| 30 | 47.74 ± 7.04 | 1.032 | 19.56 | −4.20 | −331 | 0.2291 | 22.9 | |
| C + OI | 1 | 17.64 ± 3.32 | 0.381 | 19.92 | −5.55 | −289 | 0.7151 | 71.5 |
| 2.5 | 13.32 ± 2.28 | 0.288 | 13.72 | −5.54 | −280 | 0.7848 | 78.5 | |
| 5 | 8.95 ± 1.14 | 0.193 | 14.40 | −5.36 | −289 | 0.8553 | 85.5 | |
| 10 | 8.52 ± 1.60 | 0.184 | 16.65 | −4.14 | −260 | 0.8624 | 86.2 | |
| 30 | 9.06 ± 2.49 | 0.195 | 12.39 | −4.77 | −267 | 0.8537 | 85.4 |
| Time (h) | Blank | C | IE of C (%) | OI | IE of OI (%) | C + OI | IE of C + OI (%) |
|---|---|---|---|---|---|---|---|
| 2 | 72.03 ± 1.50 | 18.76 ± 0.89 | 74.0 | 19.79 ± 2.22 | 72.5 | 0.25 ± 0.40 | 99.7 |
| 4 | 81.54 ± 0.99 | 34.28 ± 3.36 | 58.0 | 52.79 ± 7.04 | 35.3 | 1.27 ± 1.09 | 98.4 |
| 8 | 87.95 ± 1.49 | 51.91 ± 1.78 | 41.0 | 64.06 ± 5.37 | 27.2 | 2.66 ± 2.01 | 97.0 |
| 14 | 95.35 ± 0.48 | 68.38 ± 3.02 | 28.3 | 77.10 ± 6.02 | 19.1 | 9.05 ± 7.28 | 90.5 |
| 24 | 97.69 ± 0.48 | 81.57 ± 0.50 | 16.5 | 84.64 ± 7.26 | 13.4 | 19.67 ± 7.62 | 80.0 |
| Time (h) | Blank | C + OI 25 mmol/L | IE (%) | C + OI 50 mmol/L | IE (%) | C + OI 75 mmol/L | IE (%) | C + OI 100 mmol/L | IE (%) |
|---|---|---|---|---|---|---|---|---|---|
| 2 | 72.03 ± 1.50 | 55.92 ± 13.55 | 22.4 | 0.25 ± 0.40 | 99.7 | 0.00 ± 0.00 | 100 | 0.00 ± 0.00 | 100 |
| 4 | 81.54 ± 0.99 | 60.23 ± 9.69 | 26.1 | 1.27 ± 1.09 | 98.4 | 0.40 ± 0.65 | 99.5 | 0.23 ± 0.21 | 99.7 |
| 8 | 87.95 ± 1.49 | 64.66 ± 11.64 | 26.5 | 2.67 ± 2.01 | 97.0 | 2.14 ± 1.64 | 97.6 | 1.01 ± 0.65 | 98.9 |
| 14 | 95.35 ± 0.48 | 70.32 ± 9.54 | 26.3 | 9.05 ± 7.28 | 90.5 | 6.58 ± 5.69 | 93.1 | 5.39 ± 1.43 | 94.4 |
| 24 | 97.69 ± 0.48 | 72.33 ± 10.51 | 26.0 | 19.67 ± 13.62 | 80.0 | 13.98 ± 11.03 | 85.7 | 13.74 ± 2.27 | 86.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaskah, S.E.; Ehrenhaft, G.; Gollnick, J.; Fischer, C.B. N-b-Hydroxyethyl Oleyl Imidazole as Synergist to Enhance the Corrosion Protection Effect of Natural Cocoyl Sarcosine on Steel. Corros. Mater. Degrad. 2022, 3, 536-552. https://doi.org/10.3390/cmd3030029
Kaskah SE, Ehrenhaft G, Gollnick J, Fischer CB. N-b-Hydroxyethyl Oleyl Imidazole as Synergist to Enhance the Corrosion Protection Effect of Natural Cocoyl Sarcosine on Steel. Corrosion and Materials Degradation. 2022; 3(3):536-552. https://doi.org/10.3390/cmd3030029
Chicago/Turabian StyleKaskah, Saad E., Gitta Ehrenhaft, Jörg Gollnick, and Christian B. Fischer. 2022. "N-b-Hydroxyethyl Oleyl Imidazole as Synergist to Enhance the Corrosion Protection Effect of Natural Cocoyl Sarcosine on Steel" Corrosion and Materials Degradation 3, no. 3: 536-552. https://doi.org/10.3390/cmd3030029