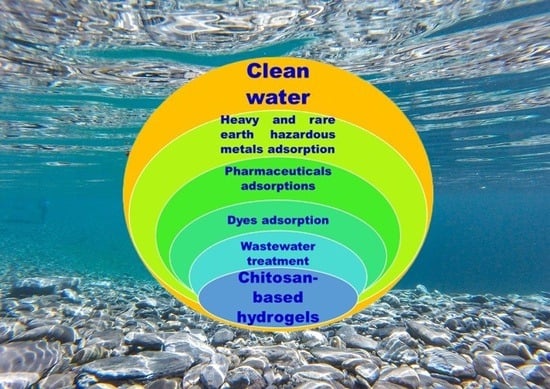
Chitosan Hydrogels for Water Purification Applications
Abstract
:1. Introduction
2. Adsorption Mechanisms of Chitosan-Based Hydrogels
3. Categories of Chitosan-Based Hydrogels for Water Purification Applications
3.1. CS-Based Composite Hydrogels
3.1.1. Composite Hydrogels Based on CS for Removing Dyes from Water
3.1.2. CS-Based Composite Hydrogels for Pharmaceuticals Adsorption
3.1.3. CS-Based Composite Hydrogels as Biosorbent of Heavy and Rare Earth Hazardous Metals
3.2. CS-Based Beads Hydrogels
3.3. CS Graft Copolymeric-Based Hydrogels
4. Different Composite Hydrogels Used for Water Purification
5. CS-Based Hydrogels Used in Various Other Applications
6. Conclusions and Prospective Studies
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bashir, I.; Lone, F.A.; Bhat, R.A.; Mir, S.A.; Dar, Z.A.; Dar, S.A. Concerns and Threats of Contamination on Aquatic Ecosystems. In Bioremediation and Biotechnology; Springer: Cham, Switzerland, 2020; Volume 27, pp. 1–26. [Google Scholar] [CrossRef]
- Chowdhary, P.; Bharagava, R.N.; Mishra, S.; Khan, N. Role of Industries in Water Scarcity and its Adverse Effects on Environment and Human Health. In Environmental Concerns and Sustainable Development; Springer: Berlin/Heidelberg, Germany, 2020; pp. 235–256. [Google Scholar] [CrossRef]
- Hampel, M.; Blasco, J.; Segner, H. Molecular and cellular effects of contamination in aquatic ecosystems. Environ. Sci. Pollut. Res. 2015, 22, 17261–17266. [Google Scholar] [CrossRef]
- Water and Sustainable Development from Vision to Action. Available online: https://www.un.org/waterforlifedecade/pdf/WaterandSD_Vision_to_Action-2.pdf (accessed on 3 July 2023).
- FAO. The State of the World’s Land and Water Resources for Food and Agriculture—Systems at breaking point; Main report; FAO: Rome, Italy, 2022; Available online: https://www.fao.org/3/cb9910en/cb9910en.pdf (accessed on 3 July 2023).
- The United Nations World Water Development Report 2022: Groundwater: Making the Invisible Visible; Executive Summary. Available online: https://unesdoc.unesco.org/ark:/48223/pf0000380726 (accessed on 3 August 2023).
- The United Nations World Water Development Report 2022: Groundwater: Making the Invisible Visible. Available online: https://unesdoc.unesco.org/ark:/48223/pf0000380721 (accessed on 3 July 2023).
- Ciccarelli, D.; Braddock, D.C.; Surman, A.J.; Vergara Arenas, B.I.; Salal, T.; Marczylo, T.; Vineis, P.; Barron, L.P. Enhanced selectivity for acidic contaminants in drinking water: From suspect screening to toxicity prediction. J. Hazard. Mater. 2023, 448, 130906. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Walker, G.W.; Muir, D.C.; Nagatani-Yoshida, K. Toward a global understanding of chemical pollution: A first comprehensive analysis of national and regional chemical inventories. Environ. Sci. Technol. 2020, 54, 2575–2584. [Google Scholar] [CrossRef] [PubMed]
- Zaldivar-Díaz, J.M.; Martínez-Miranda, V.; Castillo-Suárez, L.A.; Linares-Hernández, I.; Solache Ríos, M.J.; Alcántara-Valladolid, A.E. Synergistic electrocoagulation–precipitation process using magnesium electrodes for denim wastewater treatment: Bifunctional support electrolyte effect. J. Water Process Eng. 2023, 51, 103369. [Google Scholar] [CrossRef]
- Deng, L.; Dhar, B.R. Phosphorus recovery from wastewater via calcium phosphate precipitation: A critical review of methods, progress, and insights. Chemosphere 2023, 330, 138685. [Google Scholar] [CrossRef]
- Chai, T.; Zhang, Y.; Tan, Y.; Li, Z.; Wei, H.; Sun, S.; Jin, H.; Mu, Z.; Ma, L. Life cycle assessment of high concentration organic wastewater treatment by catalytic wet air oxidation. Chin. J. Chem. Eng. 2023, 56, 80–88. [Google Scholar] [CrossRef]
- Rikabi, A.A.K.K.; Cuciureanu, A.; Chelu, M.; Miron, A.R.; Orbeci, C.; Popa, A.G.; Craciun, M.E. Proteins ultrafiltration using polysulfone-polyaniline and polysulfone-magnetite nanoparticles composite membranes. Rev. Chim. 2015, 66, 1093–1099. [Google Scholar]
- Diaconu, I.; Aboul-Enein, H.Y.; Bunaciu, A.A.; Ruse, E.; Mirea, C.; Nechifor, G. Selective separation of acetaminophene from pharmaceutical formulations through membrane techniques. Rev. Roum. Chim. 2015, 60, 521–525. [Google Scholar]
- Rikabi, A.A.K.K.; Chelu Balaban, M.; Mariana; Harabor, I.; Albu, P.C.; Segarceanu, M.; Nechifor, G. Iono-molecular Separation with Composite Membranes I. Preparation and characterization of membranes with polysulfone matrix. Rev. Chim. 2016, 67, 1658–1665. [Google Scholar]
- Ramirez Arenas, L.; Le Coustumer, P.; Ramseier Gentile, S.; Zimmermann, S.; Stoll, S. Removal efficiency and adsorption mechanisms of CeO2 nanoparticles onto granular activated carbon used in drinking water treatment plants. Sci. Total Environ. 2023, 856, 159261. [Google Scholar] [CrossRef]
- Schumann, P.; Muschket, M.; Dittmann, D.; Rabe, L.; Reemtsma, T.; Jekel, M.; Ruhl, A.S. Is adsorption onto activated carbon a feasible drinking water treatment option for persistent and mobile substances? Water Res. 2023, 235, 119861. [Google Scholar] [CrossRef] [PubMed]
- Sim, S.I.; Teow, Y.H. Integrated Membrane-adsorption system as a sustainable development approach for semiconductor-industry wastewater treatment. Mater. Today Proc. 2023; in press. [Google Scholar] [CrossRef]
- Sillanpää, M.; Shestakova, M. Chapter 3—Emerging and Combined Electrochemical Methods. In Electrochemical Water Treatment Methods; Sillanpää, M., Shestakova, M., Eds.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 131–225. [Google Scholar] [CrossRef]
- Swanckaert, B.; Loccufier, E.; Geltmeyer, J.; Rabaey, K.; De Buysser, K.; Bonin, L.; De Clerck, K. Sulfonated silica-based cation-exchange nanofiber membranes with superior self-cleaning abilities for electrochemical water treatment applications. Sep. Purif. Technol. 2023, 309, 123001. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Rodrigo, M.A.; Sirés, I.; Scialdone, O. A critical review on latest innovations and future challenges of electrochemical technology for the abatement of organics in water. Appl. Catal. B Environ. 2023, 328, 122430. [Google Scholar] [CrossRef]
- Alrehaili, O.; Fajardo, A.S.; Garcia-Segura, S.; Westerhoff, P. Microfluidic Flow-By reactors minimize energy requirements of electrochemical water treatment without adding supporting electrolytes. Sep. Purif. Technol. 2023, 310, 123123. [Google Scholar] [CrossRef]
- Purnima, M.; Paul, T.; Pakshirajan, K.; Pugazhenthi, G. Onshore oilfield produced water treatment by hybrid microfiltration-biological process using kaolin based ceramic membrane and oleaginous Rhodococcus opacus. Chem. Eng. J. 2023, 453 Pt 2, 139850. [Google Scholar] [CrossRef]
- Dos Reis Ferreira, G.M.; Pires, J.F.; Ribeiro, L.S.; Carlier, J.D.; Costa, M.C.; Schwan, R.F.; Silva, C.F. Impact of lead (Pb2+) on the growth and biological activity of Serratia marcescens selected for wastewater treatment and identification of its zntR gene—A metal efflux regulator. World J. Microbiol. Biotechnol. 2023, 39, 91. [Google Scholar] [CrossRef] [PubMed]
- Verovšek, T.; Šuštarič, A.; Laimou-Geraniou, M.; Krizman-Matasic, I.; Prosen, H.; Eleršek, T.; Kramarič Zidar, V.; Mislej, V.; Mišmaš, B.; Stražar, M.; et al. Removal of residues of psychoactive substances during wastewater treatment, their occurrence in receiving river waters and environmental risk assessment. Sci. Total Environ. 2023, 866, 161257. [Google Scholar] [CrossRef]
- Milanović, Ž.; Dimić, D.; Klein, E.; Biela, M.; Lukeš, V.; Žižić, M.; Avdović, E.; Bešlo, D.; Vojinović, R.; Dimitrić Marković, J.; et al. Degradation Mechanisms of 4,7-Dihydroxycoumarin Derivatives in Advanced Oxidation Processes: Experimental and Kinetic DFT Study. Int. J. Environ. Res. Public Health 2023, 20, 2046. [Google Scholar] [CrossRef]
- Marcinowski, P.; Bury, D.; Krupa, M.; Ścieżyńska, D.; Prabhu, P.; Bogacki, J. Magnetite and Hematite in Advanced Oxidation Processes Application for Cosmetic Wastewater Treatment. Processes 2020, 8, 1343. [Google Scholar] [CrossRef]
- Manna, M.; Sen, S. Advanced oxidation process: A sustainable technology for treating refractory organic compounds present in industrial wastewater. Environ. Sci. Pollut. Res. 2023, 30, 25477–25505. [Google Scholar] [CrossRef] [PubMed]
- Rayaroth, M.P.; Boczkaj, G.; Aubry, O.; Aravind, U.K.; Aravindakumar, C.T. Advanced Oxidation Processes for Degradation of Water Pollutants—Ambivalent Impact of Carbonate Species: A Review. Water 2023, 15, 1615. [Google Scholar] [CrossRef]
- Rodríguez-Chueca, J.; Carbajo, J.; García-Muñoz, P. Intensification of Photo-Assisted Advanced Oxidation Processes for Water Treatment: A Critical Review. Catalysts 2023, 13, 401. [Google Scholar] [CrossRef]
- Agabo-García, C.; Repetto, G.; Albqmi, M.; Hodaifa, G. Evaluation of the olive mill wastewater treatment based on advanced oxidation processes (AOPs), flocculation, and filtration. J. Environ. Chem. Eng. 2023, 11, 109789. [Google Scholar] [CrossRef]
- Saddique, Z.; Imran, M.; Javaid, A.; Latif, S.; Hussain, N.; Kowal, P.; Boczkaj, G. Band engineering of BiOBr based materials for photocatalytic wastewater treatment via advanced oxidation processes (AOPs)—A review. Water Resour. Ind. 2023, 29, 100211. [Google Scholar] [CrossRef]
- Ning, K.; Zheng, S.; He, Y.; Hu, Y.; Hao, S.; Cui, Q.; Chen, H. Removal of fluoride from aqueous solutions through Fe(III) modified water treatment residues. J. Clean. Prod. 2023, 382, 135374. [Google Scholar] [CrossRef]
- Senanu, L.D.; Kranjac-Berisavljevic, G.; Cobbina, S.J. The use of local materials to remove heavy metals for household-scale drinking water treatment: A review. Environ. Technol. Innov. 2023, 29, 103005. [Google Scholar] [CrossRef]
- Otunola, B.O.; Aghoghovwia, M.P.; Thwala, M.; Gómez-Arias, A.; Jordaan, R.; Hernandez, J.C.; Ololade, O.O. Improving capacity for phytore-mediation of Vetiver grass and Indian mustard in heavy metal (Al and Mn) contaminated water through the application of clay minerals. Environ. Sci. Pollut. Res. 2023, 30, 53577–53588. [Google Scholar] [CrossRef]
- Riegel, M.; Haist-Gulde, B.; Sacher, F. Sorptive removal of short-chain perfluoroalkyl substances (PFAS) during drinking water treatment using activated carbon and anion exchanger. Environ. Sci. Eur. 2023, 35, 12. [Google Scholar] [CrossRef]
- Hamdan, M.A.; Sublaban, E.T.; Al-Asfar, J.J.; Banisaid, M.A. Wastewater treatment using activated carbon produced from oil shale. J. Ecol. Eng. 2023, 24, 131–139. [Google Scholar] [CrossRef]
- Saleh, T.S.; Badawi, A.K.; Salama, R.S.; Mostafa, M.M.M. Design and Development of Novel Composites Containing Nickel Ferrites Supported on Activated Carbon Derived from Agricultural Wastes and Its Application in Water Remediation. Materials 2023, 16, 2170. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, S.; Yahya, E.B.; Abdul Khalil, H.P.S.; Marwan, M.; Albadn, Y.M. Recent Advances in Carbon and Activated Carbon Nanostructured Aerogels Prepared from Agricultural Wastes for Wastewater Treatment Applications. Agriculture 2023, 13, 208. [Google Scholar] [CrossRef]
- Abd El-Ghany, N.A.; Elella, M.H.A.; Abdallah, H.M.; Mostafa, M.S.; Samy, M. Recent Advances in Various Starch Formulation for Wastewater Purification via Adsorption Technique: A Review. J. Polym. Environ. 2023, 31, 2792–2825. [Google Scholar] [CrossRef]
- Tran, T.V.; Nguyen, D.T.C.; Nguyen, T.T.T.; Nguyen, D.H.; Alhassan, M.; Jalil, A.A.; Nabgan, W.; Lee, T. A critical review on pineapple (Ananas comosus) wastes for water treatment, challenges and future prospects towards circular economy. Sci. Total Environ. 2023, 856, 158817. [Google Scholar] [CrossRef]
- Romal, J.R.A.; Ong, S.K. Marine polysaccharide-based hydrogels for critical materials selective removal and recovery: A review. Coord. Chem. Rev. 2023, 482, 215054. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Taghizadeh, M.; Taghizadeh, A. Mesoporous activated carbons of low-cost agricultural bio-wastes with high adsorption capacity: Preparation and artificial neural network modelling of dye removal from single and multicomponent (binary and ternary) systems. J. Mol. Liq. 2018, 269, 217–228. [Google Scholar] [CrossRef]
- Petrescu, S.; Avramescu, S.; Musuc, A.M.; Neatu, F.; Florea, M.; Ionita, P. Crown-ether functionalized graphene oxide for metal ions sequestration. Mater. Res. Bull. 2020, 122, 110643. [Google Scholar] [CrossRef]
- Patrinoiu, G.; Calderon-Moreno, J.M.; Birjega, R.; Culita, D.C.; Somacescu, S.; Musuc, A.M.; Spataru, T.; Carp, O. Sustainable one-pot integration of ZnO nanoparticles into carbon spheres: Manipulation of the morphological, optical and electrochemical properties. Phys. Chem. Chem. Phys. 2016, 18, 30794–30807. [Google Scholar] [CrossRef]
- Ahmad, K.; Nazir, M.A.; Qureshi, A.K.; Hussain, E.; Najam, T.; Javed, M.S.; Shah, S.S.A.; Tufail, M.K.; Hussain, S.; Khan, N.A.; et al. Engineering of Zirconium based metal-organic frameworks (Zr-MOFs) as efficient adsorbents. Mater. Sci. Eng. B 2020, 262, 114766. [Google Scholar] [CrossRef]
- Bushra, R.; Mohamad, S.; Alias, Y.; Jin, T.; Ahmad, M. Current approaches and methodologies to explore the perceptive adsorption mechanism of dyes on low-cost agricultural waste: A review. Microporous Mesoporous Mater. 2021, 319, 111040. [Google Scholar] [CrossRef]
- Mittal, H.; Alhassan, S.M.; Ray, S.S. Efficient organic dye removal from wastewater by magnetic carbonaceous adsorbent prepared from corn starch. J. Environ. Chem. Eng. 2018, 6, 7119–7131. [Google Scholar] [CrossRef]
- Mittal, H.; Morajkar, P.P.; Al Alili, A.; Alhassan, S.M. In-situ synthesis of ZnO nanoparticles using gum arabic based hydrogels as a self-template for effective malachite green dye adsorption. J. Polym. Environ. 2020, 28, 1637–1653. [Google Scholar] [CrossRef]
- Makhado, E.; Pandey, S.; Ramontja, J. Microwave assisted synthesis of xanthan gum-cl-poly (acrylic acid) based-reduced graphene oxide hydrogel composite for adsorption of methylene blue and methyl violet from aqueous solution. Int. J. Biol. Macromol. 2018, 119, 255–269. [Google Scholar] [CrossRef]
- Mittal, H.; Parashar, V.; Mishra, S.B.; Mishra, A.K. Fe3O4 MNPs and gum xanthan-based hydrogels nanocomposites for the efficient capture of malachite green from aqueous solution. Chem. Eng. J. 2014, 255, 471–482. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M.; Roy, P.; Bonilla-Petriciolet, A.; Badawi, M.; Ganachari, S.V.; Shetti, N.P.; Aminabhavi, T.M. Polymeric hydrogels-based materials for wastewater treatment. Chemosphere 2023, 331, 138743. [Google Scholar] [CrossRef]
- Flores-Valenzuela, L.E.; González-Fernández, J.V.; Carranza-Oropeza, M.V. Hydrogel Applicability for the Industrial Effluent Treatment: A Systematic Review and Bibliometric Analysis. Polymers 2023, 15, 2417. [Google Scholar] [CrossRef]
- Resende, J.F.; Paulino, I.M.R.; Bergamasco, R.; Vieira, M.F.; Vieira, A.M.S. Hydrogels produced from natural polymers: A review on its use and employment in water treatment. Braz. J. Chem. Eng. 2023, 40, 23–38. [Google Scholar] [CrossRef]
- Xu, X.; Guillomaitre, N.; Christie, K.S.S.; Bay, R.K.N.; Bizmark, N.; Datta, S.S.; Ren, Z.J.; Priestley, R.D. Quick-Release Antifouling Hydrogels for Solar-Driven Water Purification. ACS Cent. Sci. 2023, 8, 177–185. [Google Scholar] [CrossRef]
- Ahmad, S.; Tanweer, M.S.; Mir, T.A.; Alam, M.; Ikram, S.; Sheikh, J.N. Antimicrobial gum based hydrogels as adsorbents for the removal of organic and inorganic pollutants. J. Water Process Eng. 2023, 51, 103377. [Google Scholar] [CrossRef]
- Batuti, M.E.; Sadik, W.; Eldemerdash, A.G.; Hanafy, E.; Fetouh, H.A. New and innovative micro-wave-assisted technology for synthesis of guar gum-grafted acrylamide hydrogel superabsorbent for the removal of acid red 8 dye from industrial wastewater. Polym. Bull. 2023, 80, 4965–4989. [Google Scholar] [CrossRef]
- Alfei, S.; Grasso, F.; Orlandi, V.; Russo, E.; Boggia, R.; Zuccari, G. Cationic Polystyrene-Based Hydrogels as Efficient Adsorbents to Remove Methyl Orange and Fluorescein Dye Pollutants from Industrial Wastewater. Int. J. Mol. Sci. 2023, 24, 2948. [Google Scholar] [CrossRef] [PubMed]
- Baigorria, E.; Souza dos Santos, S.; de Moura, M.R.; Fraceto, L.F. Nanocomposite hydrogels 3D printed for application in water remediation. Mater. Today Chem. 2023, 30, 101559. [Google Scholar] [CrossRef]
- Liu, C.; Gao, B.; Wang, S.; Guo, K.; Shen, X.; Yue, Q.; Xu, X. Synthesis, characterization and flocculation performance of a novel sodium alginate-based flocculant. Carbohydr. Polym. 2020, 248, 116790. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yao, F.; Oderinde, O.; Zhang, Z.; Fu, G. Green synthesis of oriented xanthan gum–graphene oxide hybrid aerogels for water purification. Carbohydr. Polym. 2017, 174, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Son, N.; Kang, M. Synergistic sorption performance of karaya gum crosslink poly(acrylamide-co-acrylonitrile)@metal nanoparticle for organic pollutants. Int. J. Biol. Macromol. 2022, 210, 300–314. [Google Scholar] [CrossRef]
- Mittal, H.; Mishra, S.B.; Mishra, A.K.; Kaith, B.S.; Jindal, R. Flocculation characteristics and biodegradation studies of gum ghatti based hydrogels. Int. J. Biol. Macromol. 2013, 58, 37–46. [Google Scholar] [CrossRef]
- El Sayed, M.M. Production of Polymer Hydrogel Composites and Their Applications. J. Polym. Environ. 2023, 31, 2855–2879. [Google Scholar] [CrossRef]
- Chelu, M.; Musuc, A.M. Polymer Gels: Classification and Recent Developments in Biomedical Applications. Gels 2023, 9, 161. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Krauklis, A.E.; Karl, C.W.; Rocha, I.B.C.M.; Burlakovs, J.; Ozola-Davidane, R.; Gagani, A.I.; Starkova, O. Modelling of Environmental Ageing of Polymers and Polymer Composites-Modular and Multiscale Methods. Polymers 2022, 14, 216. [Google Scholar] [CrossRef]
- Gonçalves, J.O.; Esquerdo, V.M.; Sant’Anna Cadaval, T.R.; de Almeida Pinto, L.A. Chitosan-Based Hydrogels. In Sustainable Agriculture Reviews 36; Crini, G., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Thirupathi, K.; Raorane, C.J.; Ramkumar, V.; Ulagesan, S.; Santhamoorthy, M.; Raj, V.; Krishnakumar, G.S.; Phan, T.T.V.; Kim, S.-C. Update on Chitosan-Based Hydrogels: Preparation, Characterization, and Its Antimicrobial and Antibiofilm Applications. Gels 2023, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Carpa, R.; Farkas, A.; Dobrota, C.; Butiuc-Keul, A. Double-Network Chitosan-Based Hydrogels with Improved Mechanical, Conductive, Antimicrobial, and Antibiofouling Properties. Gels 2023, 9, 278. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hernandez, S.; Salazar, N. Biopolymer-Based Hydrogels for Harvesting Water from Humid Air: A Review. Sustainability 2023, 15, 848. [Google Scholar] [CrossRef]
- Luo, Q.; Ren, T.; Lei, Z.; Huang, Y.; Huang, Y.; Xu, D.; Wan, C.; Guo, X.; Wu, Y. Non-toxic chitosan-based hydrogel with strong adsorption and sensitive detection abilities for tetracycline. Chem. Eng. J. 2022, 427, 131738. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, Y.; Mu, X.; Li, S.; Liu, X.; Lei, Z. Research Progress of Polysaccharide-Based Natural Polymer Hydrogels in Water Purification. Gels 2023, 9, 249. [Google Scholar] [CrossRef]
- Ghiorghita, C.-A.; Dinu, M.V.; Lazar, M.M.; Dragan, E.S. Polysaccharide-Based Composite Hydrogels as Sustainable Materials for Removal of Pollutants from Wastewater. Molecules 2022, 27, 8574. [Google Scholar] [CrossRef]
- Scopus. Available online: https://www.scopus.com (accessed on 3 August 2023).
- Wang, W.; Ni, J.; Chen, L.; Ai, Z.; Zhao, Y.; Song, S. Synthesis of carboxymethyl cellulose-chitosan-montmorillonite nanosheets composite hydrogel for dye effluent remediation. Int. J. Biol. Macromol. 2020, 165, 1–10. [Google Scholar] [CrossRef]
- Wang, W.; Hu, J.; Zhang, R.; Yan, C.; Cui, L.; Zhu, J. A pH-responsive carboxymethyl cellulose/chitosan hydrogel for adsorption and desorption of anionic and cationic dyes. Cellulose 2021, 28, 897–909. [Google Scholar] [CrossRef]
- Kaur, K.; Jindal, R. Comparative study on the behaviour of Chitosan-Gelatin based Hydrogel and nanocomposite ion exchanger synthesized under microwave conditions towards photocatalytic removal of cationic dyes. Carbohydr. Polym. 2019, 207, 398–410. [Google Scholar] [CrossRef]
- Mittal, H.; Al Alili, A.; Morajkar, P.P.; Alhassan, S.M. GO crosslinked hydrogel nanocomposites of chitosan/carboxymethyl cellulose–A versatile adsorbent for the treatment of dyes contaminated wastewater. Int. J. Biol. Macromol. 2021, 167, 1248–1261. [Google Scholar] [CrossRef]
- Chan, K.; Morikawa, K.; Shibata, N.; Zinchenko, A. Adsorptive Removal of Heavy Metal Ions, Organic Dyes, and Pharmaceuticals by DNA-Chitosan Hydrogels. Gels 2021, 6, 112. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Zhou, R.; Wu, P.; Wu, L. Removal of Congo red dye from aqueous solution with hydroxyapatite/chitosan composite. Chem. Eng. J. 2012, 211, 336–342. [Google Scholar] [CrossRef]
- Poznanski, P.; Hameed, A.; Orczyk, W. Chitosan and Chitosan Nanoparticles: Parameters Enhancing Antifungal Activity. Molecules 2023, 28, 2996. [Google Scholar] [CrossRef] [PubMed]
- Piekarska, K.; Sikora, M.; Owczarek, M.; Jóźwik-Pruska, J.; Wiśniewska-Wrona, M. Chitin and Chitosan as Polymers of the Future—Obtaining, Modification, Life Cycle Assessment and Main Directions of Application. Polymers 2023, 15, 793. [Google Scholar] [CrossRef] [PubMed]
- Chopra, H.; Ruhi, G. Eco friendly chitosan: An efficient material for water purification. Pharma Innov. J. 2016, 5, 92–95. [Google Scholar]
- Bhatt, P.; Joshi, S.; Bayram, G.M.U.; Khati, P.; Simsek, H. Developments and application of chitosan-based adsorbents for wastewater treatments. Environ. Res. 2023, 226, 115530. [Google Scholar] [CrossRef]
- Zulfiani, U.; Junaidi, A.; Nareswari, C.; Ali, B.T.I.; Jaafar, J.; Widyanto, A.R.; Saiful; Dharma, H.N.C.; Widiastuti, N. Performance of a membrane fabricated from high-density polyethylene waste for dye separation in water. RSC Adv. 2023, 13, 7789–7797. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abba, A.; Carnevale Miino, M.; Damiani, S. Treatments for color removal from wastewater: State of the art. J. Environ. Manag. 2019, 236, 727–745. [Google Scholar] [CrossRef]
- Hira, N.E.; Lock, S.S.M.; Shoparwe, N.F.; Lock, I.S.M.; Lim, L.G.; Yiin, C.L.; Chan, Y.H.; Hassam, M. Review of Adsorption Studies for Contaminant Removal from Wastewater Using Molecular Simulation. Sustainability 2023, 15, 1510. [Google Scholar] [CrossRef]
- Shi, Y.; Chang, Q.; Zhang, T.; Song, G.; Sun, Y.; Ding, G. A review on selective dye adsorption by different mechanisms. J. Environ. Chem. Eng. 2022, 10, 108639. [Google Scholar] [CrossRef]
- Perju, M.M.; Dragan, E.S. Removal of azo dyes from aqueous solutions using chitosan based composite hydrogels. Ion Exch. Lett. 2010, 3, 7–11. [Google Scholar]
- Han, D.; Zhao, H.; Gao, L.; Qin, Z.; Ma, J.; Han, Y.; Jiao, T. Preparation of carboxymethyl chitosan/phytic acid composite hydrogels for rapid dye adsorption in wastewater treatment. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127355. [Google Scholar] [CrossRef]
- Wan, X.; Rong, Z.; Zhu, K.; Wu, Y. Chitosan-based dual network composite hydrogel for efficient adsorption of methylene blue dye. Int. J. Biol. Macromol. 2022, 222, 725–735. [Google Scholar] [CrossRef]
- Ma, W.; Liu, X.; Lu, H.; He, Q.; Ding, K.; Wang, X.; Wang, W.; Guo, F. Chitosan-based composite hydrogel with a rigid-in-flexible network structure for pH-universal ultra-efficient removal of dye. Int. J. Biol. Macromol. 2023, 241, 124579. [Google Scholar] [CrossRef]
- Jin, Y.; Li, Y.; Du, Q.; Chen, B.; Chen, K.; Zhang, Y.; Wang, M.; Sun, Y.; Zhao, S.; Jing, Z.; et al. Efficient adsorption of Congo red by MIL-53(Fe)/chitosan composite hydrogel spheres. Microporous Mesoporous Mater. 2023, 348, 112404. [Google Scholar] [CrossRef]
- Hingrajiya, R.D.; Patel, M.P. Fe3O4 modified chitosan based co-polymeric magnetic composite hydrogel: Synthesis, characterization and evaluation for the removal of methylene blue from aqueous solutions. Int. J. Biol. Macromol. 2023, 244, 125251. [Google Scholar] [CrossRef]
- Sethi, S.; Thakur, M.S. Synthesis and characterization of nanocomposite chitosan-gelatin hydrogel loaded with ZnO and its application in photocatalytic dye degradation. Mater. Today Proc. 2023, 78, 815–824. [Google Scholar] [CrossRef]
- Chelu, M.; Popa, M.; Calderon Moreno, J.; Leonties, A.R.; Ozon, E.A.; Pandele Cusu, J.; Surdu, V.A.; Aricov, L.; Musuc, A.M. Green Synthesis of Hydrogel-Based Adsorbent Material for the Effective Removal of Diclofenac Sodium from Wastewater. Gels 2023, 9, 454. [Google Scholar] [CrossRef] [PubMed]
- Ranjbari, S.; Tanhaei, B.; Ayati, A.; Khadempir, S.; Sillanpää, M. Efficient tetracycline adsorptive removal using tricaprylmethylammonium chloride conjugated chitosan hydrogel beads: Mechanism, kinetic, isotherms and thermodynamic study. Int. J. Biol. Macromol. 2020, 155, 421–429. [Google Scholar] [CrossRef]
- Afzal, M.Z.; Sun, X.-F.; Liu, J.; Song, C.; Wang, S.-G.; Javed, A. Enhancement of ciprofloxacin sorption on chitosan/biochar hydrogel beads. Sci. Total Environ. 2018, 639, 560–569. [Google Scholar] [CrossRef]
- Mahmoodi, H.; Fattahi, M.; Motevassel, M. Graphene oxide–chitosan hydrogel for adsorptive removal of diclofenac from aqueous solution: Preparation, characterization, kinetic and thermodynamic modelling. RSC Adv. 2021, 11, 36289–36304. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.E.; Nasef, S.M.; Gad, Y.H. Remediation of Astrazon blue and Lerui acid brilliant blue dyes from waste solutions using amphoteric superparamagnetic nanocomposite hydrogels based on chitosan prepared by gamma rays. Carbohydr. Polym. 2022, 1, 119149. [Google Scholar] [CrossRef]
- Tang, J.; Wang, L.; Qin, W.; Qing, Z.; Du, C.; Xiao, S.; Yan, B. High reusability and adsorption capacity of acid washed calcium alginate/chitosan composite hydrogel spheres in the removal of norfloxacin. Chemosphere 2023, 335, 139048. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, I.R.; Chowdhury, S.; Mazumder, M.A.J.; Al-Ahmed, A. Removal of lead ions (Pb2+) from water and wastewater: A review on the low-cost adsorbents. Appl. Water Sci. 2022, 12, 185. [Google Scholar] [CrossRef] [PubMed]
- Vieira, R.M.; Vilela, P.B.; Becegato, V.A.; Paulino, A.T. Chitosan-based hydrogel and chitosan/acid-activated montmorillonite composite hydrogel for the adsorption and removal of Pb+2 and Ni+2 ions accommodated in aqueous solutions. J. Environ. Chem. Eng. 2018, 6, 2713–2723. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, J.; Du, Y.; Zhang, M.; Yang, Z.; Su, J.; Peng, X.; Liu, X.; Sun, G.; Cui, Y. In-situ immobilization of CuMOF on sodium algi-nate/chitosan/cellulose nanofibril composite hydrogel for fast and highly efficient re-moval of Pb2+ from aqueous solutions. J. Solid State Chem. 2023, 322, 123928. [Google Scholar] [CrossRef]
- Georgaki, M.-N.; Charalambous, M.; Kazakis, N.; Talias, M.A.; Georgakis, C.; Papamitsou, T.; Mytiglaki, C. Chromium in Water and Carcinogenic Human Health Risk. Environments 2023, 10, 33. [Google Scholar] [CrossRef]
- Vilela, P.B.; Dalalibera, A.; Duminelli, E.C.; Becegato, V.A.; Paulino, A.T. Adsorption and removal of chromium (VI) contained in aqueous solutions using a chitosan-based hydrogel. Environ. Sci. Pollut. Res. 2019, 26, 28481–28489. [Google Scholar] [CrossRef] [PubMed]
- Wankar, S.; Sapre, N.; Gumathannavar, R.; Jadhav, Y.; Kulkarni, A. Silver-chitosan (Ag-CH) nanocomposite hydrogel for remediation of aqueous medium. Mater. Today Proc. 2022; in press. [Google Scholar] [CrossRef]
- Yang, S.-C.; Liao, Y.; Karthikeyan, K.G.; Pan, X.J. Mesoporous cellulose-chitosan composite hydrogel fabricated via the co-dissolution-regeneration process as biosorbent of heavy metals. Environ. Pollut. 2021, 286, 117324. [Google Scholar] [CrossRef]
- Godiya, C.B.; Revadekar, C.; Kim, J.; Park, B.J. Amine-bilayer-functionalized cellulose-chitosan composite hydrogel for the efficient uptake of hazardous metal cations and catalysis in polluted water. J. Hazard. Mater. 2022, 436, 129112. [Google Scholar] [CrossRef]
- Li, C.; Duan, L.; Cheng, X. Facile method to synthesize fluorescent chitosan hydrogels for selective detection and adsorption of Hg2+/Hg. Carbohydr. Polym. 2022, 288, 119417. [Google Scholar] [CrossRef]
- Ferreira, D.C.M.; Dos Santos, T.C.; Coimbra, J.S.D.R.; de Oliveira, E.B. Chitosan/carboxymethylcellulose polyelectrolyte complexes (PECs) are an effective material for dye and heavy metal adsorption from water. Carbohydr. Polym. 2023, 315, 120977. [Google Scholar] [CrossRef]
- Abdelmonem, I.M.; Elsharma, E.M.; Emara, A.M. Radiation synthesis of chitosan/poly(acrylamide-co-maleic acid) hydrogel for the removal of 152+154Eu (III) ions. Appl. Radiat. Isot. 2023, 197, 110801. [Google Scholar] [CrossRef] [PubMed]
- Andronescu, E.; Predoi, D.; Neacsu, I.A.; Paduraru, A.V.; Musuc, A.M.; Trusca, R.; Oprea, O.; Tanasa, E.; Vasile, O.R.; Nicoara, A.I.; et al. Photoluminescent Hydroxylapatite: Eu3+ Doping Effect on Biological Behaviour. Nanomaterials 2019, 9, 1187. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, P.; Meenakshi, S. Fabrication of hybrid chitosan encapsulated magnetic-kaolin beads for adsorption of phosphate and nitrate ions from aqueous solutions. Int. J. Biol. Macromol. 2020, 168, 750–759. [Google Scholar] [CrossRef]
- Ma, L.; Zheng, Q. Selective adsorp-tion behavior of ionimprinted magnetic chitosan beads for removal of Cu(II) ions from aqueous solution. Chin. J. Chem. Eng. 2021, 39, 103–111. [Google Scholar] [CrossRef]
- Babakhani, A.; Sartaj, M. Synthesis, characterization, and performance evaluation of ion-imprinted crosslinked chitosan (with sodium tripolyphosphate) for cadmium biosorption. J. Environ. Chem. Eng. 2022, 10, 107147. [Google Scholar] [CrossRef]
- Lan, Z.; Lin, Y.; Yang, C. Lanthanum-iron incorporated chitosan beads for adsorption of phosphate and cadmium from aqueous solutions. Chem. Eng. J. 2022, 448, 137519. [Google Scholar] [CrossRef]
- Mei, J.; Zhang, H.; Li, Z.; Ou, H. A novel crosslin-ked chitosan oligosaccharide hydrogel for total adsorption of Cr(VI). Carbohydr. Polym. 2019, 224, 115154. [Google Scholar] [CrossRef]
- Pavithra, S.; Thandapani, G.; Sugashini, S.; Sudha, P.N.; Alkhamis, H.H.; Alrefaei, A.F.; Almutairi, M.H. Batch adsorption studies on surface tailored chitosan/orange peel hydrogel composite for the removal of Cr (VI) and Cu (II) ions from synthetic wastewater. Chemosphere 2021, 271, 129415. [Google Scholar] [CrossRef]
- Tang, S.; Yang, J.; Lin, L.; Peng, K.; Chen, Y.; Jin, S.; Yao, W. Construction of physically cross-linked chitosan/sodium alginate/calcium ion double-network hydrogel and its application to heavy metal ions removal. Chem. Eng. J. 2020, 393, 124728. [Google Scholar] [CrossRef]
- Chen, X.; Song, Z.; Yuan, B.; Li, X.; Li, S.; Thang Nguyen, T.; Guo, M.; Guo, Z. Fluorescent carbon dots crosslinked cellulose Nanofibril/Chitosan interpenetrating hydrogel system for sensitive detection and efficient adsorption of Cu(II) and Cr(VI). Chem. Eng. J. 2022, 430, 133154. [Google Scholar] [CrossRef]
- Lin, Z.; Yang, Y.; Liang, Z.; Zeng, L.; Zhang, A. Preparation of Chitosan/Calcium Alginate/Bentonite Composite Hydrogel and Its Heavy Metal Ions Adsorption Properties. Polymers 2021, 13, 1891. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Khan, S.; Garg, U.; Luqman, M.; Bhagwath, S.S.; Azim, Y. Chitosan-based hydrogel system for efficient removal of Cu[II] and sustainable utilization of spent adsorbent as a catalyst for environmental applications. Int. J. Biol. Macromol. 2023, 247, 125805. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yan, Y.; Zhang, Q.; Zhang, Z.; Huang, L.; Zhang, J.; Xiong, Y.; Tan, S. Adsorption of Cd(2+) and Ni(2+) from Aqueous Single-Metal Solutions on Graphene Oxide-Chitosan-Poly(vinyl alcohol) Hydrogels. Langmuir 2019, 35, 4481–4490. [Google Scholar] [CrossRef]
- Kanungo, S.; Gupta, N.; Rawat, R.; Jain, B.; Solanki, A.; Panday, A.; Das, P.; Ganguly, S. Doped Carbon Quantum Dots Reinforced Hydrogels for Sustained Delivery of Molecular Cargo. J. Funct. Biomater. 2023, 14, 166. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huang, Z.; Luo, S.-Y.; Zong, M.-H.; Lou, W.-Y. Multi-functional magnetic hydrogels based on Millettia speciosa Champ residue cellulose and Chitosan: Highly efficient and reusable adsorbent for Congo red and Cu2+ removal. Chem. Eng. J. 2021, 423, 130198. [Google Scholar] [CrossRef]
- Jamali, M.; Akbari, A. Facile fabrication of magnetic chitosan hydrogel beads and modified by interfacial polymerization method and study of adsorption of cationic/anionic dyes from aqueous solution. J. Environ. Chem. Eng. 2021, 9, 105175. [Google Scholar] [CrossRef]
- Mustafa, F.H.A.; Gad ElRab, E.K.M.; Kamel, R.M.; Elshaarawy, R.F.M. Cost-effective removal of toxic methylene blue dye from textile effluents by new integrated crosslinked chitosan/aspartic acid hydrogels. Int. J. Biol. Macromol. 2023, 248, 125986. [Google Scholar] [CrossRef]
- Kurczewska, J. Chitosan-montmorillonite hydrogel beads for effective dye adsorption. J. Water Process Eng. 2022, 48, 102928. [Google Scholar] [CrossRef]
- Pérez-Calderón, J.; Victoria Santos, M.V.; Zaritzky, N. Synthesis, characterization and application of cross-linked chitosan/oxalic acid hydrogels to improve azo dye (Reactive Red 195) adsorption. React. Funct. Polym. 2020, 155, 104699. [Google Scholar] [CrossRef]
- da Silva, R.C.; de Aguiar, S.B.; da Cunha, P.L.R.; de Paula, R.C.M.; Feitosa, J.P.A. Effect of microwave on the synthesis of polyacrylamide-g-chitosan gel for azo dye removal. React. Funct. Polym. 2020, 148, 104491. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Phuong, V.N.; Van, T.N.; Thi, P.N.; Lan, P.D.T.; Pham, H.T.; Cao, H.T. Low-cost hydrogel derived from agro-waste for veterinary antibiotic removal: Optimization, kinetics, and toxicity evaluation. Environ. Technol. Innov. 2020, 20, 101098. [Google Scholar] [CrossRef]
- Darvishi, R.; Moghadas, H.; Moshkriz, A. Oxidized gum arabic cross-linked pectin/O-carboxymethyl chitosan: An antibiotic adsorbent hydrogel. Korean J. Chem. Eng. 2022, 39, 1350–1360. [Google Scholar] [CrossRef]
- Balakrishnan, A.; S Appunni, S.; Chinthala, M.; Jacob, M.M.; Viet, N.; Vo, D.; Reddym, S.S.; Kunne, E.S. Chitosan based beads as sustainable adsorbents for wastewater remediation: A review. Environ. Chem. Lett. 2023, 21, 1881–1905. [Google Scholar] [CrossRef]
- Saber-Samandari, S.; Saber-Samandari, S.; Nezafati, N.; Yahya, K. Efficient removal of lead (II) ions and methylene blue from aqueous solution using chitosan/Fe-hydroxyapatite nanocomposite beads. J. Environ. Manag. 2014, 146, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Iqbal, J.; Zhu, Y.; Zhang, P.; Chen, W.; Bhatnagar, A.; Du, Y. Chitosan/Ag-hydroxyapatite nanocomposite beads as a potential adsorbent for the efficient removal of toxic aquatic pollutants. Int. J. Biol. Macromol. 2018, 120, 1752–1759. [Google Scholar] [CrossRef]
- Dandil, S.; Akin Sahbaz, D.; Acikgoz, C. Adsorption of Cu(II) ions onto crosslinked chitosan/Waste Active Sludge Char (WASC) beads: Kinetic, equilibrium, and thermodynamic study. Int. J. Biol. Macromol. 2019, 136, 668–675. [Google Scholar] [CrossRef]
- Wang, G.; Yue, X.; Zhang, S.; Geng, Q.; Zheng, J.; Xu, X.; Li, T.; Pu, Y.; Li, Y.; Jia, Y.; et al. La(III) loaded Fe(III) cross–linked chitosan composites for efficient removal of phosphate from wastewater: Performance and mechanisms. J. Cleaner Prod. 2022, 379, 134833. [Google Scholar] [CrossRef]
- Koh, K.Y.; Chen, Z.; Zhang, S.; Chen, J.P. Cost-efective phosphorus removal from aqueous solution by a chitosan/lanthanum hydrogel bead: Material development, characterization of uptake process and investigation of mechanisms. Chemosphere 2022, 286, 131458. [Google Scholar] [CrossRef]
- He, W.; Yu, Q.; Wang, N.; Ouyang, X.K. Efficient adsorption of Cu(II) from aqueous solutions by acid-resistant and recyclable ethylenediamine tetraacetic acid-grafted polyvinyl alcohol/chitosan beads. J. Mol. Liq. 2020, 316, 113856. [Google Scholar] [CrossRef]
- Kekes, T.; Tzia, C. Adsorption of indigo carmine on functional chitosan and β-cyclodextrin/chitosan beads: Equilibrium, kinetics and mechanism studies. J. Environ. Manag. 2020, 262, 110372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, H.; Hu, X.; Feng, H.; Xiong, W.; Guo, W.; Zhou, J.; Mosa, A.; Peng, Y. Multicavity triethylenetetra-mine-chitosan/alginate composite beads for enhanced Cr(VI) removal. J. Clean. Prod. 2019, 231, 733–745. [Google Scholar] [CrossRef]
- Ranjbari, S.; Tanhaei, B.; Ayati, A.; Sillanpää, M. Novel Aliquat-336 impregnated chitosan beads for the adsorptive removal of anionic azo dyes. Int. J. Biol. Macromol. 2019, 125, 989–998. [Google Scholar] [CrossRef]
- Vakili, M.; Rafatullah, M.; Ibrahim, M.H.; Ab-dullah, A.Z.; Salamatinia, B.; Gholami, Z. Chitosan hydrogel beads impregnated with hexadecylamine for improved reactive blue adsorption. Carbohydr. Polym. 2016, 137, 139–146. [Google Scholar] [CrossRef]
- Sadjadi, S.; Abedian-Dehaghani, N.; Heydari, A.; Heravi, M.M. Chitosan bead containing metal–organic framework encapsulated heteropolyacid as an efficient catalyst for cascade condensation reaction. Sci. Rep. 2023, 13, 2797. [Google Scholar] [CrossRef] [PubMed]
- Abdelhameed, R.M.; El-Shahat, M.; Abdel-Gawad, H.; Hegazi, B. Efficient phenolic compounds adsorption by immobilization of copper-based metal-organic framework anchored poly-acrylonitrile/chitosan beads. Int. J. Biol. Macromol. 2023, 240, 124498. [Google Scholar] [CrossRef]
- Jansen, C.; Tran-Cong, N.M.; Schlüsener, C.; Schmitz, A.; Proksch, P.; Janiak, C. MOF@chitosan Composites with Potential Antifouling Properties for Open-Environment Applications of Met-al-Organic Frameworks. Solids 2022, 3, 35–54. [Google Scholar] [CrossRef]
- Pan, W.; Wang, J.P.; Sun, X.B.; Wang, X.X.; Jiang, J.; Zhang, Z.G.; Li, P.; Qu, C.H.; Long, Y.Z.; Yu, G.F. Ultra uniform metal−organic framework-5 loading along electrospun chitosan/polyethylene oxide membrane fibers for efficient PM2.5 removal. J. Clean. Prod. 2021, 291, 125270. [Google Scholar] [CrossRef]
- Jamshidifard, S.; Koushkbaghi, S.; Hosseini, S.; Rezaei, S.; Karamipour, A.; Rad, A.J.; Irani, M. Incorporation of UiO-66-NH2 MOF into the PAN/chitosan nanofibers for adsorption and membrane filtration of Pb (II), Cd (II) and Cr (VI) ions from aqueous solutions. J. Hazard. Mater. 2019, 368, 10. [Google Scholar] [CrossRef]
- Elanchezhiyan, S.S.; Karthikeyan, P.; Rathinam, K.; Hasmath Farzana, M.; Park, C.M. Magnetic kaolinite immobilized chitosan beads for the removal of Pb(II) and Cd(II) ions from an aqueous environment. Carbohydr. Polym. 2021, 261, 117892. [Google Scholar] [CrossRef]
- Liu, D.M.; Dong, C.; Xu, B. Preparation of magnetic kaolin embedded chitosan beads for efficient removal of hexavalent chromium from aqueous solution. J. Environ. Chem. Eng. 2021, 9, 105438. [Google Scholar] [CrossRef]
- Mishra, S.; Tripathi, A. Adsorptive removal of Pb(II) via green route using magnetic iron nanoparticle sprinkled graphene oxide chitosan beads in aqueous medium. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100632. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Z.; Peng, Y.; Feng, L.; Li, X.; Zhao, C.; Sarfaraz, K. Novel cationic polymer modified magnetic chitosan beads for efficient adsorption of heavy metals and dyes over a wide pH range. Int. J. Biol. Macromol. 2020, 156, 289–301. [Google Scholar] [CrossRef]
- Tu, S.-L.; Chen, C.-K.; Shi, S.-C.; Yang, J.H.C. Plasma-Induced Graft Polymerization of Polyethylenimine onto Chitosan/Polycaprolactone Composite Membrane for Heavy Metal Pollutants Treatment in Industrial Wastewater. Coatings 2022, 12, 1966. [Google Scholar] [CrossRef]
- Abed, K.; Ahmed, E.; Shehzad, H.; Sharif, A.; Farooqi, Z.H.; Liu, Z.; Zhou, L.; Ouyang, J.; Begum, R.; Irfan, A.; et al. An innovative approach to synthesize graft copolymerized acetylacetone chitosan/surface functionalized alginate/rutile for efficient Ni(II) uptake from aqueous medium. Int. J. Biol. Macromol. 2023, 243, 125327. [Google Scholar] [CrossRef]
- Xiao, L.; Shan, H.; Wu, Y. Chitosan cross-linked and grafted with epichlorohydrin and 2,4-dichlorobenzaldehyde as an efficient adsorbent for removal of Pb(II) ions from aqueous solution. Int. J. Biol. Macromol. 2023, 247, 125503. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Xu, Y.; Zhong, D.; Chang, H.; Mou, J.; Wang, M.H.; Shen, H. Zr4+ and glutaraldehyde cross-linked polyethyleneimine functionalized chitosan composite: Synthesis, characterization, Cr(VI) adsorption performance, mechanism and regeneration. Int. J. Biol. Macromol. 2023, 239, 124266. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Chen, W.; Peng, X.; Cao, Q.; Wang, Q.; Wang, D.; Meng, X.; Yu, G. Biosorption of lead from aqueous solutions by ion-imprinted tetraethylenepentamine modifed chitosan beads. Int. J. Biol. Macromol. 2016, 86, 562–569. [Google Scholar] [CrossRef]
- Malik, S.A.; Dar, A.A.; Banday, J.A. Rheological, morphological and swelling properties of dysprosium-based composite hydrogel beads of alginate and chitosan: A promising material for the effective cationic and anionic dye removal. Colloids Surf. A Physicochem. Eng. Asp. 2023, 663, 131046. [Google Scholar] [CrossRef]
- Khan, M.U.; Al-Asbahi, B.A.; Bibi, S.; Taimur, S.; Nawaz, M.; Yasin, T.; Farooq, W.A.; Ali, Z.; Bibi, I.; Begum, S.; et al. Investigations on amidoxime grafted sepiolite based chitosan organic–inorganic nanohybrid composite beads towards wastewater detoxification. J. King Saud Univ. Sci. 2022, 34, 101689. [Google Scholar] [CrossRef]
- Srivastava, V.; Choubey, A.K. Investigation of adsorption of organic dyes present in wastewater using chitosan beads immobilized with biofabricated CuO nanoparticles. J. Mol. Struct. 2021, 1242, 130749. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, K.; Peng, H.; Gong, Y.; Huang, Y. Imidazolium functionalized polysulfone/DTPA-chitosan composite beads for simultaneous removal of Cr(VI) and Cu(II) from aqueous solutions. Sep. Purif. Technol. 2023, 310, 123145. [Google Scholar] [CrossRef]
- Jeyaseelan, A.; Kumar, I.A.; Viswanathan, N.; Naushad, M. Rationally designed and hierarchically structured functionalized aluminium organic frameworks incorporated chitosan hybrid beads for defluoridation of water. Int. J. Biol. Macromol. 2022, 207, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Sirajudheen, P.; Raja, M.; Karthikeyan, P.; Meenakshi, S. Perceptive removal of toxic azo dyes from water using magnetic Fe3O4 reinforced graphene oxide–carboxymethyl cellulose recyclable composite: Adsorption investigation of parametric studies and their mechanisms. Surf. Interfaces 2020, 21, 100648. [Google Scholar] [CrossRef]
- Tanhaei, B.; Ayati, A.; Iakovleva, E.; Sillanpaa, M. Efficient carbon interlayed magnetic chitosan adsorbent for anionic dye removal: Synthesis, characterization and adsorption study. Int. J. Biol. Macromol. 2020, 164, 3621–3631. [Google Scholar] [CrossRef]
- Abdul Rahman, A.S.; Fizal, A.N.S.; Khalil, N.A.; Ahmad Yahaya, A.N.; Hossain, M.S.; Zulkifli, M. Fabrication and Characterization of Magnetic Cellulose–Chitosan–Alginate Composite Hydrogel Bead Bio-Sorbent. Polymers 2023, 15, 2494. [Google Scholar] [CrossRef]
- Singh, N.; Riyajuddin, S.; Ghosh, K.; Mehta, S.K.; Dan, A. Chitosan-graphene oxide hydrogels with embedded magnetic Iron oxide nanoparticles for dye removal. Appl. Nano Mater. 2019, 2, 7379–7392. [Google Scholar] [CrossRef]
- Khalil, N.A.; Rahman, A.S.A.; Huraira, A.M.A.; Janurin, S.N.D.F.; Fizal, A.N.S.; Ahmad, N.; Zulkifli, M.; Hossain, M.S.; Yahaya, A.N.A. Magnetic chitosan hydrogel beads as adsorbent for copper removal from aqueous solution. Mater. Today Proc. 2023, 74, 499–503. [Google Scholar] [CrossRef]
- Demirezen, D.A.; Yılmaz, D.D.; Yıldız, Y.S. Magnetic chitosan/calcium alginate double-network hydrogel beads: Preparation, adsorption of anionic and cationic surfactants, and reuse in the removal of methylene blue. Int. J. Biol. Macromol. 2023, 239, 124311. [Google Scholar] [CrossRef]
- Qin, L.; Zhao, Y.; Wang, L.; Zhang, L.; Kang, S.; Wang, W.; Zhang, T.; Song, S. Preparation of ion-imprinted montmorillonite nanosheets/chitosan gel beads for selective recovery of Cu(Ⅱ) from wastewater. Chemosphere 2020, 252, 126560. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Dai, Y.; Zhou, L.; Ouyang, J.; Tang, X.; Liu, Z.; Adesina, A.A. Selective biosorption of U(VI) from aqueous solution by ionimprinted honeycomb-like chitosan/kaolin clay composite foams. Int. J. Biol. Macromol. 2022, 206, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Prabaharan, M.; Reis, R.L.; Mano, J.F. Graft copolymerized chitosan—Present status and applications. Carbohydr. Polym. 2005, 62, 142–158. [Google Scholar] [CrossRef]
- Gürdağ, G.; Sarmad, S. Cellulose Graft Copolymers: Synthesis, Properties, and Applications. In Polysaccharide Based Graft Copolymers; Kalia, S., Sabaa, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Kalia, S.; Sabaa, M.W.; Kango, S. Polymer Grafting: A Versatile Means to Modify the Polysaccharides. In Polysaccharide Based Graft Copolymers; Kalia, S., Sabaa, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Michalik, R.; Wandzik, I. A Mini-Review on Chitosan-Based Hydrogels with Potential for Sustainable Agricultural Applications. Polymers 2020, 12, 2425. [Google Scholar] [CrossRef] [PubMed]
- Taokaew, S.; Kaewkong, W.; Kriangkrai, W. Recent Development of Functional Chitosan-Based Hydrogels for Pharmaceutical and Biomedical Applications. Gels 2023, 9, 277. [Google Scholar] [CrossRef]
- Hama, R.; Ulziibayar, A.; Reinhardt, J.W.; Watanabe, T.; Kelly, J.; Shinoka, T. Recent Developments in Biopolymer-Based Hydrogels for Tissue Engineering Applications. Biomolecules 2023, 13, 280. [Google Scholar] [CrossRef]
- Mourya, V.K.; Inamdar, N.N. Chitosan-modifications and applications: Opportunities galore. React. Funct. Polym. 2008, 68, 1013–1051. [Google Scholar] [CrossRef]
- Mohammadzadeh Pakdel, P.; Peighambardoust, S.J. Review on recent progress in chitosan-based hydrogels for wastewater treatment application. Carbohydr. Polym. 2018, 201, 264–279. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Z.; Wang, J.; Wang, S. Positively charged aromatic polyamide reverse osmosis membrane with high anti-fouling property prepared by polyethylenimine grafting. Desalination 2015, 365, 398–406. [Google Scholar] [CrossRef]
- Zhang, C.; Ni, C.; Liu, Z.; Wu, G.; Qin, Y. Polyethyleneimine (PEI) and Chitosan (CS) grafted with L-cysteine were used as effective materials for Hg(II) adsorption. Mater. Lett. 2023, 347, 134614. [Google Scholar] [CrossRef]
- Yusof, N.H.; Foo, K.Y.; Wilson, L.D.; Hameed, B.H.; Hussin, M.H.; Sabar, S. Microwave-Assisted Synthesis of Polyethylene-imine Grafted Chitosan Beads for the Adsorption of Acid Red 27. J. Polym. Environ. 2020, 28, 542–552. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Z.; Ouyang, X.K.; Ji, C.; Liu, Y.; Huang, F.; Yang, L. Fabrication of cross-linked chitosan beads grafted by polyethylenimine for efficient adsorption of diclofenac sodium from water. Int. J. Biol. Macromol. 2020, 145, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Esmaeildoost, F.; Shahrousvand, M.; Goudarzi, A.; Bagherieh-Najjar, M.B. Optimization of Xanthan Gum/Poly(acrylic acid)/Cloisite 15A Semi-IPN Hydrogels for Heavy Metals Removal. J. Polym. Environ. 2022, 30, 4271–4286. [Google Scholar] [CrossRef]
- Dragan, E.S.; Apopei Loghin, D.F. Enhanced sorption of methylene blue from aqueous solutions by semi-IPN composite cryogels with anionically modified potato starch entrapped in PAAm matrix. Chem. Eng. J. 2013, 234, 211–222. [Google Scholar] [CrossRef]
- Godiya, C.B.; Liang, M.; Sayed, S.M.; Li, D.; Lu, X. Novel Alginate/Polyethyleneimine Hydrogel Adsorbent for Cascaded Removal and Utilization of Cu2+ and Pb2+ ions. J. Environ. Manag. 2019, 232, 829–841. [Google Scholar] [CrossRef]
- Majhi, D.; Patra, B.N. Polyaniline and Sodium Alginate Nanocomposite: A pH-Responsive Adsorbent for the Removal of Organic Dyes from Water. RSC Adv. 2020, 10, 43904. [Google Scholar] [CrossRef]
- Gohari, R.M.; Safarnia, M.; Koohi, A.D.; Salehi, M.B. Adsorptive Removal of Cationic Dyes by Synthesized Xanthan gum-g p(AMPS-co-AAm) Hydrogel from Aqueous Media: Optimization by RSM-CCD Model. Chem. Eng. Res. Design 2022, 188, 714–728. [Google Scholar] [CrossRef]
- Ilgin, P.; Ozay, H.; Ozay, O. The Efficient Removal of Anionic and Cationic Dyes from Aqueous Media Using Hydroxyethyl Starch-Based Hydrogels. Cellulose 2020, 27, 4787–4802. [Google Scholar] [CrossRef]
- Qi, X.; Zeng, Q.; Tong, X.; Su, T.; Xie, L.; Yuan, K.; Xu, J.; Shen, J. Polydopamine/montmorillonite-embedded pullulan hydrogels as efficient adsorbents for removing crystal violet. J. Hazard. Mater. 2021, 402, 123359. [Google Scholar] [CrossRef]
- Melilli, G.; Yao, J.; Chiappone, A.; Sangermano, M.; Hakkarainen, M. Photocurable “all-lignocellulose” derived hydrogel nanocomposites for adsorption of cationic contaminants. Sustain. Mater. Technol. 2021, 27, e00243. [Google Scholar] [CrossRef]
- Veregue, F.R.; de Lima, H.H.C.; Ribeiro, S.C.; Almeida, M.S.; da Silva, C.T.P.; Guilherme, M.R.; Rinaldi, A.W. MCM-41/chondroitin sulfate hybrid hydrogels with remarkable mechanical properties and superabsorption of methylene blue. Carbohydr. Polym. 2020, 247, 116558. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Liu, Y.; Wang, X.; Yan, H.; Wang, L.; Qu, L.; Kong, D.; Qiao, M.; Wang, L. Injectable hydrogel composed of hydrophobically modified chitosan/oxidized-dextran for wound healing. Mater. Sci. Eng. C 2019, 104, 109930. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhao, L.; Gao, C.; Huang, J.; Li, Q.; Zhang, Z. Highly resilient, biocompatible, and antibacterial carbon nanotube/hydroxybutyl chitosan sponge dressing for rapid and effective hemostasis. J. Mater. Chem. B 2021, 9, 9754–9763. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Li, D.; Dai, K.; Wang, Y.; Song, P.; Li, H.; Tang, P.; Zhang, Z.; Li, Z.; Zhou, Y.; et al. Recent progress of collagen, chitosan, alginate and other hydrogels in skin repair and wound dressing applications. Int. J. Biol. Macromol. 2022, 298, 400–408. [Google Scholar] [CrossRef]
- Thambiliyagodage, C.; Jayanetti, M.; Mendis, A.; Ekanayake, G.; Liyanaarachchi, H.; Vigneswaran, S. Recent Advances in Chitosan-Based Applications—A Review. Materials 2023, 16, 2073. [Google Scholar] [CrossRef]
- Wang, X.; Song, R.; Johnson, M.; Shen, P.; Zhang, N.; Lara-Sáez, I.; Xu, Q.; Wang, W. Chitosan-Based Hydrogels for Infected Wound Treatment. Macromol. Biosci. 2023, e2300094. [Google Scholar] [CrossRef]
- Chelu, M.; Calderon Moreno, J.; Atkinson, I.; Pandele Cusu, J.; Rusu, A.; Bratan, V.; Aricov, L.; Anastasescu, M.; Seciu-Grama, A.-M.; Musuc, A.M. Green synthesis of bioinspired chitosan-ZnO-based polysaccharide gums hydrogels with propolis extract as novel functional natural biomaterials. Int. J. Biol. Macromol. 2022, 211, 410–424. [Google Scholar] [CrossRef]
- Yang, Y.; Feng, G.; Wang, J.; Zhang, R.; Zhong, S.; Wang, J.; Cui, X. Injectable chitosan-based self-healing supramolecular hydrogels with temperature and pH dual-responsivenesses. Int. J. Biol. Macromol. 2023, 227, 1038–1047. [Google Scholar] [CrossRef]
- Hamedi, H.; Moradi, S.; Hudson, S.M.; Tonelli, A.E. Chitosan based hydrogels and their applications for drug delivery in wound dressings: A review. Carbohydr. Polym. 2018, 199, 445–460. [Google Scholar] [CrossRef]
- Maur Rehman Qureshi, M.A.; Arshad, A.; Rasool, A. Graphene oxide reinforced biopolymeric (chitosan) hydrogels for controlled cephradine release. Int. J. Biol. Macromol. 2023, 242, 124948. [Google Scholar] [CrossRef]
- Manna, S.; Seth, A.; Gupta, P.; Nandi, G.; Dutta, R.; Jana, S.; Jana, S. Chitosan Derivatives as Carriers for Drug Delivery and Biomedical Applications. ACS Biomater. Sci. Eng. 2023, 9, 2181–2202. [Google Scholar] [CrossRef]
- Lei, L.; Li, X.; Xiong, T.; Yu, J.; Yu, X.; Song, Z.; Li, X. Covalently Cross-Linked Chitosan Hydrogel Sheet for Topical Ophthalmic Delivery of Levofloxacin. J. Biomed. Nanotechnol. 2018, 14, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.S.; Cho, M.O.; Li, Z.; Nurunnabi, M.; Park, S.Y.; Kang, S.-W.; Huh, K.M. Synthesis and characterization of a new photo-crosslinkable glycol chitosan thermogel for biomedical applications. Carbohydr. Polym. 2016, 144, 59–67. [Google Scholar] [CrossRef]
- Wan, Z.; Dong, Q.; Guo, X.; Bai, X.; Zhang, X.; Zhang, P.; Liu, Y.; Lv, L.; Zhou, Y. A dual-responsive polydopamine-modified hydroxybutyl chitosan hydrogel for sequential regulation of bone regeneration. Carbohydr. Polym. 2022, 297, 120027. [Google Scholar] [CrossRef] [PubMed]
- Mohan, N.; Mohanan, P.V.; Sabareeswaran, A.; Nair, P. Chitosan-hyaluronic acid hydrogel for cartilage repair. Int. J. Biol. Macromol. 2017, 104, 1936–1945. [Google Scholar] [CrossRef]
- Ranjbardamghani, F.; Eslahi, N.; Jahanmardi, R. An injectable chitosan/laponite hydrogel synthesized via hybrid cross-linking system: A smart platform for cartilage regeneration. Polym. Adv. Technol. 2023, 34, 2298–2311. [Google Scholar] [CrossRef]
- Hoveizi, E.; Naddaf, H.; Ahmadianfar, S.; Gutmann, J.L. Encapsulation of human endometrial stem cells in chitosan hydrogel containing titanium oxide nanoparticles for dental pulp repair and tissue regeneration in male Wistar rats. J. Biosci. Bioeng. 2023, 1359, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Leite, Y.K.d.C.; Oliveira, A.C.d.J.; Quelemes, P.V.; Neto, N.M.A.; Carvalho, C.E.S.d.; Soares Rodrigues, H.W.; Alves, M.M.d.M.; Carvalho, F.A.d.A.; Arcanjo, D.D.R.; Silva-Filho, E.C.d.; et al. Novel Scaffold Based on Chitosan Hydrogels/Phthalated Cashew Gum for Supporting Human Dental Pulp Stem Cells. Pharmaceuticals 2023, 16, 266. [Google Scholar] [CrossRef]
- Bhuiyan, M.H.; Clarkson, A.N.; Ali, M.A. Optimization of thermoresponsive chitosan/β-glycerophosphate hydrogels for injectable neural tissue engineering application. Colloids Surf. B Biointerfaces 2023, 224, 113193. [Google Scholar] [CrossRef]
- Neuman, K.E.; Kenny, A.; Shi, L.; Koppes, A.N.; Koppes, R.A. Complex Material Properties of Gel-Amin: A Transparent and Ionically Conductive Hydrogel for Neural Tissue Engineering. Cells Tissues Organs 2023, 212, 45–63. [Google Scholar] [CrossRef]
- Zanon, M.; Cue-López, R.; Martínez-Campos, E.; Bosch, P.; Versace, D.-L.; Hayek, H.; Garino, N.; Fabrizio Pirri, C.; Sangermano, M.; Chiappone, A. Bioderived dyes-mediated vat photopolymerization 3D printing of chitosan hydrogels for tissue engineering. Addit. Manuf. 2023, 69, 103553. [Google Scholar] [CrossRef]
- Kulka, K.; Sionkowska, A. Chitosan Based Materials in Cosmetic Applications: A Review. Molecules 2023, 28, 1817. [Google Scholar] [CrossRef] [PubMed]
- Mistry, P.A.; Konar, M.N.; Latha, S.; Chadha, U.; Bhardwaj, P.; Eticha, T.K. Chitosan superabsorbent biopolymers in sanitary and hygiene applications. Int. J. Polym. Sci. 2023, 2023, 4717905. [Google Scholar] [CrossRef]
- Cerdá-Bernad, D.; Pitterou, I.; Tzani, A.; Detsi, A.; Frutos, M.J. Novel chitosan/alginate hydrogels as carriers of phenolic-enriched extracts from saffron floral by-products using natural deep eutectic solvents as green extraction media. Curr. Res. Food Sci. 2023, 6, 100469. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, M.; Gupta, R.K.; Rani, A. Natural gums and their derivatives based hydrogels: In biomedical, environment, agriculture, and food industry. Crit. Rev. Biotechnol. 2023, 1–27. [Google Scholar] [CrossRef]
- De Oliveira, K.Á.R.; da Conceição, M.L.; de Oliveira, S.P.A.; Lima, M.D.S.; de Sousa Galvão, M.; Madruga, M.S.; Magnani, M.; de Souza, E.L. Post-harvest quality improvements in mango cultivar Tommy Atkins by chitosan coating with Mentha piperita L. essential oil. J. Hortic. Sci. Biotechnol. 2020, 95, 260–272. [Google Scholar] [CrossRef]
- Kumar, N.; Pratibha; Neeraj; Petkoska, A.T.; Al-Hilifi, S.A.; Fawole, O.A. Effect of chitosan–pullulan composite edible coating functionalized with pomegranate peel extract on the shelf life of mango (Mangifera indica). Coatings 2021, 11, 764. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Dong, Q.; Xu, C.; Deng, S.; Kang, Y.; Fan, M.; Li, L. Application of functionalized chitosan in food: A review. Int. J. Biol. Macromol. 2023, 235, 123716. [Google Scholar] [CrossRef]
- Zeng, X.; Jiang, W.; Li, H.; Li, Q.; Kokini, J.L.; Du, Z.; Xi, Y.; Li, J. Interactions of Mesona chinensis Benth polysaccharides with different polysaccharides to fabricate food hydrogels: A review. Food Hydrocoll. 2023, 139, 108556. [Google Scholar] [CrossRef]
- No, H.K.; Meyers, S.P.; Prinyawiwatkul, W.; Xu, Z. Applications of chitosan for improvement of quality and shelf life of foods: A review. J. Food Sci. 2007, 72, 87–100. [Google Scholar] [CrossRef]
- Cano Embuena, A.I.; Chafer Nacher, M.; Chiralt Boix, A.; Molina Pons, M.P.; Borras Llopis, M.; Beltran Martinez, M.C.; Gonzalez Martinez, C. Quality of goats milk cheese as affected by coating with edible chitosan-essential oil films. Int. J. Dairy Technol. 2017, 70, 68–76. [Google Scholar] [CrossRef]
- Maleki, G.; Woltering, E.J.; Mozafari, M.R. Applications of chitosan-based carrier as an encapsulating agent in food industry. Trends Food Sci. Technol. 2022, 120, 88–99. [Google Scholar] [CrossRef]
- Ortiz-Duarte, G.; Eugenia Perez-Cabrera, L.; Artes-Hernandez, F.; Martinez-Hernandez, G.B. Ag-chitosan nanocomposites in edible coatings affect the quality of fresh-cut melon. Postharvest Biol. Technol. 2019, 147, 174–184. [Google Scholar] [CrossRef]
- Jose Valera, M.; Sainz, F.; Mas, A.; Jesus Torija, M. Effect of chitosan and SO2 on viability of acetobacter strains in wine. Int. J. Food Microbiol. 2017, 246, 1–4. [Google Scholar] [CrossRef]
- Román-Doval, R.; Torres-Arellanes, S.P.; Tenorio-Barajas, A.Y.; Gómez-Sánchez, A.; Valencia-Lazcano, A.A. Chitosan: Properties and Its Application in Agriculture in Context of Molecular Weight. Polymers 2023, 15, 2867. [Google Scholar] [CrossRef]
- Ortiz, H.; Benavides Mendoza, A.; Robledo Torres, V.; Cabrera De la Fuente, M. Use of chitosan-PVA hydrogels with copper nanoparticles to improve the growth of grafted watermelon. Molecules 2017, 22, 1031. [Google Scholar]
- Choudhary, R.C.; Kumari, S.; Kumaraswamy, R.; Sharma, G.; Kumar, A.; Budhwar, S.; Pal, A.; Raliya, R.; Biswas, P. Chitosan nanomaterials for smart delivery of bioactive compounds in agriculture. In Nanoscale Engineering in Agricultural Management; Raliya, R., Ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 124–139. [Google Scholar]
- Zhang, M.; Yuan, Y.; Jin, J.; Sun, J.; Tian, X. Polyvinyl alcohol composite hydrogels/epoxidized natural rubber composites (CMCS/PVA/CS-ENR) with core-shell structure as biomass coating material for slow-release nitrogen fertilizer. Prog. Org. Coat. 2023, 183, 107744. [Google Scholar] [CrossRef]
- Ren, L.; Li, W.; Zhang, D.; Fang, W.; Yan, D.; Wang, Q.; Jin, X.; Li, Y.; Cao, A. Silica modified copper-based alginate/chitosan hybrid hydrogel to control soil fumigant release, reduce emission and enhance bioactivity. Int. J. Biol. Macromol. 2023, 244, 125132. [Google Scholar] [CrossRef]
- Santo Pereira, A.E.; Silva, P.M.; Oliveira, J.L.; Oliveira, H.C.; Fraceto, L.F. Chitosan nanoparticles as carrier systems for the plant growth hormone gibberellic acid. Colloids Surf. B Biointerfaces 2017, 150, 141–152. [Google Scholar] [CrossRef]
- Dou, Z.; Bini Farias, M.V.; Chen, W.; He, D.; Hu, Y.; Xie, X. Highly Degradable Chitosan-Montmorillonite (MMT) Nano-Composite Hydrogel for Controlled Fertilizer Release. Front. Environ. Sci. Eng. 2023, 17, 53. [Google Scholar] [CrossRef]
- El-Mekawy, R.E.; Elhady, H.A.; Al-Shareef, H.F. Highly stretchable, smooth, and biodegradable hydrogel films based on chitosan as safety food packaging. Polym. Polym. Compos. 2021, 29, 563–573. [Google Scholar] [CrossRef]
- Rodrigues, C.; de Mello, J.M.M.; Dalcanton, F.; Macuvele, D.L.P.; Padoin, N.; Fiori, M.A.; Soares, C.; Riella, H.G. Mechanical, Thermal and Antimicrobial Properties of Chitosan-Based-Nanocomposite with Potential Applications for Food Packaging. J. Polym. Environ. 2020, 28, 1216–1236. [Google Scholar] [CrossRef]
- Zárate-Moreno, J.C.; Escobar-Sierra, D.M.; Ríos-Estepa, R. Development and Evaluation of Chitosan-Based Food Coatings for Exotic Fruit Preservation. BioTech 2023, 12, 20. [Google Scholar] [CrossRef]
- Flórez, M.; Guerra-Rodríguez, E.; Cazón, P.; Vázquez, M. Chitosan for food packaging: Recent advances in active and intelligent films. Food Hydrocoll. 2022, 124, 107328. [Google Scholar] [CrossRef]
- Yong, H.; Wang, X.; Bai, R.; Miao, Z.; Zhang, X.; Liu, J. Development of Antioxidant and Intelligent PH-Sensing Packaging Films by Incorporating Purple-Fleshed Sweet Potato Extract into Chitosan Matrix. Food Hydrocoll. 2019, 90, 216–224. [Google Scholar] [CrossRef]
















| Adsorption Mechanism | Definition | Examples | Applications | References |
|---|---|---|---|---|
| hydrogen bonding | an intermolecular or intramolecular interaction with the formation of a pair of electrons–hydrogen bond acceptor | carboxymethyl cellulose-chitosan system (CMC-CS) | high removal efficiency of methylene blue (MB) | [76] |
| electrostatic interactions | the interaction between two oppositely charged molecules or/and atoms | carboxymethyl cellulose/chitosan hydrogel (CMC-CS) | removal of anionic and cationic dyes (MB and acid orange II) | [77] |
| ion exchanged | exchange of free mobile ions of an insoluble solid with different ions of a similar charge from the solution, the reaction being reversible | chitosan–gelatine ion exchanger with the addition of zirconium (IV) | an increased selectivity for cationic dyes—removal of MB | [78] |
| π–π interactions | noncovalent interactions by π-electron interactions between the acceptor and donor molecules | chitosan/carboxymethyl cellulose hydrogel using graphene oxide as chemical crosslinking agent | used to treat dyes from contaminated wastewater | [79] |
| coordination/chelation | the connection through a coordination bond of a metal ion with two or more coordination atoms (non-metal) in the same molecule to form a chelate ring structure | DNA-chitosan hydrogel | the removal of heavy metal ions, organic dyes, and pharmaceuticals | [80] |
| surface complexation | The interaction between an electron pair donor and electron acceptor in order to form various complexes | hydroxyapatite/chitosan composite (HAp-CS) | the removal of Congo red (CR) | [81] |
| Adsorbent | Pollutant | Adsorption Capacity (mg/g)/Adsorption Efficiencies (%) | Time/Equilibrium and Best-Fitted Model for Adsorption | References |
|---|---|---|---|---|
| CS magnetic-kaolin beads | Nitrate Phosphate | 74.11/ 92.05 | 40 min Freundlich (pseudo-second-order kinetic model) | [115] |
| Imprinted magnetic CS beads | Cu(II) | 78.1 | 180 min Langmuir (pseudo-second-order kinetic model) | [116] |
| Ion-imprinted crosslinked CS | Cd(II) | 1.10 mmol/g | 48 h Langmuir (pseudo-second-order kinetic model) | [117] |
| La-Fe incorporated CS beads | Cd(II) phosphate (calculated in P) | 35.5/ 52.0 | 24 h 32 h Langmuir (pseudo-second-order kinetic model) | [118] |
| CS oligosaccharide | Cr(VI) | 148.1 | 100 min Langmuir (pseudo-second-order kinetic model) | [119] |
| CS/orange peel | Cr(VI) Cu(II) | 107.5 116.6 | 240 min Freundlich (pseudo-second-order kinetic model) | [120] |
| Crosslinked CS/sodium alginate/calcium ion | Pb(II) Cu(II) Cd(II) | 176.50 70.83 81.25 | 8 h Elovich (pseudo-first-order kinetic model) (pseudo-second-order kinetic model) | [121] |
| Carbon dots crosslinked cellulose Nanofibril/CS | Cu(II) Cr(VI) | 148.3 294.46 | 4 h Langmuir (pseudo-second-order kinetic model) | [122] |
| CS/CA/Bentonite | Pb(II) Cu(II) Cd(II) | 434.89 115.30 102.38 | 1500 min Elovich (pseudo-second-order kinetic model) | [123] |
| CSGO (chitosan-graphene oxide)-R@IO (magnetically separable chitosanbased hydrogel system) | Cu(II) | 119.5 at pH 6 | 180 min Langmuir (pseudo-second-order kinetic model) | [124] |
| Graphene oxide-poly(vinyl alcohol)- chitosan GO-PVA-CS | Cd(II) Ni(II) | 172.11 70.37 | 16 h Langmuir (pseudo-second-order kinetic model) | [125] |
| Nitrogen-doped carbon dots-cellulose nanofibril-chitosan NCDs-CNF/CS | Cu(II) Cr(VI) | 148.3 294.46 | 4 h Langmuir (pseudo-second-order kinetic model) | [126] |
| Millettia speciosa Champ cellulose-CS | Congo red (CR) Cu(II) | 221.43 23.37 | 288 h, Freundlich (pseudo-second-order kinetic model) 24 h, Freundlich (pseudo-first-order kinetic model) | [127] |
| Graphene oxid-chitosan-Fe3O4 GO-CS-Fe3O4 | Methylene blue (MB) Eriochrome Black T (EBT) | 289 292 | 300 min Langmuir (pseudo-second-order kinetic model) | [128] |
| CS/aspartic acid CSAA-HG1 CSAA-HG2 | Methylene blue (MB) | 99.85% 99.88% | 30–60 min Freundlich (pseudo-second-order kinetic model) | [129] |
| CS/montmorillonite | Methyl green (MG) | 303.21 | 24 h Freundlich (pseudo-second-order kinetic model) | [130] |
| CS/oxalic acid | Reactive Red 195 RR195 | 110.7 | 16 h Redlich–Peterson | [131] |
| Polyacrylamide-chitosan-carbon PAM/CS/C PAMCS/MW-120 | Acid Blue 113 | 151.7 255.5 | 60 min, Langmuir (pseudo-second-order kinetic model) Freundlich (pseudo-second-order kinetic model) | [132] |
| Biochar/CS | Ciprofloxacin Enrofloxacin | 106.038 100.433 | 300 and 450 min Langmuir (pseudo-second-order kinetic model) | [133] |
| Pectin/O-carboxymethyl chitosan | Levofloxacin Delafloxacin | 66.3–87.5% 45–53% | 12 h SIPs model | [134] |
| Type of CS Beads | Advantages | Disadvantages | Type of Adsorbent/Pollutant | Mechanism | References |
|---|---|---|---|---|---|
| CS-based composite beads | easy to prepare, no mass loss, high surface area, and good reusability | hard selection of low-cost materials | CS/Fe-hydroxyapatite beads | chemisorption | [136] |
| CS/Ag-hydroxyapatite nanocomposite beads | ion exchange | [137] | |||
| CS/waste active sludge char beads | chelation | [138] | |||
| La-Fe incorporated CS beads | electrostatic interaction and ligand exchange | [139] | |||
| CS/lanthanum hydrogel beads | electrostatic attraction and ion exchange | [140] | |||
| Functionalized CS beads | high surface area, a multitude of available active sites, and good reusability | unclear adsorption mechanism | ethylenediamine tetraacetic acid grafted/polyvinyl alcohol/CS beads | chemisorption | [141] |
| functional β-cyclodextrin CS beads | electrostatic interaction | [142] | |||
| triethylenetetramine-CS/alginate composite beads | electrostatic interaction, hydrogen bonding, redox reaction, chelation, and cation–anion exchange | [143] | |||
| novel aliquot-336 impregnated CS conjugated beads | electrostatic interaction | [144] | |||
| CS beads impregnated hexadecyl amine | chemisorption | [145] | |||
| Metal–organic framework-based CS beads | higher porosity and surface area | low stability and low mechanical strength | MIL-101(Fe)-CS-cyclodextrin nanosponge | - | [146] |
| copper-benzenetricarboxylate (Cu-BTC), polyacrylonitrile (PAN), and CS | physical adsorption | [147] | |||
| Al-MOF@CS composites with the MOFs Alfum and MIL-160 | - | [148] | |||
| CS/PEO@MOF-5 membrane | - | [149] | |||
| PAN/CS | complexation | [150] | |||
| Magnetic CS beads | good mechanical strength, good stability, good recovery, and higher surface area | lower reusability | magnetic kaolinite immobilized CS beads | - | [151] |
| magnetic kaolin-embedded CS beads | physical adsorption | [152] | |||
| magnetic Fe3O4/graphene oxide/CS beads | - | [153] | |||
| magnetic cationic polymer CS beads | electrostatic interaction and ion exchange | [154] | |||
| Co-polymeric CS beads | a good adsorption capacity | lower stability | PEI-grafted CS beads | - | [155] |
| graft copolymerized CS with acetylacetone | complexation | [156] | |||
| epichlorohydrin-modified CS-2,4-dichlorobenzaldehyde composite | homogeneous monolayer chemical adsorption | [157] | |||
| Zr4+ and glutaraldehyde crosslinked PEI functionalized CS | electrostatic action, complexation, and reduction | [158] | |||
| Imprinted CS beads | good reusability and greater efficiency | unclear adsorption mechanism | ion-imprinted crosslinked CS | chemisorption | [117] |
| imprinted CS-acrylamide | hydrogen bond and electrostatic attraction | [156] | |||
| imprinted magnetic CS beads | enthalpy controlled process | [116] | |||
| ion-imprinted tetraethylenepentamine modified CS beads | diffusion process | [159] |
| Adsorbent | Pollutant | Adsorption Capacity (mg/g) | References |
|---|---|---|---|
| Xanthan gum-poly(acrylic acid-cloisite15A semi-IPN hydrogel (XG/PAA/Cloisite15A) | Co(II) Cu(II) Ni(II) | 436.62 530.14 511.74 | [185] |
| Poly(acrylamide) PAA-g-starch/PAA semi-IPN cryogels | Methylene blue (MB) | 667.7 | [186] |
| alginate/polyethyleneimine (ALG/PEI) hydrogels | Cu(II) Pb(II) | 322.6 344.8 | [187] |
| polyaniline/sodium alginate hydrogel (PANI/ALG) | MB Rhodamine B Orange-II Methyl orange | 555.5 434.78 476.19 146.66 | [188] |
| Xanthan gum-2-acrylamido-2-methly propane sulfonic acid-acrylamide XG-g-P(AMPS-co-AAm) | MB | 384.62 | [189] |
| hydroxyethyl starch/p(3-(acrylamidopropyl) trimethyl ammonium chloride (HESt/P(APTMACl)) hydrogel HESt/PAA hydrogels hydroxyethyl starch/p(acrylamide) (HESt/PAAm) hydrogels | Methyl violet Methyl orange Methyl violet Methyl orange Methyl violet Methyl orange | 6.62 238.1 185.2 2.84 9.17 3.33 | [190] |
| Polydopamine-montmorillonite-pullulan PDA/MMT/PUL hydrogel composites | Crystal violet | 112.45 | [191] |
| hydrogel nanocomposites based on methacrylated carboxymethyl cellulose (M-CMC) and lignosulfonate-derived carbonaceous products (All-lignocellulose) | MB Cu(II) | 350 145 | [192] |
| chondroitin sulfate/MCM-41 (chondroitin sulfate-mesoporous silica) hybrid hydrogels | MB | 3982 ± 123.6 | [193] |
| Domains | Applications | Examples | References |
|---|---|---|---|
| medicine and pharmaceutics | treating skin wounds and accelerated wound healing |
| [194,195,196,197,198] |
| wound dressing |
| [197,199] | |
| drug delivery |
| [197,200,201,202,203] | |
| lens system for eyes | release of various ophthalmic drugs (4-arm polyethylene glycol with aldehyde end groups (4-arm PEG-CHO) with glycol chitosan (GC)) | [197,204] | |
| bones and tissues engineering |
| [205,206] | |
| cartilage repair | hydrogels for in vivo cartilage regeneration (chitosan-hyaluronic acid hydrogel) | [207,208,209,210] | |
| neural tissue | injectable thermoresponsive hydrogels for neural tissue engineering (chitosan/β-glycerophosphate hydrogels) | [211,212] | |
| engineering | 3D materials for tissue engineering applications | [213] | |
| cosmetics | skin care products |
| [214,215] |
| hair care products |
| ||
| hygiene |
| ||
| food industries | food preservatives |
| [216,217,218,219,220,221,222,223,224,225,226] |
| dietary ingredient |
| ||
| color stabilization in foods |
| ||
| controlling and stabilizing agent |
| ||
| agriculture | removal of pesticides and herbicides | antifungal control | [217,227,228,229,230,231,232,233] |
| plant growth |
| ||
| enhancing the soil quality | for biologically transmitting active nutrients | ||
| packaging industry | packaging for food |
| [234,235,236,237,238] |
| active/preservative coatings |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chelu, M.; Musuc, A.M.; Popa, M.; Calderon Moreno, J.M. Chitosan Hydrogels for Water Purification Applications. Gels 2023, 9, 664. https://doi.org/10.3390/gels9080664
Chelu M, Musuc AM, Popa M, Calderon Moreno JM. Chitosan Hydrogels for Water Purification Applications. Gels. 2023; 9(8):664. https://doi.org/10.3390/gels9080664
Chicago/Turabian StyleChelu, Mariana, Adina Magdalena Musuc, Monica Popa, and Jose M. Calderon Moreno. 2023. "Chitosan Hydrogels for Water Purification Applications" Gels 9, no. 8: 664. https://doi.org/10.3390/gels9080664