Nanocomposites of Ferrites with TiO2, SiO2 and Carbon Quantum Dots as Photocatalysts for Degradation of Organic Pollutants and Microbes
Abstract
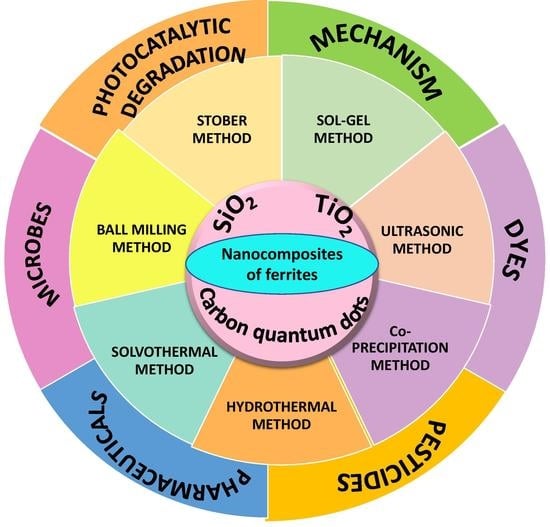
:1. Introduction
2. Synthesis and Structural Features of Ferrite-Based Nanocomposites
2.1. Magnetic Features of Ferrite Nanoparticles
2.2. Nanocomposites of Ferrites with TiO2
2.2.1. Sol–Gel Method
2.2.2. Ultrasonic Method
2.2.3. Coprecipitation Method
2.2.4. Hydrothermal Method
2.2.5. Solvothermal Method
2.3. Nanocomposites of Ferrites with Silica
2.3.1. Sol–Gel Auto-Combustion Method
2.3.2. Stöber Method
2.3.3. Coprecipitation Method
2.3.4. Ball-Milling Method
2.4. Ferrites with Carbon Quantum Dots
3. Applications
- (1)
- Cell membranes and cell walls are destroyed.
- (2)
- The release of reactive oxygen species (ROS) in order to kill the cells.
- (3)
- Inhibiting cell proliferation by interacting with nucleic material (DNA/RNA).
3.1. Photocatalytic Applications for Degradation of Organic Compounds
3.2. Photocatalytic Applications for Degradation of Microbes
4. Challenges and Opportunities
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Drinking Water. 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/drinking-water (accessed on 27 March 2019).
- Asfaram, A.; Ghaedi, M.; Goudarzi, A.; Hajati, S. Ultrasound-Assisted Binary Adsorption of Dyes onto Mn@ CuS/ZnS-NC-AC as a Novel Adsorbent: Application of Chemometrics for Optimization and Modeling. J. Ind. Eng. Chem. 2017, 54, 377–388. [Google Scholar] [CrossRef]
- Chung, K.-T. Azo Dyes and Human Health: A Review. J. Environ. Sci. Health C 2016, 34, 233–261. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lu, J.; Zhou, Y.; Liu, Y. Recent Advances for Dyes Removal Using Novel Adsorbents: A Review. Environ. Pollut. 2019, 252, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Ojemaye, M.O.; Okoh, A.I. Multiple Nitrogen Functionalized Magnetic Nanoparticles as an Efficient Adsorbent: Synthesis, Kinetics, Isotherm and Thermodynamic Studies for the Removal of Rhodamine B from Aqueous Solution. Sci. Rep. 2019, 9, 9672. [Google Scholar] [CrossRef] [PubMed]
- Mazaheri, H.; Ghaedi, M.; Ahmadi Azqhandi, M.H.; Asfaram, A. Application of Machine/Statistical Learning, Artificial Intelligence and Statistical Experimental Design for the Modeling and Optimization of Methylene Blue and Cd(ii) Removal from a Binary Aqueous Solution by Natural Walnut Carbon. Phys. Chem. Chem. Phys. 2017, 19, 11299–11317. [Google Scholar] [CrossRef] [PubMed]
- Gerbaldo, M.V.; Marchetti, S.G.; Elías, V.R.; Mendieta, S.N.; Crivello, M.E. Degradation of Anti-Inflammatory Drug Diclofenac Using Cobalt Ferrite as Photocatalyst. Chem. Eng. Res. Des. 2021, 166, 237–247. [Google Scholar] [CrossRef]
- Tabasum, A.; Alghuthaymi, M.; Qazi, U.Y.; Shahid, I.; Abbas, Q.; Javaid, R.; Nadeem, N.; Zahid, M. UV-Accelerated Photocatalytic Degradation of Pesticide over Magnetite and Cobalt Ferrite Decorated Graphene Oxide Composite. Plants 2020, 10, 6. [Google Scholar] [CrossRef]
- Chatterjee, S.; Guha, N.; Krishnan, S.; Singh, A.K.; Mathur, P.; Rai, D.K. Selective and Recyclable Congo Red Dye Adsorption by Spherical Fe3O4 Nanoparticles Functionalized with 1,2,4,5-Benzenetetracarboxylic Acid. Sci. Rep. 2020, 10, 111. [Google Scholar] [CrossRef]
- Anantha, M.S.; Olivera, S.; Hu, C.; Jayanna, B.K.; Reddy, N.; Venkatesh, K.; Muralidhara, H.B.; Naidu, R. Comparison of the Photocatalytic, Adsorption and Electrochemical Methods for the Removal of Cationic Dyes from Aqueous Solutions. Environ. Technol. Innov. 2020, 17, 100612. [Google Scholar] [CrossRef]
- Bilińska, L.; Gmurek, M.; Ledakowicz, S. Textile Wastewater Treatment by AOPs for Brine Reuse. Process. Saf. Environ. Prot. 2017, 109, 420–428. [Google Scholar] [CrossRef]
- Boutra, B.; Güy, N.; Özacar, M.; Trari, M. Magnetically Separable MnFe2O4/TA/ZnO Nanocomposites for Photocatalytic Degradation of Congo Red under Visible Light. J. Magn. Magn. Mater. 2020, 497, 165994. [Google Scholar] [CrossRef]
- Ali, T.; Tripathi, P.; Azam, A.; Raza, W.; Ahmed, A.S.; Ahmed, A.; Muneer, M. Photocatalytic Performance of Fe-Doped TiO2 Nanoparticles under Visible-Light Irradiation. Mater. Res. Express 2017, 4, 015022. [Google Scholar] [CrossRef]
- Bora, L.V.; Mewada, R.K. Visible/Solar Light Active Photocatalysts for Organic Effluent Treatment: Fundamentals, Mechanisms and Parametric Review. Renew. Sustain. Energy Rev. 2017, 76, 1393–1421. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Jeng, M.-J.; Wung, Y.-L.; Chang, L.-B.; Chow, L. Particle Size Effects of TiO2 Layers on the Solar Efficiency of Dye-Sensitized Solar Cells. Int. J. Photoenergy 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Thao, L.; Dang, T.; Khanitchaidecha, W.; Channei, D.; Nakaruk, A. Photocatalytic Degradation of Organic Dye under UV-A Irradiation Using TiO2-Vetiver Multifunctional Nano Particles. Materials 2017, 10, 122. [Google Scholar] [CrossRef]
- Saliu, O.D.; Olatunji, G.A.; Yakubu, A.; Arowona, M.T.; Mohammed, A.A. Catalytic Crosslinking of a Regenerated Hydrophobic Benzylated Cellulose and Nano TiO2 Composite for Enhanced Oil Absorbency. e-Polymers 2017, 17, 295–302. [Google Scholar] [CrossRef]
- Li, H.; Zhang, T.; Pan, C.; Pu, C.; Hu, Y.; Hu, X.; Liu, E.; Fan, J. Self-Assembled Bi2MoO6/TiO2 Nanofiber Heterojunction Film with Enhanced Photocatalytic Activities. Appl. Surf. Sci. 2017, 391, 303–310. [Google Scholar] [CrossRef]
- Wang, Y.; Sunarso, J.; Zhao, B.; Ge, C.; Chen, G. One-Dimensional BiOBr Nanosheets/TiO2 Nanofibers Composite: Controllable Synthesis and Enhanced Visible Photocatalytic Activity. Ceram. Int. 2017, 43, 15769–15776. [Google Scholar] [CrossRef]
- Cheng, R.; Fan, X.; Wang, M.; Li, M.; Tian, J.; Zhang, L. Facile Construction of CuFe2O4/g-C3N4 Photocatalyst for Enhanced Visible-Light Hydrogen Evolution. RSC Adv. 2016, 6, 18990–18995. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Mamba, B.B.; Msagati, T.A.M. Application of Spinel Ferrite Nanoparticles in Water and Wastewater Treatment: A Review. Sep. Purif. Technol. 2017, 188, 399–422. [Google Scholar] [CrossRef]
- Choi, H.; Lee, S.; Kouh, T.; Kim, S.J.; Kim, C.S.; Hahn, E. Synthesis and Characterization of Co-Zn Ferrite Nanoparticles for Application to Magnetic Hyperthermia. J. Korean Phys. Soc. 2017, 70, 89–92. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, Y. Sol-Gel Synthesized Magnetic MnFe2O4 Spinel Ferrite Nanoparticles as Novel Catalyst for Oxidative Degradation of Methyl Orange. J. Nanomater. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Waag, F.; Gökce, B.; Kalapu, C.; Bendt, G.; Salamon, S.; Landers, J.; Hagemann, U.; Heidelmann, M.; Schulz, S.; Wende, H.; et al. Adjusting the Catalytic Properties of Cobalt Ferrite Nanoparticles by Pulsed Laser Fragmentation in Water with Defined Energy Dose. Sci. Rep. 2017, 7, 13161. [Google Scholar] [CrossRef] [PubMed]
- Qu, Q.; Zhu, A.; Shao, X.; Shi, G.; Tian, Y. Development of a Carbon Quantum Dots-Based Fluorescent Cu2+ Probe Suitable for Living Cell Imaging. Chem. Commun. 2012, 48, 5473. [Google Scholar] [CrossRef]
- Mirtchev, P.; Henderson, E.J.; Soheilnia, N.; Yip, C.M.; Ozin, G.A. Solution Phase Synthesis of Carbon Quantum Dots as Sensitizers for Nanocrystalline TiO2 Solar Cells. J. Mater. Chem. 2012, 22, 1265–1269. [Google Scholar] [CrossRef]
- Li, H.; Kang, Z.; Liu, Y.; Lee, S.-T. Carbon Nanodots: Synthesis, Properties and Applications. J. Mater. Chem. 2012, 22, 24230. [Google Scholar] [CrossRef]
- Di, J.; Xia, J.; Ge, Y.; Li, H.; Ji, H.; Xu, H.; Zhang, Q.; Li, H.; Li, M. Novel Visible-Light-Driven CQDs/Bi2WO6 Hybrid Materials with Enhanced Photocatalytic Activity toward Organic Pollutants Degradation and Mechanism Insight. Appl. Catal. B Environ. 2015, 168–169, 51–61. [Google Scholar] [CrossRef]
- Mamba, G.; Mishra, A. Advances in Magnetically Separable Photocatalysts: Smart, Recyclable Materials for Water Pollution Mitigation. Catalysts 2016, 6, 79. [Google Scholar] [CrossRef]
- Govan, J.; Gun’ko, Y. Recent Advances in the Application of Magnetic Nanoparticles as a Support for Homogeneous Catalysts. Nanomaterials 2014, 4, 222–241. [Google Scholar] [CrossRef]
- Rodriguez-Arco, L.; Rodriguez, I.A.; Carriel, V.; Bonhome-Espinosa, A.B.; Campos, F.; Kuzhir, P.; Duran, J.D.G.; Lopez-Lopez, M.T. Biocompatible Magnetic Core–Shell Nanocomposites for Engineered Magnetic Tissues. Nanoscale 2016, 8, 8138–8150. [Google Scholar] [CrossRef] [PubMed]
- Hakami, T.M.; Davarpanah, A.M.; Rahdar, A.; Barrett, S.D. Structural and Magnetic Study and Cytotoxicity Evaluation of Tetra-Metallic Nanoparticles of Co0.5Ni0.5CrxFe2-XO4 Prepared by Co-Precipitation. J. Mol. Struct. 2018, 1165, 344–348. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, X.; Yi, Z.; Wu, X.; Liu, G.; Pu, Z.; Yang, H. Construction of a Z-Scheme Ag 2 MoO4/BiOBr Heterojunction for Photocatalytically Removing Organic Pollutants. Dalton Trans. 2022, 51, 18652–18666. [Google Scholar] [CrossRef] [PubMed]
- Ghamkhari, A.; Mohamadi, L.; Kazemzadeh, S.; Zafar, M.N.; Rahdar, A.; Khaksefidi, R. Synthesis and Characterization of Poly(Styrene-Block-Acrylic Acid) Diblock Copolymer Modified Magnetite Nanocomposite for Efficient Removal of Penicillin G. Compos. B: Eng. 2020, 182, 107643. [Google Scholar] [CrossRef]
- Mohammadi, L.; Rahdar, A.; Khaksefidi, R.; Ghamkhari, A.; Fytianos, G.; Kyzas, G.Z. Polystyrene Magnetic Nanocomposites as Antibiotic Adsorbents. Polymers 2020, 12, 1313. [Google Scholar] [CrossRef]
- Ahmadi, S.; Mohammadi, L.; Rahdar, A.; Rahdar, S.; Dehghani, R.; Adaobi Igwegbe, C.; Kyzas, G.Z. Acid Dye Removal from Aqueous Solution by Using Neodymium(III) Oxide Nanoadsorbents. Nanomaterials 2020, 10, 556. [Google Scholar] [CrossRef]
- Rahdar, S.; Rahdar, A.; Sattari, M.; Hafshejani, L.D.; Tolkou, A.K.; Kyzas, G.Z. Barium/Cobalt@Polyethylene Glycol Nanocomposites for Dye Removal from Aqueous Solutions. Polymers 2021, 13, 1161. [Google Scholar] [CrossRef]
- Mohafez, F.S.; Davarpanah, A.M.; Rahdar, A.; Beyzaei, H.; Zeybek, O.; Barrett, S.D. Structural, Magnetic, and in Vitro Inhibitory Characteristics of Ce-Substituted MnFe2O4 Nanoparticles. Appl. Phys. A 2021, 127, 600. [Google Scholar] [CrossRef]
- Davarpanah, A.M.; Rahdar, A.; Dastnae, M.A.; Zeybek, O.; Beyzaei, H. (1−x)BaFe12O19/xCoFe2O4 Hard/Soft Magnetic Nanocomposites: Synthesis, Physical Characterization, and Antibacterial Activities Study. J. Mol. Struct. 2019, 1175, 445–449. [Google Scholar] [CrossRef]
- Ahmadian-Fard-Fini, S.; Salavati-Niasari, M.; Safardoust-Hojaghan, H. Hydrothermal Green Synthesis and Photocatalytic Activity of Magnetic CoFe2O4–Carbon Quantum Dots Nanocomposite by Turmeric Precursor. J. Mater. Sci. Mater. Electron. 2017, 28, 16205–16214. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Mamba, B.B. Photocatalytic Application of Spinel Ferrite Nanoparticles and Nanocomposites in Wastewater Treatment: Review. Sustain. Mater. Technol. 2020, 23, e00140. [Google Scholar] [CrossRef]
- Ghasemi, A. Magnetic Ferrites and Related Nanocomposites, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2021; p. 300. [Google Scholar]
- Katal, R.; Masudy-Panah, S.; Tanhaei, M.; Farahani, M.H.D.A.; Jiangyong, H. A Review on the Synthesis of the Various Types of Anatase TiO2 Facets and Their Applications for Photocatalysis. Chem. Eng. J. 2020, 384, 123384. [Google Scholar] [CrossRef]
- Kim, C.-S.; Shin, J.-W.; Cho, Y.-H.; Jang, H.-D.; Byun, H.-S.; Kim, T.-O. Synthesis and Characterization of Cu/N-Doped Mesoporous TiO2 Visible Light Photocatalysts. Appl. Catal. A Gen. 2013, 455, 211–218. [Google Scholar] [CrossRef]
- Min, K.S.; Kumar, R.S.; Lee, J.H.; Kim, K.S.; Lee, S.G.; Son, Y.-A. Synthesis of New TiO2/Porphyrin-Based Composites and Photocatalytic Studies on Methylene Blue Degradation. Dyes Pigm. 2019, 160, 37–47. [Google Scholar] [CrossRef]
- Min, K.S.; Manivannan, R.; Son, Y.-A. Porphyrin Dye/TiO2 Imbedded PET to Improve Visible-Light Photocatalytic Activity and Organosilicon Attachment to Enrich Hydrophobicity to Attain an Efficient Self-Cleaning Material. Dyes Pigm. 2019, 162, 8–17. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, Z. The Preparation of Self-Floating Sm/N Co-Doped TiO2/Diatomite Hybrid Pellet with Enhanced Visible-Light-Responsive Photoactivity and Reusability. Adv. Powder Technol. 2019, 30, 415–422. [Google Scholar] [CrossRef]
- Wang, G.; Ma, X.; Wei, S.; Li, S.; Qiao, J.; Wang, J.; Song, Y. Highly Efficient Visible-Light Driven Photocatalytic Hydrogen Production from a Novel Z-Scheme Er3+:YAlO3/Ta2O5-V5+||Fe3+-TiO2/Au Coated Composite. J. Power Sources 2018, 373, 161–171. [Google Scholar] [CrossRef]
- Lee, H.U.; Lee, S.C.; Choi, S.; Son, B.; Lee, S.M.; Kim, H.J.; Lee, J. Efficient Visible-Light Induced Photocatalysis on Nanoporous Nitrogen-Doped Titanium Dioxide Catalysts. Chem. Eng. J. 2013, 228, 756–764. [Google Scholar] [CrossRef]
- Krasil’Nikov, V.N.; Shalaeva, E.V.; Baklanova, I.V.; Melkozerova, M.A.; Kuznetsov, M.V.; Zabolotskaya, E.V.; Gyrdasova, O.I.; Buldakova, L.Y.; Murzakaev, A.M. Synthesis, Structure and Spectroscopic Characteristics of Ti(O,C)2/Carbon Nanostructured Globules with Visible Light Photocatalytic Activity. Bull. Mater. Sci. 2016, 39, 1569–1579. [Google Scholar] [CrossRef]
- Ju, T.; Lee, H.; Kang, M. The Photovoltaic Efficiency of Dye Sensitized Solar Cell Assembled Using Carbon Capsulated TiO2 Electrode. J. Ind. Eng. Chem. 2014, 20, 2636–2640. [Google Scholar] [CrossRef]
- Chen, P.; Wang, F.; Chen, Z.-F.; Zhang, Q.; Su, Y.; Shen, L.; Yao, K.; Liu, Y.; Cai, Z.; Lv, W.; et al. Study on the Photocatalytic Mechanism and Detoxicity of Gemfibrozil by a Sunlight-Driven TiO2/Carbon Dots Photocatalyst: The Significant Roles of Reactive Oxygen Species. Appl. Catal. B Environ. 2017, 204, 250–259. [Google Scholar] [CrossRef]
- Surenjan, A.; Sambandam, B.; Pradeep, T.; Philip, L. Synthesis, Characterization and Performance of Visible Light Active C-TiO2 for Pharmaceutical Photodegradation. J. Environ. Chem. Eng. 2017, 5, 757–767. [Google Scholar] [CrossRef]
- Li, X.-W.; Yuan, Y.; Huang, X.; Lin, K.-F. Visible-Infrared Optical Properties Measurement of the Mesoporous TiO2/Carbon Materials. Int. J. Hydrog. Energy 2016, 41, 15638–15645. [Google Scholar] [CrossRef]
- Wanag, A.; Kusiak-Nejman, E.; Kapica-Kozar, J.; Morawski, A.W. Photocatalytic Performance of Thermally Prepared TiO2/C Photocatalysts under Artificial Solar Light. Micro Nano Lett. 2016, 11, 202–206. [Google Scholar] [CrossRef]
- Neville, E.M.; Ziegler, J.; Don MacElroy, J.M.; Ravindranathan Thampi, K.; Sullivan, J.A. Serendipity Following Attempts to Prepare C-Doped Rutile TiO2. Appl. Catal. A Gen. 2014, 470, 434–441. [Google Scholar] [CrossRef]
- Teh, C.Y.; Wu, T.Y.; Juan, J.C. An Application of Ultrasound Technology in Synthesis of Titania-Based Photocatalyst for Degrading Pollutant. Chem. Eng. J. 2017, 317, 586–612. [Google Scholar] [CrossRef]
- Reli, M.; Kobielusz, M.; Matějová, L.; Daniš, S.; Macyk, W.; Obalová, L.; Kuśtrowski, P.; Rokicińska, A.; Kočí, K. TiO2 Processed by Pressurized Hot Solvents as a Novel Photocatalyst for Photocatalytic Reduction of Carbon Dioxide. Appl. Surf. Sci. 2017, 391, 282–287. [Google Scholar] [CrossRef]
- Rahimi, N.; Pax, R.A.; Gray, E.; Mac, A. Review of Functional Titanium Oxides. I: TiO2 and Its Modifications. Prog. Solid State Chem. 2016, 44, 86–105. [Google Scholar] [CrossRef]
- Mikrut, P.; Kobielusz, M.; Macyk, W. Spectroelectrochemical Characterization of Euhedral Anatase TiO2 Crystals—Implications for Photoelectrochemical and Photocatalytic Properties of {001} {100} and {101} Facets. Electrochim. Acta 2019, 310, 256–265. [Google Scholar] [CrossRef]
- Surówka, M.; Kobielusz, M.; Trochowski, M.; Buchalska, M.; Kruczała, K.; Broś, P.; Macyk, W. Iron and Other Metal Species as Phase-Composition Controllers Influencing the Photocatalytic Activity of TiO2 Materials. Appl. Catal. B Environ. 2019, 247, 173–181. [Google Scholar] [CrossRef]
- Sadowski, R.; Wach, A.; Buchalska, M.; Kuśtrowski, P.; Macyk, W. Photosensitized TiO2 Films on Polymers—Titania-Polymer Interactions and Visible Light Induced Photoactivity. Appl. Surf. Sci. 2019, 475, 710–719. [Google Scholar] [CrossRef]
- Karthik, K.; Vijayalakshmi, S.; Phuruangrat, A.; Revathi, V.; Verma, U. Multifunctional Applications of Microwave-Assisted Biogenic TiO2 Nanoparticles. J. Clust. Sci. 2019, 30, 965–972. [Google Scholar] [CrossRef]
- Mahdavi-Shakib, A.; Husremovic, S.; Ki, S.; Glynn, J.; Babb, L.; Sempel, J.; Stavrinoudis, I.; Arce-Ramos, J.-M.; Nelson, R.; Grabow, L.C.; et al. Titania Surface Chemistry and Its Influence on Supported Metal Catalysts. Polyhedron 2019, 170, 41–50. [Google Scholar] [CrossRef]
- Fatima, R.; Afridi, M.N.; Kumar, V.; Lee, J.; Ali, I.; Kim, K.-H.; Kim, J.-O. Photocatalytic Degradation Performance of Various Types of Modified TiO2 against Nitrophenols in Aqueous Systems. J. Clean. Prod. 2019, 231, 899–912. [Google Scholar] [CrossRef]
- Boro, B.; Gogoi, B.; Rajbongshi, B.M.; Ramchiary, A. Nano-Structured TiO2/ZnO Nanocomposite for Dye-Sensitized Solar Cells Application: A Review. Renew. Sustain. Energy Rev. 2018, 81, 2264–2270. [Google Scholar] [CrossRef]
- Akhter, P.; Farkhondehfal, M.A.; Hernández, S.; Hussain, M.; Fina, A.; Saracco, G.; Khan, A.U.; Russo, N. Environmental Issues Regarding CO2 and Recent Strategies for Alternative Fuels through Photocatalytic Reduction with Titania-Based Materials. J. Environ. Chem. Eng. 2016, 4, 3934–3953. [Google Scholar] [CrossRef]
- Ashraf, S.; Siddiqa, A.; Shahida, S.; Qaisar, S. Titanium-Based Nanocomposite Materials for Arsenic Removal from Water: A Review. Heliyon 2019, 5, e01577. [Google Scholar] [CrossRef]
- Etacheri, V.; Michlits, G.; Seery, M.K.; Hinder, S.J.; Pillai, S.C. A Highly Efficient TiO2–xCx Nano-Heterojunction Photocatalyst for Visible Light Induced Antibacterial Applications. ACS Appl. Mater. Interfaces 2013, 5, 1663–1672. [Google Scholar] [CrossRef]
- Di Valentin, C.; Pacchioni, G. Trends in Non-Metal Doping of Anatase TiO2: B, C, N and F. Catal. Today 2013, 206, 12–18. [Google Scholar] [CrossRef]
- Tatarchuk, T.; Mironyuk, I.; Kotsyubynsky, V.; Shyichuk, A.; Myslin, M.; Boychuk, V. Structure, Morphology and Adsorption Properties of Titania Shell Immobilized onto Cobalt Ferrite Nanoparticle Core. J. Mol. Liq. 2020, 297, 111757. [Google Scholar] [CrossRef]
- Dadfar, M.R.; Seyyed Ebrahimi, S.A.; Masoudpanah, S.M. Sol–Gel Synthesis and Characterization of SrFe12O19/TiO2 Nanocomposites. J. Supercond. Nov. Magn. 2015, 28, 89–94. [Google Scholar] [CrossRef]
- Xu, S.; Feng, D.; Shangguan, W. Preparations and Photocatalytic Properties of Visible-Light-Active Zinc Ferrite-Doped TiO2 Photocatalyst. J. Phys. Chem. C 2009, 113, 2463–2467. [Google Scholar] [CrossRef]
- Lahijani, B.; Hedayati, K.; Goodarzi, M. Magnetic PbFe12O19-TiO2 Nanocomposites and Their Photocatalytic Performance in the Removal of Toxic Pollutants. Main Group Met. Chem. 2018, 41, 53–62. [Google Scholar] [CrossRef]
- De Lucena, P.R.; Pessoa-Neto, O.D.; Dos Santos, I.M.G.; Souza, A.G.; Longo, E.; Varela, J.A. Synthesis by the Polymeric Precursor Method and Characterization of Undoped and Sn, Cr and V-Doped ZrTiO. J. Alloys Compd. 2005, 397, 255–259. [Google Scholar] [CrossRef]
- Mourão, H.A.J.L.; Malagutti, A.R.; Ribeiro, C. Synthesis of TiO2-Coated CoFe2O4 Photocatalysts Applied to the Photodegradation of Atrazine and Rhodamine B in Water. Appl. Catal. A Gen. 2010, 382, 284–292. [Google Scholar] [CrossRef]
- Mohanty, P.; Mahapatra, R.; Padhi, P.; Ramana, C.V.V.; Mishra, D.K. Ultrasonic Cavitation: An Approach to Synthesize Uniformly Dispersed Metal Matrix Nanocomposites—A Review. Nano Struct. Nano Objects 2020, 23, 100475. [Google Scholar] [CrossRef]
- Ley, S.V.; Low, C.M.R. Ultrasound in Synthesis; Springer: Berlin/Heidelberg, Germany, 1989; p. 133. [Google Scholar]
- Ao, Y.; Xu, J.; Fu, D.; Shen, X.; Yuan, C. A Novel Magnetically Separable Composite Photocatalyst: Titania-Coated Magnetic Activated Carbon. Sep. Purif. Technol. 2008, 61, 436–441. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, X.; Gong, X.; Yan, F.; Zhang, Z. Sonochemical Synthesis and Characterization of Magnetic Separable Fe3O4-TiO2 Nanocomposites and Their Catalytic Properties. Int. J. Smart Nano Mater. 2010, 1, 278–287. [Google Scholar] [CrossRef]
- Rane, A.V.; Kanny, K.; Abitha, V.K.; Thomas, S. Synthesis of Inorganic Nanomaterials; Bhagyaraj, S.M., Oluwafemi, O.S., Kalarikkal, N., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 121–139. [Google Scholar]
- Sathishkumar, P.; Mangalaraja, R.V.; Anandan, S.; Ashokkumar, M. CoFe2O4/TiO2 Nanocatalysts for the Photocatalytic Degradation of Reactive Red 120 in Aqueous Solutions in the Presence and Absence of Electron Acceptors. Chem. Eng. J. 2013, 220, 302–310. [Google Scholar] [CrossRef]
- Chandrika, M.; Ravindra, A.V.; Rajesh, C.; Ramarao, S.D.; Ju, S. Studies on Structural and Optical Properties of Nano ZnFe2O4 and ZnFe2O4-TiO2 Composite Synthesized by Co-Precipitation Route. Mater. Chem. Phys. 2019, 230, 107–113. [Google Scholar] [CrossRef]
- Haw, C.; Chiu, W.; Abdul Rahman, S.; Khiew, P.; Radiman, S.; Abdul Shukor, R.; Hamid, M.A.A.; Ghazali, N. The Design of New Magnetic-Photocatalyst Nanocomposites (CoFe2O4–TiO2) as Smart Nanomaterials for Recyclable-Photocatalysis Applications. New J. Chem. 2016, 40, 1124–1136. [Google Scholar] [CrossRef]
- Allaedini, G.; Tasirin, S.M.; Aminayi, P. Magnetic Properties of Cobalt Ferrite Synthesized by Hydrothermal Method. Int. Nano Lett. 2015, 5, 183–186. [Google Scholar] [CrossRef]
- Aziz, A.A.; Puma, G.L.; Ibrahim, S.; Saravanan, P. Preparation, Characterisation and Solar Photoactivity of Titania Supported Strontium Ferrite Nanocomposite Photocatalyst. J. Exp. Nanosci. 2013, 8, 295–310. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Doong, R. Fabrication of Highly Visible-Light-Responsive ZnFe2O4/TiO2 Heterostructures for the Enhanced Photocatalytic Degradation of Organic Dyes. RSC Adv. 2016, 6, 103428–103437. [Google Scholar] [CrossRef]
- Pongwan, P.; Inceesungvorn, B.; Phanichphant, S.; Kangwansupamonkon, W.; Wetchakun, N. Synthesis and Characterization of a Magnetically Separable CoFe2O4/TiO2 Nanocomposite for the Photomineralization of Formic Acid. Ferroelectrics 2013, 453, 133–140. [Google Scholar] [CrossRef]
- Sun, J.; Fu, Y.; Xiong, P.; Sun, X.; Xu, B.; Wang, X. A Magnetically Separable P25/CoFe2O4/Graphene Catalyst with Enhanced Adsorption Capacity and Visible-Light-Driven Photocatalytic Activity. RSC Adv. 2013, 3, 22490. [Google Scholar] [CrossRef]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-Light Activation of TiO2 Photocatalysts: Advances in Theory and Experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Atacan, K.; Güy, N.; Çakar, S. Preparation and Antibacterial Activity of Solvothermal Synthesized ZnFe2O4/Ag-TiO2 Nanocomposite. Sakarya Uni. J. Sci. 2018, 22, 1720–1726. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Doong, R. Heterostructured ZnFe2O4/TiO2 Nanocomposites with a Highly Recyclable Visible-Light-Response for Bisphenol A Degradation. RSC Adv. 2017, 7, 50006–50016. [Google Scholar] [CrossRef]
- Deng, Y.-H.; Wang, C.-C.; Hu, J.-H.; Yang, W.-L.; Fu, S.-K. Investigation of Formation of Silica-Coated Magnetite Nanoparticles via Sol–Gel Approach. Colloids Surf. A Physicochem. Eng. Asp. 2005, 262, 87–93. [Google Scholar] [CrossRef]
- Sounderya, N.; Zhang, Y. Use of Core/Shell Structured Nanoparticles for Biomedical Applications. Biomedical 2008, 1, 34–42. [Google Scholar] [CrossRef]
- Morales, A.E.; Mora, E.S.; Pal, U. Use of diffuse reflectance spectroscopy for optical characterization of unsupported nanostructures. Rev. Mex. Fis. 2007, 53, 18–22. [Google Scholar]
- Bardapurkar, P.P.; Shewale, S.S.; Barde, N.P.; Jadhav, K.M. Structural, Magnetic and Catalytical Properties of Cobalt Ferrite Nanoparticles Dispersed in Silica Matrix. Mater. Res. Express. 2019, 6, 045055. [Google Scholar] [CrossRef]
- Gharagozlou, M. Influence of Calcination Temperature on Structural and Magnetic Properties of Nanocomposites Formed by Co-Ferrite Dispersed in Sol-Gel Silica Matrix Using Tetrakis(2-Hydroxyethyl) Orthosilicate as Precursor. Chem. Cent. J. 2011, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Pansambal, S.; Ghotekar, S.; Shewale, S.; Deshmukh, K.; Barde, N.; Bardapurkar, P. Efficient Synthesis of Magnetically Separable CoFe2O4@SiO2 Nanoparticles and Its Potent Catalytic Applications for the Synthesis of 5-Aryl-1,2,4-Triazolidine-3-Thione Derivatives. J. Water Environ. Nanotechnol. 2019, 4, 174–186. [Google Scholar] [CrossRef]
- Dippong, T.; Levei, E.A.; Cadar, O. Preparation of CoFe2O4/SiO2 Nanocomposites at Low Temperatures Using Short Chain Diols. J. Chem. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled Growth of Monodisperse Silica Spheres in the Micron Size Range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Ehi-Eromosele, C.O.; Ita, B.I.; Iweala, E.E.J.; Ogunniran, K.O.; Adekoya, J.A.; Siyanbola, T.O. Silica Functionalized Magnesium Ferrite Nanocomposites for Potential Biomedical Applications: Preparation, Characterization and Enhanced Colloidal Stability Studies. J. Nano Res. 2016, 40, 146–157. [Google Scholar] [CrossRef]
- Khanna, L.; Verma, N.K. Silica/Potassium Ferrite Nanocomposite: Structural, Morphological, Magnetic, Thermal and in Vitro Cytotoxicity Analysis. Mater. Sci. Eng. B 2013, 178, 1230–1239. [Google Scholar] [CrossRef]
- Flood-Garibay, J.A.; Méndez-Rojas, M.A. Synthesis and Characterization of Magnetic Nanoparticles of Cobalt Ferrite Coated with Silica. Biointerface Res. Appl. Chem. 2019, 10, 4908–4913. [Google Scholar] [CrossRef]
- Girgis, E.; Wahsh, M.M.; Othman, A.G.; Bandhu, L.; Rao, K. Synthesis, Magnetic and Optical Properties of Core/Shell Co1−xZnx Fe2O4/SiO2 Nanoparticles. Nanoscale Res. Lett. 2011, 6, 460. [Google Scholar] [CrossRef] [PubMed]
- Rohilla, S.; Kumar, S.; Aghamkar, P.; Sunder, S.; Agarwal, A. Investigations on Structural and Magnetic Properties of Cobalt Ferrite/Silica Nanocomposites Prepared by the Coprecipitation Method. J. Magn. Magn. Mater. 2011, 323, 897–902. [Google Scholar] [CrossRef]
- Bansal, M.; Ahlawat, D.S.; Singh, A.; Kumar, V.; Rathee, S.P. Effect of Heat Treatment on the Microstructural Properties of Silica Embedded Cobalt Ferrite Nanocomposites. Nanocomposites 2020, 6, 158–164. [Google Scholar] [CrossRef]
- Yakob, M.; Umar, H.; Wahyuningsih, P.; Putra, R.A. Characterization of microstructural and optical CoFe2O4/SiO2 ferrite nanocomposite for photodegradation of methylene blue. AIMS Mater. Sci. 2019, 6, 45–51. [Google Scholar] [CrossRef]
- Sharma, S.; Rohilla, S. Synthesis of Nanocomposites of NiFe2O4/SiO2 through Coprecipitation Method and Structural Characterization Using Rietveld Refinement. In Proceedings of the AIP Conference Proceedings, Seoul, Republic of Korea, 2 November 2020; p. 110023. [Google Scholar]
- Kong, L.B.; Zhang, T.S.; Ma, J.; Boey, F. Progress in Synthesis of Ferroelectric Ceramic Materials via High-Energy Mechanochemical Technique. Prog. Mater. Sci. 2008, 53, 207–322. [Google Scholar] [CrossRef]
- Aguilar-González, M.A.; Mendoza-Suárez, G.; Padmasree, K.P. Synthesis and Characterization of Barium Ferrite–Silica Nanocomposites. Mater. Charact. 2013, 84, 175–181. [Google Scholar] [CrossRef]
- Scano, A.; Cabras, V.; Marongiu, F.; Peddis, D.; Pilloni, M.; Ennas, G. New Opportunities in the Preparation of Nanocomposites for Biomedical Applications: Revised Mechanosynthesis of Magnetite–Silica Nanocomposites. Mater. Res. Express 2017, 4, 025004. [Google Scholar] [CrossRef]
- Xu, Y.; Tang, C.-J.; Huang, H.; Sun, C.-Q.; Zhang, Y.-K.; Ye, Q.-F.; Wang, A.-J. Green Synthesis of Fluorescent Carbon Quantum Dots for Detection of Hg2+. Chin. J. Anal. Chem. 2014, 42, 1252–1258. [Google Scholar] [CrossRef]
- De, B.; Karak, N. A Green and Facile Approach for the Synthesis of Water Soluble Fluorescent Carbon Dots from Banana Juice. RSC Adv. 2013, 3, 8286. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, X.; Chang, J.; Wu, D.; Wang, L.; Liu, X.; Xu, F.; Guo, Y.; Jiang, K. Fluorescent Carbon Quantum Dots, Capacitance and Catalysis Active Porous Carbon Microspheres from Beer. RSC Adv. 2015, 5, 48665–48674. [Google Scholar] [CrossRef]
- Wee, S.S.; Ng, Y.H.; Ng, S.M. Synthesis of Fluorescent Carbon Dots via Simple Acid Hydrolysis of Bovine Serum Albumin and Its Potential as Sensitive Sensing Probe for Lead (II) Ions. Talanta 2013, 116, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, C.; Shen, G.; Liu, H.; Fu, H.; Cui, D. Fluorescent Carbon Dots as an Efficient SiRNA Nanocarrier for Its Interference Therapy in Gastric Cancer Cells. J. Nanobiotechnol. 2014, 12, 58. [Google Scholar] [CrossRef] [PubMed]
- Vitha, T.S. Quantitative Evaluation of Pytocompounds from Terminalia Chebula by High Performance Thin Layer Chromatography (Hptlc) Method and Its Antibiofilm Activity. Int. J. Pharma Bio Sci. 2017, 8, 214–224. [Google Scholar] [CrossRef]
- Moradi, B.; Nabiyouni, G.; Ghanbari, D. Rapid Photo-Degradation of Toxic Dye Pollutants: Green Synthesis of Mono-Disperse Fe3O4–CeO2 Nanocomposites in the Presence of Lemon Extract. J. Mater. Sci. Mater. Electron. 2018, 29, 11065–11080. [Google Scholar] [CrossRef]
- Barman, M.K.; Patra, A. Current Status and Prospects on Chemical Structure Driven Photoluminescence Behaviour of Carbon Dots. J. Photochem. Photobiol. C 2018, 37, 1–22. [Google Scholar] [CrossRef]
- Das, R.; Bandyopadhyay, R.; Pramanik, P. Carbon Quantum Dots from Natural Resource: A Review. Mater. Today Chem. 2018, 8, 96–109. [Google Scholar] [CrossRef]
- Ahmadian-Fard-Fini, S.; Salavati-Niasari, M.; Ghanbari, D. Hydrothermal Green Synthesis of Magnetic Fe3O4-Carbon Dots by Lemon and Grape Fruit Extracts and as a Photoluminescence Sensor for Detecting of E. Coli Bacteria. Spectrochim. Acta Mol. Biomol. Spectrosc. 2018, 203, 481–493. [Google Scholar] [CrossRef]
- Baragau, I.-A.; Power, N.P.; Morgan, D.J.; Heil, T.; Lobo, R.A.; Roberts, C.S.; Titirici, M.-M.; Dunn, S.; Kellici, S. Continuous Hydrothermal Flow Synthesis of Blue-Luminescent, Excitation-Independent Nitrogen-Doped Carbon Quantum Dots as Nanosensors. J. Mater. Chem. A 2020, 8, 3270–3279. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, A. Carbon Quantum Dots: Synthesis, Properties and Applications. J. Mater. Chem. C 2014, 2, 6921. [Google Scholar] [CrossRef]
- Liu, J.-H.; Cao, L.; LeCroy, G.E.; Wang, P.; Meziani, M.J.; Dong, Y.; Liu, Y.; Luo, P.G.; Sun, Y.-P. Carbon “Quantum” Dots for Fluorescence Labeling of Cells. ACS Appl. Mater. Interfaces 2015, 7, 19439–19445. [Google Scholar] [CrossRef]
- Dong, X.; Liang, W.; Meziani, M.J.; Sun, Y.-P.; Yang, L. Carbon Dots as Potent Antimicrobial Agents. Theranostics 2020, 10, 671–686. [Google Scholar] [CrossRef]
- Ahmadian-Fard-Fini, S.; Ghanbari, D.; Salavati-Niasari, M. Photoluminescence Carbon Dot as a Sensor for Detecting of Pseudomonas Aeruginosa Bacteria: Hydrothermal Synthesis of Magnetic Hollow NiFe2O4-Carbon Dots Nanocomposite Material. Compos. Part B Eng. 2019, 161, 564–577. [Google Scholar] [CrossRef]
- Huang, Y.; Liang, Y.; Rao, Y.; Zhu, D.; Cao, J.; Shen, Z.; Ho, W.; Lee, S.C. Environment-Friendly Carbon Quantum Dots/ZnFe2O4 Photocatalysts: Characterization, Biocompatibility, and Mechanisms for NO Removal. Environ. Sci. Technol. 2017, 51, 2924–2933. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Fan, T. Study on Carbon Quantum Dots/BiFeO3 Heterostructures and Their Enhanced Photocatalytic Activities under Visible Light Irradiation. J. Mater. Sci. Mater. Electron. 2017, 28, 10019–10027. [Google Scholar] [CrossRef]
- Nabiyouni, G.; Ghanbari, D. Hydrothermal Synthesis of Magnetic and Photoluminescence CuFe2O4-Carbon Dots Nanocomposite as a Sensor for Detecting of HgII Ions. J. Nanostruct. 2020, 10, 760–768. [Google Scholar] [CrossRef]
- Rahman, I.A.; Padavettan, V. Synthesis of Silica Nanoparticles by Sol-Gel: Size-Dependent Properties, Surface Modification, and Applications in Silica-Polymer Nanocomposites—A Review. J. Nanomater. 2012, 2012, 1–15. [Google Scholar] [CrossRef]
- Hagens, W.I.; Oomen, A.G.; De Jong, W.H.; Cassee, F.R.; Sips, A.J.A.M. What Do We (Need to) Know about the Kinetic Properties of Nanoparticles in the Body? J. Regul. Toxicol. Pharmacol. 2007, 49, 217–229. [Google Scholar] [CrossRef]
- Mathiazhagan, A.; Joseph, R. Nanotechnology—A New Prospective in Organic Coating-Review. Int. J. Chem. Eng. Appl. 2011, 2, 225–237. [Google Scholar] [CrossRef]
- Nalathambi, V.; Suresh, G. Contribution of Nanotechnology in The Paints and Coatings. Int. J. Chem. Eng. Res. 2014, 1, 116–186. [Google Scholar] [CrossRef]
- Singh, R.; Thirupathi, G. Manganese-Zinc Spinel Ferrite Nanoparticles and Ferrofluids; Singh, R., Thirupathied, G., Eds.; InTech: London, UK, 2017; pp. 140–159. [Google Scholar]
- Peng, Y.; Wang, Z.; Liu, W.; Zhang, H.; Zuo, W.; Tang, H.; Chen, F.; Wang, B. Size-and Shape-Dependent Peroxidase-like Catalytic Activity of MnFe2O4 Nanoparticles and Their Applications in Highly Efficient Colorimetric Detection of Target Cancer Cells. Dalton Trans. 2015, 44, 12871–12877. [Google Scholar] [CrossRef]
- Reddy, S.; Kumara Swamy, B.E.; Chandra, U.; Mahathesha, K.R.; Sathisha, T.V.; Jayadevappa, H. Synthesis of MgFe2O4 Nanoparticles and MgFe2O4 Nanoparticles/CPE for Electrochemical Investigation of Dopamine. Anal. Methods 2011, 3, 2792. [Google Scholar] [CrossRef]
- Céspedes, E.; Byrne, J.M.; Farrow, N.; Moise, S.; Coker, V.S.; Bencsik, M.; Lloyd, J.R.; Telling, N.D. Bacterially Synthesized Ferrite Nanoparticles for Magnetic Hyperthermia Applications. Nanoscale 2014, 6, 12958–12970. [Google Scholar] [CrossRef] [PubMed]
- Pardeshi, S.K.; Pawar, R.Y. SrFe2O4 Complex Oxide an Effective and Environmentally Benign Catalyst for Selective Oxidation of Styrene. J. Mol. Catal. A Chem. 2011, 334, 35–43. [Google Scholar] [CrossRef]
- Khedr, M.H.; Abdel Halim, K.S.; Soliman, N.K. Synthesis and Photocatalytic Activity of Nano-Sized Iron Oxides. Mater. Lett. 2009, 63, 598–601. [Google Scholar] [CrossRef]
- Surendra, B.S. Green Engineered Synthesis of Ag-Doped CuFe2O4: Characterization, Cyclic Voltammetry and Photocatalytic Studies. J. Sci. Adv. Mater. Dev. 2018, 3, 44–50. [Google Scholar] [CrossRef]
- Diao, Y.; Yan, Z.; Guo, M.; Wang, X. Magnetic Multi-Metal Co-Doped Magnesium Ferrite Nanoparticles: An Efficient Visible Light-Assisted Heterogeneous Fenton-like Catalyst Synthesized from Saprolite Laterite Ore. J. Hazard. Mater. 2018, 344, 829–838. [Google Scholar] [CrossRef]
- Han, X.; Zhang, H.; Chen, T.; Zhang, M.; Guo, M. Facile Synthesis of Metal-Doped Magnesium Ferrite from Saprolite Laterite as an Effective Heterogeneous Fenton-like Catalyst. J. Mol. Liq. 2018, 272, 43–52. [Google Scholar] [CrossRef]
- Feng, Y.; Liao, C.; Shih, K. Copper-Promoted Circumneutral Activation of H2O2 by Magnetic CuFe2O4 Spinel Nanoparticles: Mechanism, Stoichiometric Efficiency, and Pathway of Degrading Sulfanilamide. Chemosphere 2016, 154, 573–582. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, D.; Kou, F.; Ouyang, Q.; Chen, J.; Fang, Z. Removal of Norfloxacin by Surface Fenton System (MnFe2O4/H2O2): Kinetics, Mechanism and Degradation Pathway. Chem. Eng. J. 2018, 351, 747–755. [Google Scholar] [CrossRef]
- Guo, X.; Wang, D. Photo-Fenton Degradation of Methylene Blue by Synergistic Action of Oxalic Acid and Hydrogen Peroxide with NiFe2O4 Hollow Nanospheres Catalyst. J. Environ. Chem. Eng. 2019, 7, 102814. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; López-Ramón, M.V.; Fontecha-Cámara, M.Á.; Álvarez, M.A.; Mateus, L. Removal of Phenolic Compounds from Water Using Copper Ferrite Nanosphere Composites as Fenton Catalysts. Nanomaterials 2019, 9, 901. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Fan, M.; Wang, Y.; Luo, G.; Fei, W. Magnetic Titanium Dioxide Based Nanomaterials: Synthesis, Characteristics, and Photocatalytic Application in Pollutant Degradation. J. Mater. Chem. A 2015, 3, 17511–17524. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Yeganeh, M.; Shahtahmasebi, N.; Kompany, A.; Karimipour, M.; Razavi, F.; Nasralla, N.H.S.; Šiller, L. The Magnetic Characterization of Fe Doped TiO2 Semiconducting Oxide Nanoparticles Synthesized by Sol–Gel Method. Physical. B Condens. Matter 2017, 511, 89–98. [Google Scholar] [CrossRef]
- Lin, Z.; Xu, Y.; Zhen, Z.; Fu, Y.; Liu, Y.; Li, W.; Luo, C.; Ding, A.; Zhang, D. Application and Reactivation of Magnetic Nanoparticles in Microcystis Aeruginosa Harvesting. Bioresour. Technol. 2015, 190, 82–88. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, X.; Li, H.; Jia, J.; Liu, Y.; Ejenavi, O.; Ding, A.; Sun, Y.; Zhang, D. Separating and Characterizing Functional Alkane Degraders from Crude-Oil-Contaminated Sites via Magnetic Nanoparticle-Mediated Isolation. Res. Microbiol. 2016, 167, 731–744. [Google Scholar] [CrossRef]
- Zhang, P.; Mo, Z.; Han, L.; Zhu, X.; Wang, B.; Zhang, C. Preparation and Photocatalytic Performance of Magnetic TiO2/Montmorillonite/Fe3O4 Nanocomposites. Ind. Eng. Chem. Res. 2014, 53, 8057–8061. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, Y.; Geng, B.; Wang, M.; Sun, X. Photocatalytic Performance and Magnetic Separation of TiO2-Functionalized γ-Fe2O3, Fe, and Fe/Fe2O3 Magnetic Particles. J. Alloys Compd. 2017, 700, 113–121. [Google Scholar] [CrossRef]
- Chen, C.-C.; Jaihindh, D.; Hu, S.-H.; Fu, Y.-P. Magnetic Recyclable Photocatalysts of Ni-Cu-Zn Ferrite@SiO2@TiO2@Ag and Their Photocatalytic Activities. J. Photochem. Photobiol. A Chem. 2017, 334, 74–85. [Google Scholar] [CrossRef]
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Electrochemical Advanced Oxidation Processes: A Review on Their Application to Synthetic and Real Wastewaters. Appl. Catal. B Environ. 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Boczkaj, G.; Fernandes, A. Wastewater Treatment by Means of Advanced Oxidation Processes at Basic PH Conditions: A Review. Chem. Eng. J. 2017, 320, 608–633. [Google Scholar] [CrossRef]
- Dewil, R.; Mantzavinos, D.; Poulios, I.; Rodrigo, M.A. New Perspectives for Advanced Oxidation Processes. J. Environ. Manag. 2017, 195, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Araki, S.; Yamamoto, H. Evaluation of Advanced Oxidation Processes (AOP) Using O3, UV, and TiO2 for the Degradation of Phenol in Water. J. Water Process. Eng. 2015, 7, 54–60. [Google Scholar] [CrossRef]
- Moellmann, J.; Ehrlich, S.; Tonner, R.; Grimme, S. A DFT-D Study of Structural and Energetic Properties of TiO2 Modifications. J. Phys. Condens. Matter. 2012, 24, 424206. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, D.O.; Dunnill, C.W.; Buckeridge, J.; Shevlin, S.A.; Logsdail, A.J.; Woodley, S.M.; Catlow, C.R.A.; Powell Michael, J.; Palgrave, R.G.; Parkin, I.P.; et al. Band Alignment of Rutile and Anatase TiO2. Nat. Mater. 2013, 12, 798–801. [Google Scholar] [CrossRef]
- Graça, M.P.F.; Nico, C.; Peres, M.; Valente, M.A.; Monteiro, T. Study of the Optical and Dielectric Properties of TiO2 Nanocrystals Prepared by the Pechini Method. J. Nanosci. Nanotechnol. 2012, 12, 8600–8606. [Google Scholar] [CrossRef]
- Habila, M.A.; ALOthman, Z.A.; El-Toni, A.M.; Labis, J.P.; Soylak, M. Synthesis and Application of Fe3O4@SiO2@TiO2 for Photocatalytic Decomposition of Organic Matrix Simultaneously with Magnetic Solid Phase Extraction of Heavy Metals Prior to ICP-MS Analysis. Talanta 2016, 154, 539–547. [Google Scholar] [CrossRef]
- Wilson, M.; Cheng, C.Y.C.; Oswald, G.; Srivastava, R.; Beaumont, S.K.; Badyal, J.P.S. Magnetic Recyclable Microcomposite Silica-Steel Core with TiO2 Nanocomposite Shell Photocatalysts for Sustainable Water Purification. Colloids Surf. A Physicochem. Eng. Asp. 2017, 523, 27–37. [Google Scholar] [CrossRef]
- Chung, I.-M.; Park, I.; Seung-Hyun, K.; Thiruvengadam, M.; Rajakumar, G. Plant-Mediated Synthesis of Silver Nanoparticles: Their Characteristic Properties and Therapeutic Applications. Nanoscale Res. Lett. 2016, 11, 40. [Google Scholar] [CrossRef]
- Ravikumar, P.; Kumar, S.S. Antifungal activity of extracellularly synthesized silver nanoparticles from Morinda citrifolia. Int. J. Tech. Res. Appl. 2014, 2, 108–111. [Google Scholar]
- Inbathamizh, L.; Ponnu, T.M.; Mary, E.J. In Vitro Evaluation of Antioxidant and Anticancer Potential of Morinda Pubescens Synthesized Silver Nanoparticles. J. Pharm. Res. 2013, 6, 32–38. [Google Scholar] [CrossRef]
- He, Z.; Cai, Q.; Fang, H.; Situ, G.; Qiu, J.; Song, S.; Chen, J. Photocatalytic Activity of TiO2 Containing Anatase Nanoparticles and Rutile Nanoflower Structure Consisting of Nanorods. J. Environ. Sci. 2013, 25, 2460–2468. [Google Scholar] [CrossRef] [PubMed]
- Hooshmand, S.; Kargozar, S.; Ghorbani, A.; Darroudi, M.; Keshavarz, M.; Baino, F.; Kim, H.-W. Biomedical Waste Management by Using Nanophotocatalysts: The Need for New Options. Materials 2020, 13, 3511. [Google Scholar] [CrossRef] [PubMed]
- Vimbela, G.V.; Ngo, S.M.; Fraze, C.; Yang, L.; Stout, D.A. Antibacterial properties and toxicity from metallic nanomaterials. Int. J. Nanomed. 2017, 12, 3941–3965. [Google Scholar] [CrossRef] [PubMed]
- Verdier, T.; Coutand, M.; Bertron, A.; Roques, C. Antibacterial Activity of TiO2 Photocatalyst Alone or in Coatings on E. Coli: The Influence of Methodological Aspects. Coatings 2014, 4, 670–686. [Google Scholar] [CrossRef]
- Xie, J.; Hung, Y.-C. Methodology to Evaluate the Antimicrobial Effectiveness of UV-Activated TiO2 Nanoparticle-Embedded Cellulose Acetate Film. Food Control 2019, 106, 106690. [Google Scholar] [CrossRef]
- Reddy, A.K.; Kambalyal, P.B.; Shanmugasundaram, K.; Rajesh, V.; Donthula, S.; Patil, S.R. Comparative Evaluation of Antimicrobial Efficacy of Silver, Titanium Dioxide and Zinc Oxide Nanoparticles against Streptococcus Mutans. Pesqui. Bras. Odontopediatria Clín. Integr. 2018, 18, e4150. [Google Scholar] [CrossRef]
- Long, M.; Wang, J.; Zhuang, H.; Zhang, Y.; Wu, H.; Zhang, J. Performance and Mechanism of Standard Nano-TiO2 (P-25) in Photocatalytic Disinfection of Foodborne Microorganisms—Salmonella Typhimurium and Listeria Monocytogenes. Food Control 2014, 39, 68–74. [Google Scholar] [CrossRef]
- Altın, İ.; Sökmen, M. Preparation of TiO2-Polystyrene Photocatalyst from Waste Material and Its Usability for Removal of Various Pollutants. Appl. Catal. B Environ. 2014, 144, 694–701. [Google Scholar] [CrossRef]
- Bonetta, S.; Bonetta, S.; Motta, F.; Strini, A.; Carraro, E. Photocatalytic Bacterial Inactivation by TiO2-Coated Surfaces. AMB Expr. 2013, 3, 59. [Google Scholar] [CrossRef]
- Yao, N.; Lun Yeung, K. Investigation of the Performance of TiO2 Photocatalytic Coatings. Chem. Eng. J. 2011, 167, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.S.; Ye, Q.Y.; Li, D.D. Influence of nano-TiO2 modified LDPE film packaging on quality of strawberry. Mod. Food Sci. Technol. 2013, 29, 2340–2344. [Google Scholar]
- Gumiero, M.; Peressini, D.; Pizzariello, A.; Sensidoni, A.; Iacumin, L.; Comi, G.; Toniolo, R. Effect of TiO2 Photocatalytic Activity in a HDPE-Based Food Packaging on the Structural and Microbiological Stability of a Short-Ripened Cheese. Food Chem. 2013, 138, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, C.; Zhuang, H.; Zhang, J. Photocatalytic Degradation of Methylene Blue and Inactivation of Gram-Negative Bacteria by TiO2 Nanoparticles in Aqueous Suspension. Food Control 2013, 34, 372–377. [Google Scholar] [CrossRef]
- Yue, Q.; Sun, J.; Kang, Y.; Deng, Y. Advances in the Interfacial Assembly of Mesoporous Silica on Magnetite Particles. Angew. Chem. Int. Ed. 2020, 59, 15804–15817. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Liang, Z.; Zou, R.; Zhao, Y. Heterogeneous Catalysis in Zeolites, Mesoporous Silica, and Metal-Organic Frameworks. Adv. Mater. 2017, 29, 1701139. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, K.; Kuwahara, Y.; Sumida, Y.; Yamashita, H. Fabrication of Photocatalytic Paper Using TiO2 Nanoparticles Confined in Hollow Silica Capsules. Langmuir 2017, 33, 288–295. [Google Scholar] [CrossRef]
- Wang, D.; Han, D.; Shi, Z.; Wang, J.; Yang, J.; Li, X.; Song, H. Optimized Design of Three-Dimensional Multi-Shell Fe3O4/SiO2/ZnO/ZnSe Microspheres with Type II Heterostructure for Photocatalytic Applications. Appl. Catal. B Environ. 2018, 227, 61–69. [Google Scholar] [CrossRef]
- Tang, X.; Feng, Q.; Liu, K.; Luo, X.; Huang, J.; Li, Z. A simple and innovative route to remarkably enhance the photocatalytic performance of TiO2: Using micro-meso porous silica nanofibers as carrier to support highly-dispersed TiO2 nanoparticles. Micropor. Mesopor. Mater. 2018, 258, 251–261. [Google Scholar] [CrossRef]
- Mohanty, S.; Babu, P.; Parida, K.; Naik, B. Surface-Plasmon-Resonance-Induced Photocatalysis by Core–Shell SiO2@Ag NCs@Ag3PO4 toward Water-Splitting and Phenol Oxidation Reactions. Inorg. Chem. 2019, 58, 9643–9654. [Google Scholar] [CrossRef]
- Singh, R.; Bapat, R.; Qin, L.; Feng, H.; Polshettiwar, V. Atomic Layer Deposited (ALD) TiO2 on Fibrous Nano-Silica (KCC-1) for Photocatalysis: Nanoparticle Formation and Size Quantization Effect. ACS Catal. 2016, 6, 2770–2784. [Google Scholar] [CrossRef]
- Yin, Q.; Wu, W.; Qiao, R.; Ke, X.; Hu, Y.; Li, Z. Glucose-Assisted Transformation of Ni-Doped-ZnO@carbon to a Ni-Doped-ZnO@void@SiO2 Core–Shell Nanocomposite Photocatalyst. RSC Adv. 2016, 6, 38653–38661. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, H.; Liu, J.; Liu, Y.; Kang, Z. Carbon Quantum Dots Serving as Spectral Converters through Broadband Upconversion of Near-Infrared Photons for Photoelectrochemical Hydrogen Generation. J. Mater. Chem. A 2013, 1, 11529. [Google Scholar] [CrossRef]
- Rajendiran, K.; Zhao, Z.; Pei, D.-S.; Fu, A. Antimicrobial Activity and Mechanism of Functionalized Quantum Dots. Polymers 2019, 11, 1670. [Google Scholar] [CrossRef]
- Moradlou, O.; Rabiei, Z.; Banazadeh, A.; Warzywoda, J.; Zirak, M. Carbon Quantum Dots as Nano-Scaffolds for α-Fe2O3 Growth: Preparation of Ti/CQD@α-Fe2O3 Photoanode for Water Splitting under Visible Light Irradiation. Appl. Catal. B Environ. 2018, 227, 178–189. [Google Scholar] [CrossRef]
- Lops, C.; Ancona, A.; Di Cesare, K.; Dumontel, B.; Garino, N.; Canavese, G.; Hérnandez, S.; Cauda, V. Sonophotocatalytic Degradation Mechanisms of Rhodamine B Dye via Radicals Generation by Micro-and Nano-Particles of ZnO. Appl. Catal. B Environ. 2019, 243, 629–640. [Google Scholar] [CrossRef]
- Madhav, S.; Ahamad, A.; Singh, P.; Mishra, P.K. A Review of Textile Industry: Wet Processing, Environmental Impacts, and Effluent Treatment Methods. Environ. Qual. Manag. 2018, 27, 31–41. [Google Scholar] [CrossRef]
- Wang, S.; Xu, M.; Peng, T.; Zhang, C.; Li, T.; Hussain, I.; Wang, J.; Tan, B. Porous Hypercrosslinked Polymer-TiO2-Graphene Composite Photocatalysts for Visible-Light-Driven CO2 Conversion. Nat. Commun. 2019, 10, 676. [Google Scholar] [CrossRef]
- Tang, Y.; Zhou, P.; Wang, K.; Lin, F.; Lai, J.; Chao, Y.; Li, H.; Guo, S. BiOCl/Ultrathin Polyaniline Core/Shell Nanosheets with a Sensitization Mechanism for Efficient Visible-Light-Driven Photocatalysis. Sci. China Mater. 2019, 62, 95–102. [Google Scholar] [CrossRef]
- Fu, J.; Yu, J.; Jiang, C.; Cheng, B. G-C3N4-Based Heterostructured Photocatalysts. Adv. Energy Mater. 2018, 8, 1701503. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, W.; Zhang, S.; Wen, B.; Su, Z. Advanced 3D Nanohybrid Foam Based on Graphene Oxide: Facile Fabrication Strategy, Interfacial Synergetic Mechanism, and Excellent Photocatalytic Performance. Sci. China Mater. 2019, 62, 1888–1897. [Google Scholar] [CrossRef]
- Chen, S.; Yu, J.; Zhang, J. Enhanced Photocatalytic CO2 Reduction Activity of MOF-Derived ZnO/NiO Porous Hollow Spheres. J. CO2 Util. 2018, 24, 548–554. [Google Scholar] [CrossRef]
- Teimouri, M.; Husain, S.W.; Saber-Tehrani, M.; Aberoomand-Azar, P. Preparation of Novel Ni/Co Co-Doping Fe3O4/TiO2 Core–Shell Nanocomposites and Their Use in Effective Photocatalytic Degradation of Amlodipine Drug. Sep. Sci. Technol. 2019, 54, 634–641. [Google Scholar] [CrossRef]
- Stefan, M.; Leostean, C.; Pana, O.; Toloman, D.; Popa, A.; Perhaita, I.; Senilă, M.; Marincas, O.; Barbu-Tudoran, L. Magnetic Recoverable Fe3O4-TiO2:Eu Composite Nanoparticles with Enhanced Photocatalytic Activity. Appl. Surf. Sci. 2016, 390, 248–259. [Google Scholar] [CrossRef]
- Abbas, M.; Rao, B.P.; Reddy, V.; Kim, C. Fe3O4/TiO2 Core/Shell Nanocubes: Single-Batch Surfactantless Synthesis, Characterization and Efficient Catalysts for Methylene Blue Degradation. Ceram. Int. 2014, 40, 11177–11186. [Google Scholar] [CrossRef]
- Golshan, M.; Kakavandi, B.; Ahmadi, M.; Azizi, M. Photocatalytic Activation of Peroxymonosulfate by TiO2 Anchored on Cupper Ferrite (TiO2@CuFe2O4) into 2,4-D Degradation: Process Feasibility, Mechanism and Pathway. J. Hazard. Mater. 2018, 359, 325–337. [Google Scholar] [CrossRef]
- Dung, N.T. Preparation of Magnetic Antibacterial Composite Beads Fe3O4/Alginate/Ag. Vietnam. J. Sci. Technol. 2018, 56, 192. [Google Scholar] [CrossRef]
- Moosavi, S.M.; Molla-Abbasi, P.; Haji-Aghajani, Z. Photo-Catalyst CoFe2O4–TiO2: Application in Photo-Degradation of Organic Dyes and Magnetic Nanocomposite Preparation. J. Mater. Sci. Mater. Electron. 2016, 27, 4879–4886. [Google Scholar] [CrossRef]
- Bavarsiha, F.; Rajabi, M.; Montazeri-Pour, M. Synthesis of SrFe12O19/SiO2/TiO2 Composites with Core/Shell/Shell Nano-Structure and Evaluation of Their Photo-Catalytic Efficiency for Degradation of Methylene Blue. J. Mater. Sci. Mater. Electron. 2018, 29, 1877–1887. [Google Scholar] [CrossRef]
- Chen, C.-C.; Fu, Y.-P.; Hu, S.-H. Characterizations of TiO2/SiO2/Ni-Cu-Zn Ferrite Composite for Magnetic Photocatalysts. J. Am. Ceram. Soc. 2015, 98, 2803–2811. [Google Scholar] [CrossRef]
- Coromelci, C.; Neamtu, M.; Ignat, M.; Samoila, P.; Zaltariov, M.F.; Palamaru, M. Ultrasound Assisted Synthesis of Heterostructured TiO2/ZnFe2O4 and TiO2/ZnFe1.98La0.02O4 Systems as Tunable Photocatalysts for Efficient Organic Pollutants Removal. Ceram. Int. 2022, 48, 4829–4840. [Google Scholar] [CrossRef]
- Mandal, S.; Adhikari, S.; Pu, S.; Wang, X.; Kim, D.-H.; Patel, R.K. Interactive Fe2O3/Porous SiO2 Nanospheres for Photocatalytic Degradation of Organic Pollutants: Kinetic and Mechanistic Approach. Chemosphere 2019, 234, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Grewal, J.K.; Kaur, M.; Sharma, R.K.; Oliveira, A.C.; Garg, V.K.; Sharma, V.K. Structural and Photocatalytic Studies on Oxygen Hyperstoichiometric Titanium-Substituted Strontium Ferrite Nanoparticles. Magnetochemistry 2022, 8, 120. [Google Scholar] [CrossRef]
- Singh, G.; Kaur, M.; Kumar Garg, V.; Oliveira, A.C. Oxygen Hyper Stoichiometric Trimetallic Titanium Doped Magnesium Ferrite: Structural and Photocatalytic Studies. Ceram. Int. 2022, 48, 24476–24484. [Google Scholar] [CrossRef]
- Grewal, J.K.; Kaur, M.; Ubhi, M.K.; Oliveira, A.C.; Garg, V.K.; Sharma, V.K. Structural, Magnetic, and Photocatalytic Properties of Core–Shell Reversal Nanocomposites of Titanium-Doped Strontium Ferrite and Silica. J. Mater. Res. 2023, 38, 1019–1034. [Google Scholar] [CrossRef]
- Redfield, R.R. Antibiotic Resistance Threats in the United States. (U.S. Centers for Disease Control and Prevention). Am. Fam. Physician 2014, 89, 938–941. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. London: Review on Antimicrobial Resistance; Government of United Kingdom: London, UK, 2016; pp. 1–84. [Google Scholar]
- Chen, W.-J.; Tsai, P.-J.; Chen, Y.-C. Functional Fe3O4/TiO2 Core/Shell Magnetic Nanoparticles as Photokilling Agents for Pathogenic Bacteria. Small 2008, 4, 485–491. [Google Scholar] [CrossRef]
- Cui, B.; Peng, H.; Xia, H.; Guo, X.; Guo, H. Magnetically Recoverable Core–Shell Nanocomposites γ-Fe2O3@SiO2@TiO2–Ag with Enhanced Photocatalytic Activity and Antibacterial Activity. Separ. Purif. Technol. 2013, 103, 251–257. [Google Scholar] [CrossRef]
- Yang, J.; Gao, G.; Zhang, X.; Ma, Y.-H.; Chen, X.; Wu, F.-G. One-Step Synthesis of Carbon Dots with Bacterial Contact-Enhanced Fluorescence Emission: Fast Gram-Type Identification and Selective Gram-Positive Bacterial Inactivation. Carbon 2019, 146, 827–839. [Google Scholar] [CrossRef]
- Moradlou, O.; Rabiei, Z.; Delavari, N. Antibacterial Effects of Carbon Quantum Dots@hematite Nanostructures Deposited on Titanium against Gram-Positive and Gram-Negative Bacteria. J. Photochem. Photobiol. A Chem. 2019, 379, 144–149. [Google Scholar] [CrossRef]
- Kooti, M.; Gharineh, S.; Mehrkhah, M.; Shaker, A.; Motamedi, H. Preparation and Antibacterial Activity of CoFe2O4/SiO2/Ag Composite Impregnated with Streptomycin. Chem. Eng. J. 2015, 259, 34–42. [Google Scholar] [CrossRef]

















| S.No. | Ferrites | Preparation Method | Size (nm) | Bandgap (eV) | Crystal Structure | Degradation (%) | References |
|---|---|---|---|---|---|---|---|
| 1. | Fe2O3 | Coprecipitation | 35 | - | cubic | 77 (Congo red) | [140] |
| 2. | Codoped MgFe2O4 | Hydrothermal | 52 | 1.92 | cubic | 96.8 (Rhodamine B) | [142] |
| 3. | Doped MgFe2O4 | Hydrothermal | 96 | - | cubic | 97.8 (Rhodamine B) | [143] |
| 4. | CuFe2O4 | Sol–gel | 15 | - | cubic | 100 (Sulfanilamide) | [144] |
| 5. | MnFe2O4 | Sol–gel | 281.1 | 2.8 | cubic | 90.6 (Norfloxacin) | [145] |
| 6. | NiFe2O4 | Coprecipitation | 155–185 | 1.56 | cubic | 98.5 (Methylene blue) | [146] |
| 7. | CuFe2O4 | Solvothermal | 150 | - | cubic | 95 (Phenolic compound) | [147] |
| S. No. | Nanocomposites of Ferrites with TiO2 | Bandgap (eV) | References |
|---|---|---|---|
| 1. | TiO2 | 3.2 | [35] |
| 2. | CoFe2O4/TiO2 | 2.8 | [66] |
| 3. | ZnFe2O4-TiO2 | 2.3 | [74] |
| 4. | TiO2/ZnFe2O4 | 1.92 | [75] |
| 5. | TiO2-SrFe12O19 | 2.26 | [78] |
| 6. | ZnFe2O4-TiO2 | 1.9 | [79] |
| S.No. | Photocatalyst | Pollutant | Degradation (%) | Time (min) | Light Source | References |
|---|---|---|---|---|---|---|
| 1. | CoFe2O4 -CQD | Acid black 24, acid brown 14, acid red 1 | 95, 90, 65 | 60, 90, 120 | UV | [41] |
| 2. | CoFe2O4-TiO2 | Reactive red 120 | 98.89 | 360 | UV | [85] |
| 3. | TiO2/SrFe12O19 | 2,4-dichlorophenoxyacetic acid | 100 | 240 | sunlight | [87] |
| 4. | CoFe2O4/SiO2 | Methylene blue | 80.6 | 120 | UV | [108] |
| 5. | CQD/BiFeO3 | Rhodamine B | - | 60 | Visible | [129] |
| 6.. | Ni/Co-Fe3O4/TiO2 | Amlodipine drug | 92.49 | 90 | UV | [198] |
| 7. | Fe3O4/TiO2: Eu | Rhodamine B | 85.3 | 180 | Visible | [199] |
| 8. | Fe3O4/TiO2 | Methylene blue | 81 | 6 | UV | [201] |
| 9. | TiO2@CuFe2O4 | 2,4-dichlorophenoxyacetic acid | 69.7 | 60 | UV | [202] |
| 10. | NiFe2O4@TiO2/Pt | Methyl orange, acid brown | 45, 65 | 90 | UV | [203] |
| 11. | SrFe12O19/SiO2/TiO2 | Methylene blue | 80 | 180 | UV | [205] |
| 12. | TiO2/SiO2/Ni–Cu–Zn ferrite | Methylene blue | 83.9 | 360 | Visible | [206] |
| 13. | Fe2O3/SiO2 | Methylene blue and congo red | 88, 88 | 180, 240 | Visible | [208] |
| 14. | Sr1−xTixFe2O4+δ | p-nitrophenol, pendimethalin, martius yellow | 91.3, 94.4, 87.2 | 120 | Visible | [209] |
| 15. | Mg1−xTixFe2O4+δ | Rhodamine-B | 98 | 120 | Visible | [210] |
| 16. | Sr0.4Ti0.6Fe2O4.6@SiO2 | pendimethalin | 96 | 120 | Visible | [211] |
| 17. | γ-Fe2O3@SiO2@TiO2-Ag | Methyl orange | 84 | 60 | UV | [215] |
| S. No. | Photocatalyst | Microbes | Degradation | Light Source | References |
|---|---|---|---|---|---|
| 1. | ZnFe2O4/Ag-TiO2 | S. aureus, E. coli | Zone of inhibition 15 ± 0.2, 12 ± 0.3 mm respectively | Dark | [92] |
| 2. | Ig-G-Fe3O4/TiO2 | Staphylococcus saprophyticus, Streptococcus pyogenes, S. aureus | 79.15%, 82.87% 82.40% | - | [214] |
| 3. | γ-Fe2O3@SiO2@TiO2-Ag | E. coli | 75%, 97% | Dark, visible | [215] |
| 4. | Ti/CQD@hematite | S. aureus, E.coli | 70%, 20% 80%, 35% | Dark, Visible | [217] |
| 5. | CoFe2O4/SiO2/Ag | S. aureus, Bacillus subtills, E. coli, Pseudomonas aeruginosa | Diameter of inhibition zone 20, 21, 17, 18 mm respectively | - | [218] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, A.; Kaur, M.; Singh, V.; Vyas, P. Nanocomposites of Ferrites with TiO2, SiO2 and Carbon Quantum Dots as Photocatalysts for Degradation of Organic Pollutants and Microbes. Magnetochemistry 2023, 9, 127. https://doi.org/10.3390/magnetochemistry9050127
Kaur A, Kaur M, Singh V, Vyas P. Nanocomposites of Ferrites with TiO2, SiO2 and Carbon Quantum Dots as Photocatalysts for Degradation of Organic Pollutants and Microbes. Magnetochemistry. 2023; 9(5):127. https://doi.org/10.3390/magnetochemistry9050127
Chicago/Turabian StyleKaur, Ajaypal, Manpreet Kaur, Vasundhara Singh, and Pratibha Vyas. 2023. "Nanocomposites of Ferrites with TiO2, SiO2 and Carbon Quantum Dots as Photocatalysts for Degradation of Organic Pollutants and Microbes" Magnetochemistry 9, no. 5: 127. https://doi.org/10.3390/magnetochemistry9050127