Journal Description
Corrosion and Materials Degradation
Corrosion and Materials Degradation
is an international, peer-reviewed, open access journal on corrosion, environment-assisted degradation, corrosion mitigation, corrosion mechanism and corrosion monitoring, published quarterly online by MDPI.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within ESCI (Web of Science), Scopus, EBSCO, and other databases.
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 20.2 days after submission; acceptance to publication is undertaken in 5.6 days (median values for papers published in this journal in the second half of 2023).
- Recognition of Reviewers: APC discount vouchers, optional signed peer review, and reviewer names published annually in the journal.
Latest Articles
In-Situ AFM Studies of Surfactant Adsorption on Stainless Steel Surfaces during Electrochemical Polarization
Corros. Mater. Degrad. 2024, 5(2), 224-240; https://doi.org/10.3390/cmd5020009 - 07 Apr 2024
Abstract
►
Show Figures
Corrosion inhibitors are one of the best practices to prevent the far-reaching negative impacts of corrosion on ferrous alloys. A thorough understanding of their corrosion-inhibiting effects is essential for a sustainable economy and environment. Anionic surfactants are known to act efficiently as corrosion
[...] Read more.
Corrosion inhibitors are one of the best practices to prevent the far-reaching negative impacts of corrosion on ferrous alloys. A thorough understanding of their corrosion-inhibiting effects is essential for a sustainable economy and environment. Anionic surfactants are known to act efficiently as corrosion inhibitors. Here, we present that in-situ atomic force microscopy (AFM) measurements can provide deep insights into the adsorption and inhibition mechanism of surfactants on stainless steel surfaces during local corrosion. These include the configuration of surfactant molecules on the surface and how the microstructure of the stainless steel surface influences the inhibition process. Three different anionic surfactants, namely palm kernel oil (PKO), linear alkylbenzene sulfonate (LAS), and fatty alcohol ether sulfate (FAES), were investigated on a titanium-stabilized ferritic stainless steel (1.4510) in NaCl solution. For PKO, the results show random adsorption of bi- and multilayer whereas LAS and FAES adsorb only as local corrosion occurs. Thereby, LAS accumulates only locally and especially at the titanium precipitates of the 1.4510 and FAES forms a densely packed monolayer on the surface. This leads to better corrosion inhibiting properties for LAS and FAES compared to PKO.
Full article
Open AccessArticle
Dissociative Adsorption of Hydrogen Molecules at Al2O3 Inclusions in Steels and Its Implications for Gaseous Hydrogen Embrittlement of Pipelines
by
Yinghao Sun and Frank Cheng
Corros. Mater. Degrad. 2024, 5(2), 200-223; https://doi.org/10.3390/cmd5020008 - 02 Apr 2024
Abstract
Hydrogen embrittlement (HE) of steel pipelines in high-pressure gaseous environments is a potential threat to the pipeline integrity. The occurrence of gaseous HE is subjected to associative adsorption of hydrogen molecules (H2) at specific “active sites”, such as grain boundaries and
[...] Read more.
Hydrogen embrittlement (HE) of steel pipelines in high-pressure gaseous environments is a potential threat to the pipeline integrity. The occurrence of gaseous HE is subjected to associative adsorption of hydrogen molecules (H2) at specific “active sites”, such as grain boundaries and dislocations on the steel surface, to generate hydrogen atoms (H). Non-metallic inclusions are another type of metallurgical defect potentially serving as “active sites” to cause the dissociative adsorption of H2. Al2O3 is a common inclusion contained in pipeline steels. In this work, the dissociative adsorption of hydrogen at the
(This article belongs to the Special Issue Corrosion Mechanisms and Electrochemical Interfaces: In Honor of Prof. Digby Macdonald)
►▼
Show Figures
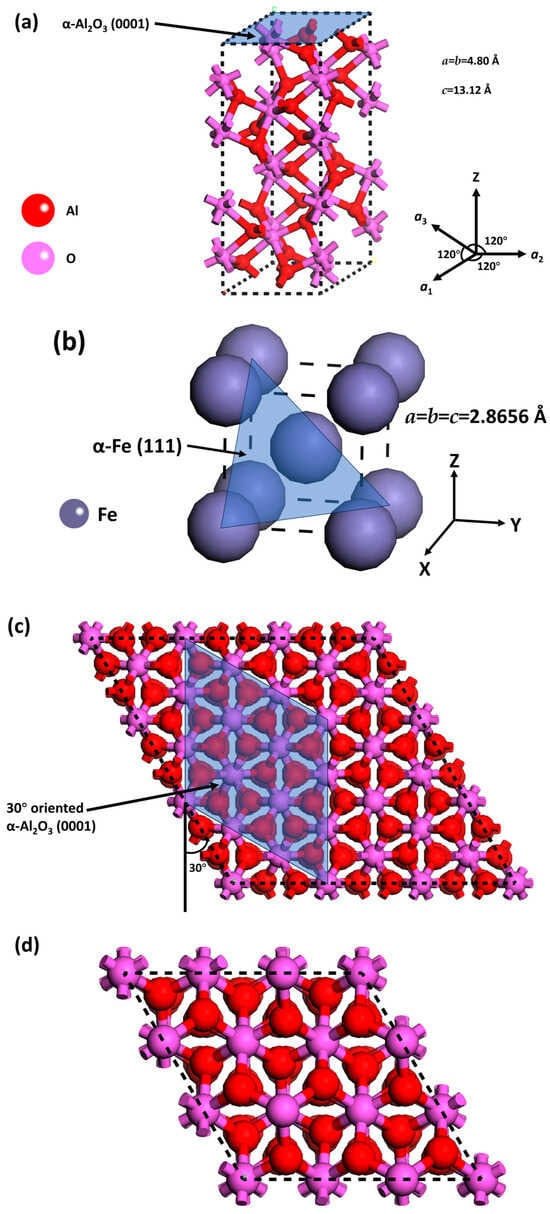
Figure 1
Open AccessFeature PaperReview
Review of the Modelling of Corrosion Processes and Lifetime Prediction for HLW/SF Containers—Part 1: Process Models
by
Fraser King, Miroslav Kolàř, Scott Briggs, Mehran Behazin, Peter Keech and Nikitas Diomidis
Corros. Mater. Degrad. 2024, 5(2), 124-199; https://doi.org/10.3390/cmd5020007 - 28 Mar 2024
Abstract
The disposal of high-level radioactive waste (HLW) and spent nuclear fuel (SF) presents a unique challenge for the prediction of the long-term performance of corrodible structures since HLW/SF containers are expected, in some cases, to have lifetimes of one million years or longer.
[...] Read more.
The disposal of high-level radioactive waste (HLW) and spent nuclear fuel (SF) presents a unique challenge for the prediction of the long-term performance of corrodible structures since HLW/SF containers are expected, in some cases, to have lifetimes of one million years or longer. Various empirical and deterministic models have been developed over the past 45 years for making predictions of long-term corrosion behaviour, including models for uniform and localised corrosion, environmentally assisted cracking, microbiologically influenced corrosion, and radiation-induced corrosion. More recently, fracture-mechanics-based approaches have been developed to account for joint mechanical–corrosion degradation modes. Regardless of whether empirical or deterministic models are used, it is essential to be able to demonstrate a thorough mechanistic understanding of the corrosion processes involved. In addition to process models focused on specific corrosion mechanisms, there is also a need for performance-assessment models as part of the overall demonstration of the safety of a deep geological repository. Performance-assessment models are discussed in Part 2 of this review.
Full article
(This article belongs to the Special Issue Mechanism and Predictive/Deterministic Aspects of Corrosion)
►▼
Show Figures
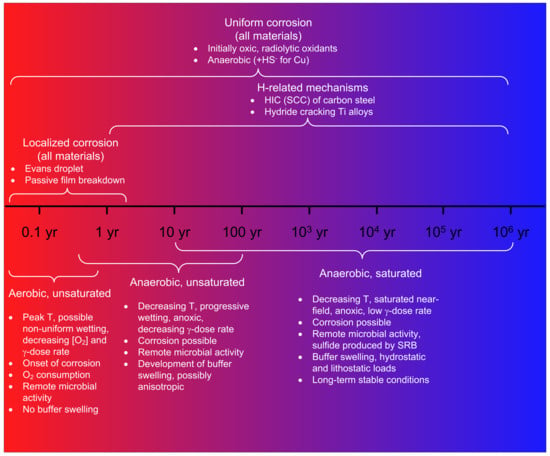
Figure 1
Open AccessReview
Distinctive Oxide Films Develop on the Surface of FeCrAl as the Environment Changes for Nuclear Fuel Cladding
by
Haozheng Qu, Liang Yin, Michael Larsen and Raul B. Rebak
Corros. Mater. Degrad. 2024, 5(1), 109-123; https://doi.org/10.3390/cmd5010006 - 18 Mar 2024
Abstract
The corrosion-resistant properties of IronChromium–Aluminum (FeCrAl) alloys have been known for nearly a century. Since the 1950s, they have been explored for application in the generation of nuclear power. In the last decade, the focus has been on the use of FeCrAl as
[...] Read more.
The corrosion-resistant properties of IronChromium–Aluminum (FeCrAl) alloys have been known for nearly a century. Since the 1950s, they have been explored for application in the generation of nuclear power. In the last decade, the focus has been on the use of FeCrAl as cladding for uranium dioxide fuel in light water reactors (LWRs). The corrosion resistance of this alloy depends on the oxide that it can develop on the surface. In LWRs in the vicinity of 300 °C, the external surface oxide of the FeCrAl cladding could be rich in Fe under oxidizing conditions but rich in Cr under reducing conditions. If there is an accident and the cladding is exposed to superheated steam, the cladding will protect itself by developing an alpha aluminum film on the surface.
Full article
(This article belongs to the Special Issue Corrosion Mechanisms and Electrochemical Interfaces: In Honor of Prof. Digby Macdonald)
►▼
Show Figures
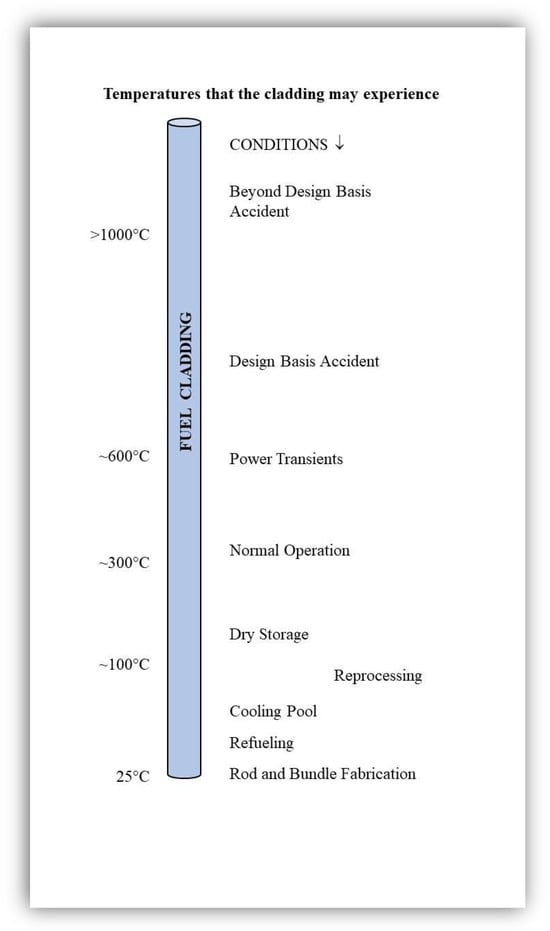
Figure 1
Open AccessArticle
The AA7075–CS1018 Galvanic Couple under Evaporating Droplets
by
Marvin Montoya, Juan Genesca and Rodrigo Montoya
Corros. Mater. Degrad. 2024, 5(1), 92-108; https://doi.org/10.3390/cmd5010005 - 07 Mar 2024
Abstract
The galvanic corrosion behavior of the AA7075–CS1018 couple was examined in dynamic electrolytes using the ZRA technique. A modified electrochemical setup was developed to support the use of thin-film gel and liquid electrolytes on metallic surfaces. This allowed the collection of chemical information,
[...] Read more.
The galvanic corrosion behavior of the AA7075–CS1018 couple was examined in dynamic electrolytes using the ZRA technique. A modified electrochemical setup was developed to support the use of thin-film gel and liquid electrolytes on metallic surfaces. This allowed the collection of chemical information, left behind by the liquid electrolyte during evaporation, through a thin-film gel. The analysis of the gel electrolyte film confirmed the acidification on AA7075 and the alkalinization on CS1018 but also offered novel insights on their dependence on the galvanic current. The galvanic current was proportional to the initial NaCl concentration in the range of 0.01 to 0.06 M. However, due to continuous evaporation, the NaCl concentration increased, limiting oxygen diffusion and decreasing the galvanic current, especially for electrolytes exceeding 0.06 M. The galvanic current was determined by considering the dynamic evolution (caused by the evaporation of the electrolyte film) of both the thickness of the electrolyte and its concentration.
Full article
(This article belongs to the Special Issue Corrosion Mechanisms and Electrochemical Interfaces: In Honor of Prof. Digby Macdonald)
►▼
Show Figures
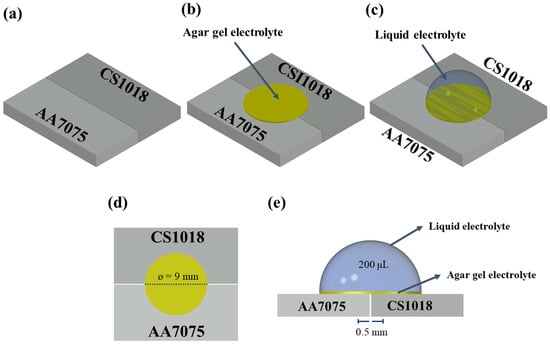
Figure 1
Open AccessArticle
Performance of Phenolic-Epoxy Coatings after Exposure to High Temperatures
by
Saleh Ahmed, Katerina Lepkova, Xiao Sun, William D. A. Rickard and Thunyaluk Pojtanabuntoeng
Corros. Mater. Degrad. 2024, 5(1), 73-91; https://doi.org/10.3390/cmd5010004 - 29 Feb 2024
Abstract
Phenolic-epoxy coatings, which are designed to protect substrates from thermal damage, are widely applied in many fields. There remains an inadequate understanding of how such coatings change during their service life after exposure to various temperature conditions. To further elucidate this issue, this
[...] Read more.
Phenolic-epoxy coatings, which are designed to protect substrates from thermal damage, are widely applied in many fields. There remains an inadequate understanding of how such coatings change during their service life after exposure to various temperature conditions. To further elucidate this issue, this case study investigated the effects of high temperatures on carbon steel panels coated with phenolic epoxy and exposed to different heating conditions. A general trend of decreasing barrier performance was observed after exposure to 150 °C for 3 d, as evidenced by the appearance of cracks on the panel surfaces. In contrast, the coating performance improved after exposure to isothermal conditions (120 °C) or thermal cycling from room temperature to 120 °C, as indicated by the increased low-frequency impedance modulus values of the coating. This unexpected improvement was further examined by characterising the coatings using transform infrared spectroscopy (FTIR), thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), pull-off adhesion tests, and time-of-flight secondary ion mass spectrometry (ToF-SIMS). The maximum pull-off adhesion force (24.9 ± 3.6 MPa) was measured after thermal cycling for 40 d.
Full article
(This article belongs to the Special Issue Advances in Corrosion Protection by Coatings)
►▼
Show Figures
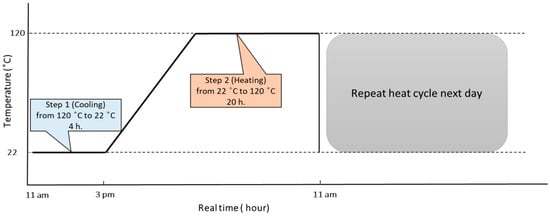
Figure 1
Open AccessFeature PaperReview
Corrosion at the Steel–Medium Interface
by
Robert E. Melchers
Corros. Mater. Degrad. 2024, 5(1), 52-72; https://doi.org/10.3390/cmd5010003 - 29 Jan 2024
Abstract
Corrosion on the interface between a metal alloy, such as steel, and a wet, permeable non-metallic medium is of considerable practical interest. Examples include the interface between steel and water, the atmosphere or concrete, as for steel reinforcement bars; between metal and soil,
[...] Read more.
Corrosion on the interface between a metal alloy, such as steel, and a wet, permeable non-metallic medium is of considerable practical interest. Examples include the interface between steel and water, the atmosphere or concrete, as for steel reinforcement bars; between metal and soil, as for buried cast iron or steel pipes; deposits of some type, as in under-deposit corrosion; and the interface with insulation, protective coatings, or macro- or micro-biological agents. In all cases, corrosion initiation depends on the characteristics of the interfacial zone, both of the metal and the medium, and the spatial variability. For (near-)homogeneous semi-infinite media with good interfacial contact, the pitting, crevices and general corrosion of the metal will be largely controlled by the metal (micro-)characteristics, including its inclusions, imperfections and surface roughness. In other cases, these may be overshadowed by the macro-characteristics of the medium and the degree of interfacial contact, possibly with severe resulting corrosion. Where the build-up of corrosion products can occur at the interface, they will dominate longer-term corrosion and govern the long-term corrosion rate. For media of finite thickness, diffusion issues and material deterioration may also be involved. The practical implications are outlined. It is argued that with the presence of a suitable medium, it is possible to achieve negligible long-term corrosion but only if certain practical actions are taken.
Full article
(This article belongs to the Special Issue Exclusive Papers Collection of Editorial Board Members of Corrosion and Materials Degradation 2023)
►▼
Show Figures
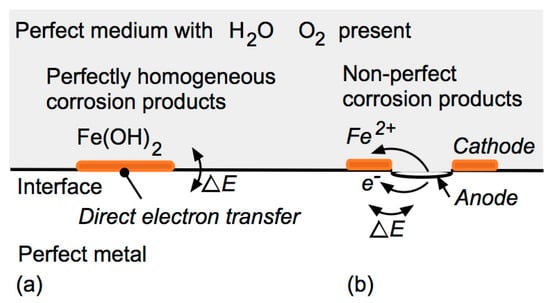
Figure 1
Open AccessArticle
Effect of Microstructure on Corrosion Behavior of Cold Sprayed Aluminum Alloy 5083
by
Munsu Kim, Lorena Perez-Andrade, Luke N. Brewer and Gregory W. Kubacki
Corros. Mater. Degrad. 2024, 5(1), 27-51; https://doi.org/10.3390/cmd5010002 - 16 Jan 2024
Abstract
This paper investigates the effect of the microstructure on the corrosion behavior of cold sprayed (CS) AA5083 compared to its wrought counterpart. It has been shown that the microstructure of CS aluminum alloys, such as AA2024, AA6061, and AA7075, affects their corrosion behavior;
[...] Read more.
This paper investigates the effect of the microstructure on the corrosion behavior of cold sprayed (CS) AA5083 compared to its wrought counterpart. It has been shown that the microstructure of CS aluminum alloys, such as AA2024, AA6061, and AA7075, affects their corrosion behavior; however, investigations of the corrosion behavior of CS AA5083 with a direct comparison to wrought AA5083 have been limited. The microstructure and corrosion behavior of CS AA5083 were examined by scanning electron microscopy (SEM), transmission electron microscopy (TEM), energy dispersive X-ray spectroscopy (EDS), electron backscattered diffraction (EBSD), electrochemical and immersion tests, and ASTM G67. The CS process resulted in microstructural changes, such as the size and spatial distribution of intermetallic particles, grain size, and misorientation. The refined grain size and intermetallic particles along prior particle boundaries stimulated the initiation and propagation of localized corrosion. Electrochemical tests presented enhanced anodic kinetics with high pitting susceptibility, giving rise to extensive localized corrosion in CS AA5083. The ASTM G67 test demonstrated significantly higher mass loss for CS AA5083 compared to its wrought counterpart due to preferential attack within prior particle boundary regions in the CS microstructure. Possible mechanisms of intergranular corrosion (IGC) propagation at prior particle boundary regions have been discussed.
Full article
(This article belongs to the Special Issue Advances in Corrosion Protection by Coatings)
►▼
Show Figures
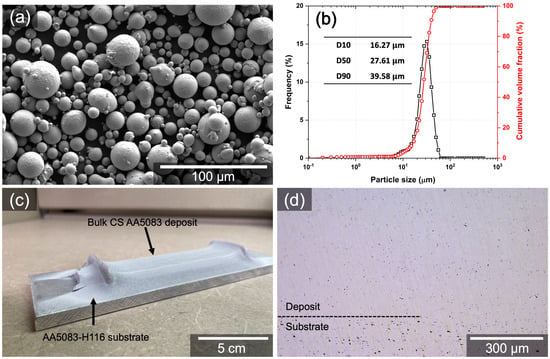
Figure 1
Open AccessArticle
Microbial Communities in Model Seawater-Compensated Fuel Ballast Tanks: Biodegradation and Biocorrosion Stimulated by Marine Sediments
by
Kathleen E. Duncan, Lina E. Dominici, Mark A. Nanny, Irene A. Davidova, Brian H. Harriman and Joseph M. Suflita
Corros. Mater. Degrad. 2024, 5(1), 1-26; https://doi.org/10.3390/cmd5010001 - 03 Jan 2024
Abstract
►▼
Show Figures
Some naval vessels add seawater to carbon steel fuel ballast tanks to maintain stability during fuel consumption. Marine sediments often contaminate ballast tank fluids and have been implicated in stimulating fuel biodegradation and enhancing biocorrosion. The impact of the marine sediment was evaluated
[...] Read more.
Some naval vessels add seawater to carbon steel fuel ballast tanks to maintain stability during fuel consumption. Marine sediments often contaminate ballast tank fluids and have been implicated in stimulating fuel biodegradation and enhancing biocorrosion. The impact of the marine sediment was evaluated in model ballast tank reactors containing seawater, fuel (petroleum-F76, Fischer–Tropsch F76, or a 1:1 mixture), and carbon steel coupons. Control reactors did not receive fuel. The marine sediment was added to the reactors after 400 days and incubated for another year. Sediment addition produced higher estimated bacterial numbers and enhanced sulfate reduction. Ferrous sulfides were detected on all coupons, but pitting corrosion was only identified on coupons exposed to FT-F76. Aerobic hydrocarbon-degrading bacteria increased, and the level of dissolved iron decreased, consistent with the stimulation of aerobic hydrocarbon degradation by iron. We propose that sediments provide an inoculum of hydrocarbon-degrading microbes that are stimulated by dissolved iron released during steel corrosion. Hydrocarbon degradation provides intermediates for use by sulfate-reducing bacteria and reduces the level of fuel components inhibitory to anaerobic bacteria. The synergistic effect of dissolved iron produced by corrosion, biodegradable fuels, and iron-stimulated hydrocarbon-degrading microbes is a poorly recognized but potentially significant biocorrosion mechanism.
Full article
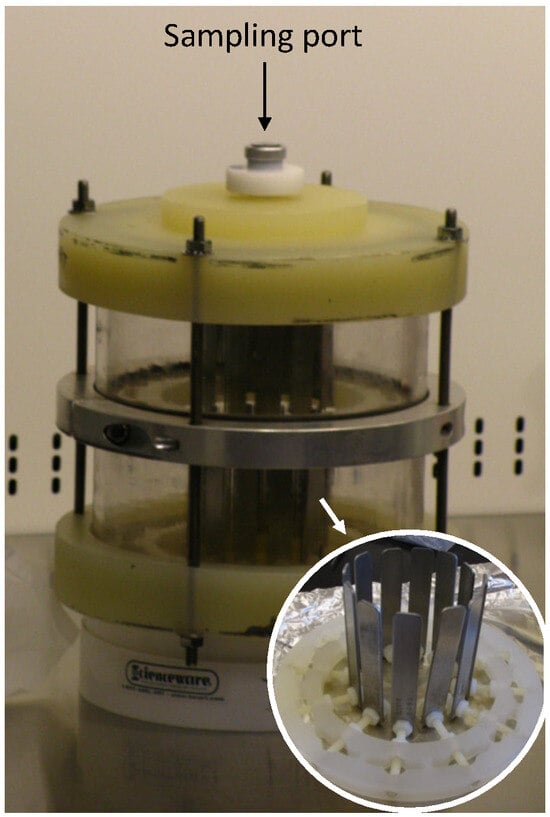
Figure 1
Open AccessArticle
Data Mining Applied to the Electrochemical Noise Technique in the Time/Frequency Domain for Stress Corrosion Cracking Recognition
by
Luigi Calabrese, Massimiliano Galeano and Edoardo Proverbio
Corros. Mater. Degrad. 2023, 4(4), 659-679; https://doi.org/10.3390/cmd4040034 - 06 Dec 2023
Abstract
In this paper, time/frequency domain data processing was proposed to analyse the EN signal recorded during stress corrosion cracking on precipitation-hardening martensitic stainless steel in a chloride environment. Continuous Wavelet Transform, albeit with some limitations, showed a suitable support in the discriminatory capacity
[...] Read more.
In this paper, time/frequency domain data processing was proposed to analyse the EN signal recorded during stress corrosion cracking on precipitation-hardening martensitic stainless steel in a chloride environment. Continuous Wavelet Transform, albeit with some limitations, showed a suitable support in the discriminatory capacity among transient signals related to the different stress corrosion cracking mechanisms. In particular, the aim is to propose the analysis of electrochemical noise signals under stress corrosion cracking conditions in the time–frequency domain by using the Hilbert–Huang approach. The Hilbert–Huang Transform (performed by the Empirical Mode Decomposition approach) was finally proposed to carry out an identification of the corrosion mechanisms in comparison to conventional data processing methods. By using this approach, a detailed simultaneous decomposition of the original electrochemical noise data in the time and frequency domain was carried out. The method gave useful information about transitions among different corrosion mechanisms, allowing us to (i) identify a specific characteristic response for each corrosion damaging phenomenon induced by stress corrosion cracking, (ii) time each corrosion of the damaging phenomenon, and (iii) provide a topological description of the advancing SCC damaging stages. This characteristic evidences that the Hilbert–Huang Transform is a very powerful technique to potentially recognize and distinguish the different corrosion mechanisms occurring during stress corrosion cracking.
Full article
(This article belongs to the Special Issue Exclusive Papers Collection of Editorial Board Members of Corrosion and Materials Degradation 2023)
►▼
Show Figures
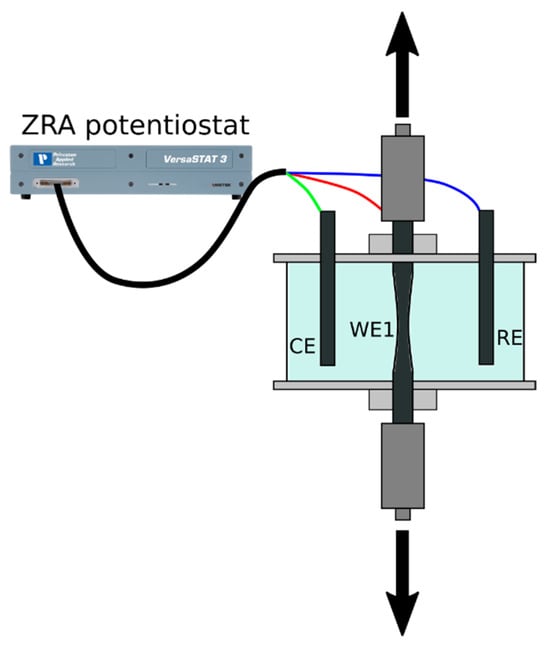
Figure 1
Open AccessReview
Review on Corrosion, Tribocorrosion and Osseointegration of Titanium Alloys as Biomaterials
by
Jamal Takadoum
Corros. Mater. Degrad. 2023, 4(4), 644-658; https://doi.org/10.3390/cmd4040033 - 28 Nov 2023
Abstract
►▼
Show Figures
When introduced into the body, the implant interacts with biological environment and may suffer corrosion. In addition, when this implant is submitted to friction, it may degrade by tribocorrosion due to the simultaneous action of corrosion by the body liquid and mechanical wear.
[...] Read more.
When introduced into the body, the implant interacts with biological environment and may suffer corrosion. In addition, when this implant is submitted to friction, it may degrade by tribocorrosion due to the simultaneous action of corrosion by the body liquid and mechanical wear. Both corrosion and tribocorrosion are connected to the presence of proteins that cover the surface implant. The latter plays an ambiguous role on corrosion since dozens of contradictory papers pointed out their beneficial or detrimental effect. After its introduction into the body, the implant should form a direct interface with bone through structural and functional connection. The osseointegration and the strength of interfacial bond depend on surface properties of the implant, namely, its topographical and physico-chemical properties. In addition, since bone cells are sensitive to the species produced during the implant corrosion, when corrosion occurs, this may lead to impact osseointegration and to cause implant loosening. There is a strong connection between corrosion and osseointegration, both of which are worth discussion. That is the object of the present narrative review where we will discuss: (1) corrosion and tribocorrosion of titanium alloys used as biomaterials paying particular attention to the influence of proteins, (2) the effect of implant roughness and surface energy on osseointegration.
Full article
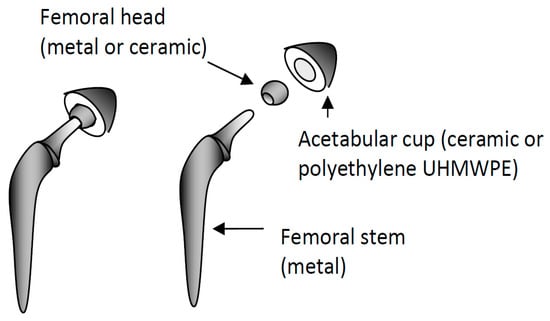
Figure 1
Open AccessArticle
Improving the Mechanical and Electrochemical Performance of Additively Manufactured 8620 Low Alloy Steel via Boriding
by
Ezazul Haque Sabuz, Mohammed Noor-A-Alam, Waseem Haider and Ishraq Shabib
Corros. Mater. Degrad. 2023, 4(4), 623-643; https://doi.org/10.3390/cmd4040032 - 06 Nov 2023
Cited by 1
Abstract
►▼
Show Figures
In this study, mechanical and electrochemical performance of borided additively manufactured (AM) and wrought 8620 low alloy steel were investigated and compared to their bare counterparts. The microstructure of borided 8620 exhibited the presence of FeB and Fe2B phases with a
[...] Read more.
In this study, mechanical and electrochemical performance of borided additively manufactured (AM) and wrought 8620 low alloy steel were investigated and compared to their bare counterparts. The microstructure of borided 8620 exhibited the presence of FeB and Fe2B phases with a saw tooth morphology. Both AM and wrought samples with boride layers showed a similar performance in hardness, wear, potentiodynamic polarization (PD), electrochemical impedance spectroscopy (EIS), and linear polarization resistance (LPR) experiments. However, borided steels exhibited about an 8-fold increase in Vickers hardness and about a 6-fold enhancement in wear resistance compared to bare ones. Electrochemical experiments of borided specimens (both AM and wrought) in 0.1 M Na2S2O3 + 1 M NH4Cl solution revealed a 3–6-fold lower corrosion current density, about a 6-fold higher charge transfer resistance, and about a 6-fold lower double-layer capacitance, demonstrating an improved corrosion resistance compared to their bare counterparts. Post-corrosion surface analysis revealed the presence of thick sulfide and oxide layers on the bare steels, whereas dispersed corrosion particles were observed on the borided samples. The enhanced wear and electrochemical performance of the borided steels were attributed to the hard FeB/Fe2B layers and the reduced amount of adsorbed sulfur on their surface.
Full article
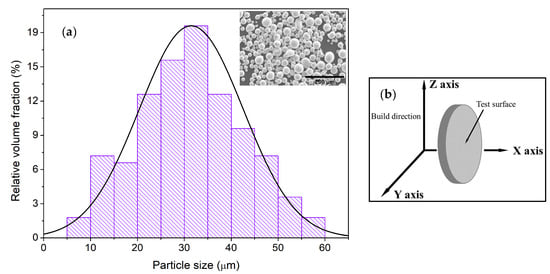
Figure 1
Open AccessReview
A Review on Tribocorrosion Behavior of Aluminum Alloys: From Fundamental Mechanisms to Alloy Design Strategies
by
Zhengyu Zhang, Raja Shekar Bhupal Dandu, Edwin Eyram Klu and Wenjun Cai
Corros. Mater. Degrad. 2023, 4(4), 594-622; https://doi.org/10.3390/cmd4040031 - 18 Oct 2023
Cited by 1
Abstract
Tribocorrosion, a research field that has been evolving for decades, has gained renewed attention in recent years, driven by increased demand for wear- and corrosion-resistant materials from biomedical implants, nuclear power generation, advanced manufacturing, batteries, marine and offshore industries, etc. In the United
[...] Read more.
Tribocorrosion, a research field that has been evolving for decades, has gained renewed attention in recent years, driven by increased demand for wear- and corrosion-resistant materials from biomedical implants, nuclear power generation, advanced manufacturing, batteries, marine and offshore industries, etc. In the United States, wear and corrosion are estimated to cost nearly USD 300 billion per year. Among various important structural materials, passive metals such as aluminum alloys are most vulnerable to tribocorrosion due to the wear-accelerated corrosion as a result of passive film removal. Thus, designing aluminum alloys with better tribocorrosion performance is of both scientific and practical importance. This article reviews five decades of research on the tribocorrosion of aluminum alloys, from experimental to computational studies. Special focus is placed on two aspects: (1) The effects of alloying and grain size on the fundamental wear, corrosion, and tribocorrosion mechanisms; and (2) Alloy design strategies to improve the tribocorrosion resistance of aluminum alloys. Finally, the paper sheds light on the current challenges faced and outlines a few future research directions in the field of tribocorrosion of aluminum alloys.
Full article
(This article belongs to the Special Issue Mechanism and Predictive/Deterministic Aspects of Corrosion)
►▼
Show Figures
Figure 1
Open AccessArticle
Evaluating the Impact of Redox Potential on the Corrosion of Q125, 316L, and C276 Steel in Low-Temperature Geothermal Systems
by
Samuel Bowman, Vikas Agrawal and Shikha Sharma
Corros. Mater. Degrad. 2023, 4(4), 573-593; https://doi.org/10.3390/cmd4040030 - 08 Oct 2023
Cited by 2
Abstract
►▼
Show Figures
Time series experiments were used to explore the fluid redox impact on the corrosion of Q125, 316L, and C276 steels in low-ionic-strength and neutral water at temperature and pressure conditions associated with low-temperature geothermal systems. After exposing polished samples of each steel grade
[...] Read more.
Time series experiments were used to explore the fluid redox impact on the corrosion of Q125, 316L, and C276 steels in low-ionic-strength and neutral water at temperature and pressure conditions associated with low-temperature geothermal systems. After exposing polished samples of each steel grade to an oxidizing (H2O2) and a reducing (Zn-doped) fluid for intervals of 24 h, 1 week, and 6 weeks, the atomic force microscopy results revealed general corrosion for Q125, while 316L and C276 exhibited pitting, crevice expansion, and edge attack corrosion. Secondary depositional features are frequently found as topographic highs, adjacent to pitting corrosion. These features may be identified as there is a very strong spatial correlation between the height retrace and phase retrace surface maps. All steels became progressively rougher over time after exposure to both fluids, while the corrosion rates were more complex. Samples exposed to the reducing fluid experienced an increase in the corrosion rate over time, while C276 and 316L experienced a decrease in the corrosion rate. Finally, a novel data validation technique was developed to address the intrinsic scalability of corrosion. The results indicate that the AFM scan area does not affect the measured surface roughness over nearly three orders of magnitude.
Full article
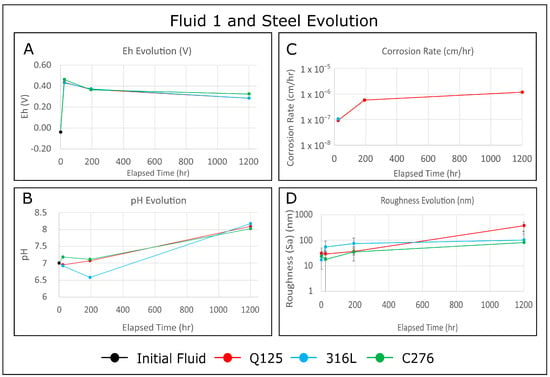
Figure 1
Open AccessFeature PaperReview
State-of-the-Art Review of Aliphatic Polyesters and Polyolefins Biodeterioration by Microorganisms: From Mechanism to Characterization
by
Shiva Khoshtinat
Corros. Mater. Degrad. 2023, 4(4), 542-572; https://doi.org/10.3390/cmd4040029 - 04 Oct 2023
Cited by 2
Abstract
►▼
Show Figures
As a result of the exponential growth in the production of plastics and their extended degradation period, strong environmental concerns in association with the disposal of plastic waste have emerged. Pursuing sustainable solutions for managing plastic waste has led to significant interest in
[...] Read more.
As a result of the exponential growth in the production of plastics and their extended degradation period, strong environmental concerns in association with the disposal of plastic waste have emerged. Pursuing sustainable solutions for managing plastic waste has led to significant interest in plastic biodegradation research, with a specific focus on biodeterioration facilitated by microorganisms. The biodeterioration of plastic by microorganisms is a complex phenomenon that can be influenced by a variety of environmental factors such as humidity, temperature, and pH, as well as polymer properties such as molecular structure, molecular weight, and crystallinity. Toward a better understanding of this phenomenon for resolving the issue of plastic waste, this review article focuses on the biodeterioration of synthetic polymers, in particular aliphatic polyesters and polyolefins, through the enzymatic activities of microorganisms. First, the mechanism of polymer biodegradation via enzymatic activity is discussed, followed by the physical properties of polymers and environmental conditions that influence their biodegradability rates. Then, an overview of experimental approaches and standardized protocols used to assess the biodegradability of polymers by these degrading agents is provided. Finally, current developments in employing biodeterioration for the degradation of aliphatic polyesters and polyolefins are reviewed. The review concludes with a discussion on the complexity of biodegradation by microorganisms, the necessity of proper engineering of polymer properties during production to enhance their biodegradability, and the need for further research to discover sustainable and environmentally acceptable alternatives.
Full article
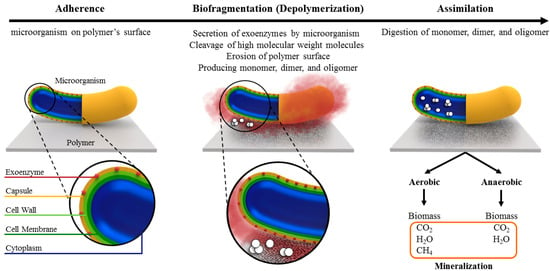
Figure 1
Open AccessArticle
Oxidation Behavior at 1000 °C of Low-Mn High-Cr Cantor’s HEA-Based Alloys Strengthened or Not by MC Carbides
by
Pauline Spaeter, Corentin Gay, Nassima Chenikha, Ghouti Medjahdi, Anne Vernière, Christophe Rapin, Lionel Aranda and Patrice Berthod
Corros. Mater. Degrad. 2023, 4(4), 528-541; https://doi.org/10.3390/cmd4040028 - 25 Sep 2023
Abstract
►▼
Show Figures
A conventionally cast CoNiFeMn0.5Cr1.5 alloy and two versions with 0.25 C & 3.7 Ta or 0.25 C & 3.7 Hf were tested in oxidation at 1000 °C for 50 h with thermogravimetric recording of the oxidation kinetic. In all cases,
[...] Read more.
A conventionally cast CoNiFeMn0.5Cr1.5 alloy and two versions with 0.25 C & 3.7 Ta or 0.25 C & 3.7 Hf were tested in oxidation at 1000 °C for 50 h with thermogravimetric recording of the oxidation kinetic. In all cases, the obtained mass gain curve is parabolic. The parabolic constants are much lower than the Kp previously determined for the original alloys with an equimolar base (CoNiFeMnCr). However, the post-mortem exploitation of the oxidized samples revealed analogous oxidation features on the surface and the subsurface, also with external oxide strata on the surface with different Mn and Cr contents, and rather great Mn depletion, in addition to a moderate Cr depletion, in the subsurface. Globally, the oxidation behavior is significantly better than was earlier observed for the equimolar version of these alloys.
Full article
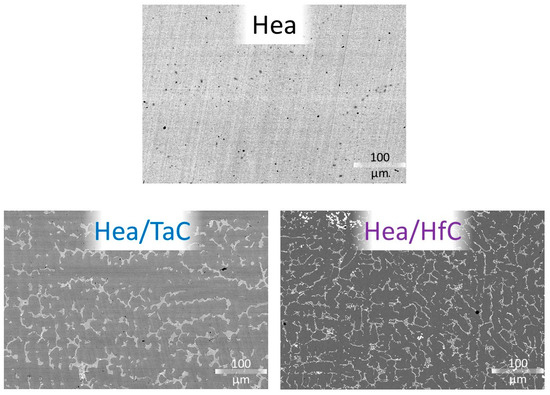
Figure 1
Open AccessArticle
Self-Healing Coatings Consisting of an Outer Electrodeposited Epoxy Resin Layer and an Inner Porous Anodic Oxide Layer with Healing Agents for the Corrosion Protection of Al Alloys
by
Rin Takada, Kota Hirasawa, Hideaki Takahashi and Makoto Chiba
Corros. Mater. Degrad. 2023, 4(3), 516-527; https://doi.org/10.3390/cmd4030027 - 18 Sep 2023
Abstract
►▼
Show Figures
Recently, new surface treatments for the corrosion protection of Al alloys by forming self-healing layers have attracted the attention of many researchers. The authors of this paper have previously developed self-healing polyurethane coatings with micro-capsules containing healing agents and porous anodic oxide films
[...] Read more.
Recently, new surface treatments for the corrosion protection of Al alloys by forming self-healing layers have attracted the attention of many researchers. The authors of this paper have previously developed self-healing polyurethane coatings with micro-capsules containing healing agents and porous anodic oxide films filled with healing agents. In this study, self-healing coatings consisting of an outer electrodeposited epoxy resin layer and an inner porous anodic oxide layer with healing agents were developed for the corrosion protection of Al alloys. The corrosion protection abilities of the self-healing coating were shown in Cu2+/Cl− solutions after damaging with indenters and were affected by freezing treatments and the tip angles of the indenter.
Full article
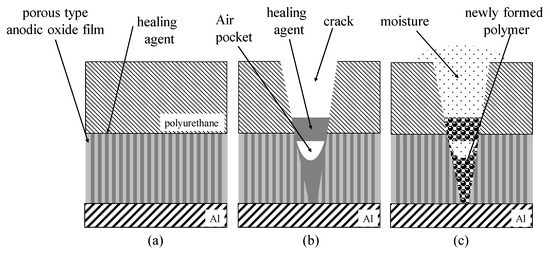
Figure 1
Open AccessArticle
The Effect of Microstructure on Local Corrosion Product Formation during Initial SO2-Induced Atmospheric Corrosion of ZnAlMg Coating Studied by FTIR-ATR FPA Chemical Imaging
by
Dan Persson, Dominique Thierry and Nathalie LeBozec
Corros. Mater. Degrad. 2023, 4(3), 503-515; https://doi.org/10.3390/cmd4030026 - 08 Sep 2023
Abstract
The initial atmospheric corrosion of ZM (ZnAlMg)-coated steel in humid air (85% RH) and humid argon (85% RH) containing 320 ppb SO2 was studied using in situ infrared reflection absorption spectroscopy (IRRAS), FTIR-ATR focal plane array (FPA) imaging and SEM-EDS. The corrosion
[...] Read more.
The initial atmospheric corrosion of ZM (ZnAlMg)-coated steel in humid air (85% RH) and humid argon (85% RH) containing 320 ppb SO2 was studied using in situ infrared reflection absorption spectroscopy (IRRAS), FTIR-ATR focal plane array (FPA) imaging and SEM-EDS. The corrosion products formed in humid air containing SO2 are mainly composed of magnesium sulphites and sulphates, with sulphite-containing corrosion products formed initially while the contribution from sulphates increased with exposure time. The results from FTIR-FPA imaging and SEM-EDS showed that the magnesium sulphite and sulphate are formed mainly on eutectic phases with a higher quantity of corrosion products formed on the binary eutectic (Zn-MgZn2) phases. This is due to presence of microgalvanic elements with the zinc-rich phases as the main sites for the cathodic oxygen reduction while the anodic reactions take place on the eutectic areas. Sulphate content is the highest on the binary eutectic phases, due to the microgalvanic effects and the production of oxidants by the cathodic reaction, which increases the oxidation of sulphite to sulphate.
Full article
(This article belongs to the Special Issue Atmospheric Corrosion of Materials)
►▼
Show Figures
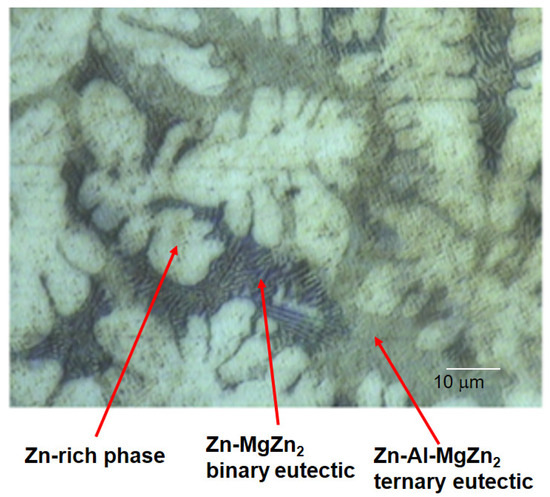
Figure 1
Open AccessFeature PaperArticle
Plasma Electrolytic Oxidation on Magnesium AZ31 with Sepiolite as Inhibitor Carrier for Improved Corrosion Protection
by
Robert Sottor, Ricarda Gruen, Kerstin Kremmer, Stephan Lederer, Michael Schneider and Wolfram Fuerbeth
Corros. Mater. Degrad. 2023, 4(3), 488-502; https://doi.org/10.3390/cmd4030025 - 30 Aug 2023
Abstract
►▼
Show Figures
Plasma electrolytic oxidation (PEO) in an alkaline silicate electrolyte containing nanosized sepiolite fibers was carried out on magnesium alloy AZ31. The mineral fibers were loaded with different corrosion inhibitors and incorporated in situ during the PEO treatment. The composition and microstructure of the
[...] Read more.
Plasma electrolytic oxidation (PEO) in an alkaline silicate electrolyte containing nanosized sepiolite fibers was carried out on magnesium alloy AZ31. The mineral fibers were loaded with different corrosion inhibitors and incorporated in situ during the PEO treatment. The composition and microstructure of the PEO coatings were investigated by SEM. It was shown that the fibers are located on the surface as well as inside the “weak spots” of the coating, i.e., pores and discharge channels. The fixation of the particles is caused by sintering due to the heat developed during the PEO treatment. Investigations using electrochemical impedance spectroscopy and linear sweep voltammetry in 0.01 M NaCl solution confirmed an improvement of the corrosion protection. The use of the inhibitors shifts the critical pitting potential in the anodic direction. Regarding efficiency, cerium-loaded sepiolite showed the best behavior by shifting the pitting potential by +0.9 V.
Full article
Figure 1
Open AccessFeature PaperReview
Improved and Innovative Accident-Tolerant Nuclear Fuel Materials Considered for Retrofitting Light Water Reactors—A Review
by
Raul B. Rebak
Corros. Mater. Degrad. 2023, 4(3), 466-487; https://doi.org/10.3390/cmd4030024 - 24 Aug 2023
Cited by 1
Abstract
Since 2011, there has been an international effort to evaluate the behavior of newer fuel rod materials for the retrofitting of existing light water reactors (LWR). These materials include concepts for the cladding of the fuel and for the fuel itself. The materials
[...] Read more.
Since 2011, there has been an international effort to evaluate the behavior of newer fuel rod materials for the retrofitting of existing light water reactors (LWR). These materials include concepts for the cladding of the fuel and for the fuel itself. The materials can be broadly categorized into evolutionary or improved existing materials and revolutionary or innovative materials. The purpose of the newer materials or accident-tolerant fuels (ATF) is to make the LWRs more resistant to loss-of-coolant accidents and thus increase their operation safety. The benefits and detriments of the three main concepts for the cladding are discussed. These include (i) coatings for existing zirconium alloys; (ii) monolithic iron–chromium–aluminum alloys; and (iii) composites based on silicon carbide. The use of ATF materials may help extend the life of currently operating LWRs, while also being a link to material development for future commercial reactors.
Full article
(This article belongs to the Special Issue Mechanism and Predictive/Deterministic Aspects of Corrosion)
►▼
Show Figures
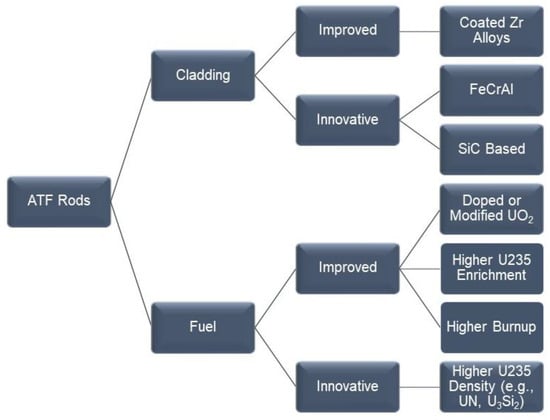
Figure 1
Highly Accessed Articles
Latest Books
E-Mail Alert
News
Topics
Topic in
Coatings, CMD, Materials, Metals, Molecules
Corrosion and Protection of Metallic Materials, 2nd Edition
Topic Editors: Sebastian Feliú, Jr., Federico R. García-Galván, Lucien VelevaDeadline: 31 July 2024

Conferences
Special Issues
Special Issue in
CMD
Corrosion Mechanisms and Electrochemical Interfaces: In Honor of Prof. Digby Macdonald
Guest Editors: David M. Bastidas, Raman SinghDeadline: 31 July 2024
Special Issue in
CMD
Advances in Corrosion Protection by Coatings
Guest Editors: Wolfram Fürbeth, Marjorie OlivierDeadline: 30 September 2024
Special Issue in
CMD
Corrosion and Corrosion Protection Strategies in the Marine Environment
Guest Editor: Ladislav VrsalovićDeadline: 30 November 2024