Rheological Considerations of Pharmaceutical Formulations: Focus on Viscoelasticity
Abstract
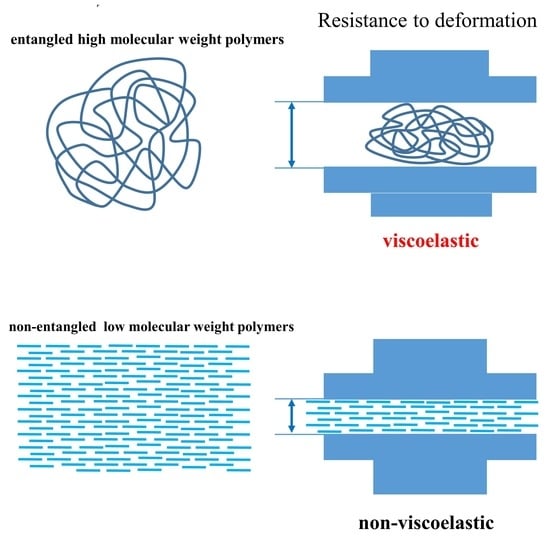
:1. Introduction
2. Results and Discussion
2.1. Hyaluronic Acid (HA)
2.2. Methylcellulose (MC)
2.3. Alginate Gels
2.4. Gellan Gum (GG)
2.5. Pectin
2.6. Chitosan
2.7. Polymethyl Methacrylate (PMMA)
2.8. Gelatin
3. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, J.; Papautsky, I. Viscoelastic microfluidics: Progress and challenges. Microsyst. Nanoeng. 2020, 6, 113. [Google Scholar] [CrossRef] [PubMed]
- Swann, D.A.; Radin, E.L. Role of hyaluronic acid in joint lubrication. Ann. Rheum. Dis. 1974, 33, 318–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiffany, J.M. The viscosity of human tears. Int. Ophthalmol. 1991, 15, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Recchioni, A.; Mocciardini, E. Viscoelastic properties of the human tear film. Exp. Eye Res. 2022, 219, 109083. [Google Scholar] [CrossRef]
- Georgiev, G.A.; Eftimov, P. Contribution of Mucins towards the Physical Properties of the Tear Film: A Modern Update. Int. J. Mol. Sci. 2019, 20, 6132. [Google Scholar] [CrossRef] [Green Version]
- Echeverría, C.; Mijangos, C. Rheology Applied to Microgels: Brief (Revision of the) State of the Art. Polymers 2022, 14, 1279. [Google Scholar] [CrossRef]
- Everett, J.S.; Sommers, M.S. Skin viscoelasticity: Physiologic mechanisms, measurement issues, and application to nursing science. Biol. Res. Nurs. 2013, 15, 338–346. [Google Scholar] [CrossRef]
- Mattei, G.; Cacopardo, L. Engineering Gels with Time-Evolving Viscoelasticity. Materials 2020, 13, 438. [Google Scholar] [CrossRef] [Green Version]
- Sathaye, S.; Mbi, A. Rheology of peptide- and protein-based physical hydrogels: Are everyday measurements just scratching the surface? Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 34–68. [Google Scholar] [CrossRef]
- Alonso, J.M.; Andrade Del Olmo, J. Injectable Hydrogels: From Laboratory to Industrialization. Polymers 2021, 13, 650. [Google Scholar] [CrossRef]
- Mastropietro, D. Rheology in Pharmaceutical Formulations—A Perspective. J. Dev. Drugs 2013, 2, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Sun, H. The Relation between the Rheological Properties of Gels and the Mechanical Properties of Their Corresponding Aerogels. Gels 2018, 4, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasqui, D.; De Cagna, M. Polysaccharide-Based Hydrogels: The Key Role of Water in Affecting Mechanical Properties. Polymers 2012, 4, 1517–1534. [Google Scholar] [CrossRef]
- Lee, C.H.; Moturi, V. Thixotropic property in pharmaceutical formulations. J. Control. Release 2009, 136, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Bianco, S.; Panja, S. Using Rheology to Understand Transient and Dynamic Gels. Gels 2022, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Picout, D.R.; Ross-Murphy, S.B. Rheology of biopolymer solutions and gels. Sci. World J. 2003, 3, 105–121. [Google Scholar] [CrossRef]
- Ferrari, F.; Rossi, S. Rheological and mechanical properties of pharmaceutical gels. Part I: Non-medicated systems. Boll. Chim. Farm. 2001, 140, 329–336. [Google Scholar]
- You, J.; Zhou, J. Rheological study of physical cross-linked quaternized cellulose hydrogels induced by β-glycerophosphate. Langmuir 2012, 28, 4965–4973. [Google Scholar] [CrossRef]
- Camponeschi, F.; Atrei, A. New Formulations of Polysaccharide-Based Hydrogels for Drug Release and Tissue Engineering. Gels 2015, 1, 3–23. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Yang, T. Thermally Tunable Dynamic and Static Elastic Properties of Hydrogel Due to Volumetric Phase Transition. Polymers 2020, 12, 1462. [Google Scholar] [CrossRef]
- Jin, Y.; Heo, H. The effects of temperature and frequency dispersion on sound speed in bulk poly (vinyl alcohol) poly (N-isopropylacrylamide) hydrogels caused by the phase transition. Ultrasonics 2020, 104, 105931. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.; Reyes, D. Anomalous temperature dependence of speed of sound of bulk poly(N-isopropylacrylamide) hydrogels near the phase transition. Ultrasonics 2014, 54, 1337–1340. [Google Scholar] [CrossRef]
- Zuidema, J.M.; Rivet, C.J. A protocol for rheological characterization of hydrogels for tissue engineering strategies. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Demkin, V.P.; Mel’nichuk, S.V. Determination of Viscoelastic Characteristics of Whole Blood Based on the Low-Frequency Piezotromboelastography Method. Russ. Phys. J. 2020, 62, 2219–2227. [Google Scholar] [CrossRef]
- Karim, M.; Kohale, S.C. Determination of viscoelastic properties by analysis of probe-particle motion in molecular simulations. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2012, 86, 051501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, T.; Jin, Y. Acoustic Attenuation and Dispersion in Fatty Tissues and Tissue Phantoms Influencing Ultrasound Biomedical Imaging. ACS Omega 2023, 8, 1319–1330. [Google Scholar] [CrossRef]
- Chiarelli, P.; Lanatà, A. Acoustic waves in hydrogels: A bi-phasic model for ultrasound tissue-mimicking phantom. Mater. Sci. Eng. C 2009, 29, 899–907. [Google Scholar] [CrossRef]
- Walker, J.M.; Myers, A.M. Nondestructive evaluation of hydrogel mechanical properties using ultrasound. Ann. Biomed. Eng. 2011, 39, 2521–2530. [Google Scholar] [CrossRef] [Green Version]
- Ruland, A.; Onofrillo, C. Standardised quantitative ultrasound imaging approach for the contact-less three-dimensional analysis of neocartilage formation in hydrogel-based bioscaffolds. Acta Biomater. 2022, 147, 129–146. [Google Scholar] [CrossRef]
- Fan, Z.; Cheng, P. Rheological insight of polysaccharide/protein based hydrogels in recent food and biomedical fields: A review. Int. J. Biol. Macromol. 2022, 222, 1642–1664. [Google Scholar] [CrossRef]
- Zulkiflee, I.; Fauzi, M.B. Gelatin-Polyvinyl Alcohol Film for Tissue Engineering: A Concise Review. Biomedicines 2021, 9, 979. [Google Scholar] [CrossRef] [PubMed]
- Tussupova, B.; Yermagambetova, A. Preparation and regulation of structuralmechanical properties of biodegradable films based on starch and agar. East. Eur. J. Enterp. Technol. 2020, 5, 40–48. [Google Scholar]
- Nueraji, M.; Toktarbay, Z. Mechanically-robust electrospun nanocomposite fiber membranes for oil and water separation. Environ. Res. 2023, 220, 115212. [Google Scholar] [CrossRef] [PubMed]
- Stojkov, G.; Niyazov, Z. Relationship between Structure and Rheology of Hydrogels for Various Applications. Gels 2021, 7, 255. [Google Scholar] [CrossRef] [PubMed]
- Borkenstein, A.F.; Borkenstein, E.M. Ophthalmic Viscosurgical Devices (OVDs) in Challenging Cases: A Review. Ophthalmol. 2021, 10, 831–843. [Google Scholar] [CrossRef] [PubMed]
- Mitura, S.; Sionkowska, A. Biopolymers for hydrogels in cosmetics: Review. J. Mater. Sci. Mater. Med. 2020, 31, 50. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, M.; Azadi, A. Hydrogel nanoparticles in drug delivery. Adv. Drug. Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef]
- Mantha, S.; Pillai, S. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef] [Green Version]
- Slaughter, B.V.; Khurshid, S.S. Hydrogels in regenerative medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef] [Green Version]
- Khademhosseini, A.; Langer, R. A decade of progress in tissue engineering. Nat. Protoc. 2016, 11, 1775–1781. [Google Scholar] [CrossRef]
- Leijten, J.; Seo, J. Spatially and Temporally Controlled Hydrogels for Tissue Engineering. Mater. Sci. Eng. R Rep. 2017, 119, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Higashide, T.; Sugiyama, K. Use of viscoelastic substance in ophthalmic surgery—Focus on sodium hyaluronate. Clin. Ophthalmol. 2008, 2, 21–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valachová, K.; Šoltés, L. Versatile Use of Chitosan and Hyaluronan in Medicine. Molecules 2021, 26, 1195. [Google Scholar] [CrossRef]
- Pitarresi, G.; Palumbo, F.S. Medicated hydrogels of hyaluronic acid derivatives for use in orthopedic field. Int. J. Pharm. 2013, 449, 84–94. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Hydrogels for tissue engineering. Chem. Rev. 2001, 101, 1869–1879. [Google Scholar] [CrossRef]
- Tamer, T.M. Hyaluronan and synovial joint: Function, distribution and healing. Interdiscip. Toxicol. 2013, 6, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Fakhari, A.; Berkland, C. Applications and emerging trends of hyaluronic acid in tissue engineering, as a dermal filler and in osteoarthritis treatment. Acta Biomater. 2013, 9, 7081–7092. [Google Scholar] [CrossRef] [Green Version]
- Christensen, L.H. Host tissue interaction, fate, and risks of degradable and nondegradable gel fillers. Derm. Surg. 2009, 35 (Suppl. S2), 1612–1619. [Google Scholar] [CrossRef]
- Sun, S.F.; Chou, Y.J. Hyaluronic acid as a treatment for ankle osteoarthritis. Curr. Rev. Musculoskelet. Med. 2009, 2, 78–82. [Google Scholar] [CrossRef] [Green Version]
- Watterson, J.R.; Esdaile, J.M. Viscosupplementation: Therapeutic mechanisms and clinical potential in osteoarthritis of the knee. J. Am. Acad. Orthop. Surg. 2000, 8, 277–284. [Google Scholar] [CrossRef]
- Finelli, I.; Chiessi, E. A new viscosupplement based on partially hydrophobic hyaluronic acid: A comparative study. Biorheology 2011, 48, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Fam, H.; Bryant, J.T. Rheological properties of synovial fluids. Biorheology 2007, 44, 59–74. [Google Scholar] [PubMed]
- Rebenda, D.; Vrbka, M. On the Dependence of Rheology of Hyaluronic Acid Solutions and Frictional Behavior of Articular Cartilage. Materials 2020, 13, 2659. [Google Scholar] [CrossRef] [PubMed]
- Chernos, M.; Grecov, D. Rheological study of hyaluronic acid derivatives. Biomed. Eng. Lett. 2017, 7, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, M.; Manjoo, A. Rheological Properties of Commercially Available Hyaluronic Acid Products in the United States for the Treatment of Osteoarthritis Knee Pain. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2018, 11, 1–5. [Google Scholar] [CrossRef]
- Zerbinati, N.; Sommatis, S. Toward Physicochemical and Rheological Characterization of Different Injectable Hyaluronic Acid Dermal Fillers Cross-Linked with Polyethylene Glycol Diglycidyl Ether. Polymers 2021, 13, 948. [Google Scholar] [CrossRef]
- La Gatta, A.; Bedini, E. Hyaluronan Hydrogels: Rheology and Stability in Relation to the Type/Level of Biopolymer Chemical Modification. Polymers 2022, 14, 2402. [Google Scholar] [CrossRef]
- Desbrieres, J.; Hirrien, M. Thermogelation of methylcellulose: Rheological considerations. Polymers 2000, 41, 2451–2461. [Google Scholar] [CrossRef]
- Shimokawa, K.; Saegusa, K. Rheological properties of reversible thermo-setting in situ gelling solutions with the methylcellulose-polyethylene glycol-citric acid ternary system (2): Effects of various water-soluble polymers and salts on the gelling temperature. Colloids Surf. B Biointerfaces 2009, 74, 56–58. [Google Scholar] [CrossRef]
- Demir Oğuz, Ö.; Ege, D. Rheological and Mechanical Properties of Thermoresponsive Methylcellulose/Calcium Phosphate-Based Injectable Bone Substitutes. Materials 2018, 11, 604. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Yao, P. Injectable thermo-responsive hydrogel composed of xanthan gum and methylcellulose double networks with shear-thinning property. Carbohydr. Polym. 2015, 132, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Tator, C.H. Fast-gelling injectable blend of hyaluronan and methylcellulose for intrathecal, localized delivery to the injured spinal cord. Biomaterials 2006, 27, 2370–2379. [Google Scholar] [CrossRef]
- Eskens, O.; Villani, G. Rheological Investigation of Thermoresponsive Alginate-Methylcellulose Gels for Epidermal Growth Factor Formulation. Cosmetics 2021, 8, 3. [Google Scholar] [CrossRef]
- Liang, H.F.; Hong, M.H. Novel method using a temperature-sensitive polymer (methylcellulose) to thermally gel aqueous alginate as a pH-sensitive hydrogel. Biomacromolecules 2004, 5, 1917–1925. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Mollah, M.Z.I.; Zahid, H.M. The Usages and Potential Uses of Alginate for Healthcare Applications. Front. Mol. Biosci. 2021, 8, 719972. [Google Scholar] [CrossRef]
- Hariyadi, D.M.; Islam, N. Current Status of Alginate in Drug Delivery. Adv. Pharm. Pharm. Sci. 2020, 2020, 8886095. [Google Scholar] [CrossRef]
- Ching, S.H.; Bansal, N. Alginate gel particles—A review of production techniques and physical properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1133–1152. [Google Scholar] [CrossRef]
- Liparoti, S.; Speranza, V. Alginate hydrogel: The influence of the hardening on the rheological behaviour. J. Mech. Behav. Biomed. Mater. 2021, 116, 104341. [Google Scholar] [CrossRef]
- Jain, D.; Bar-Shalom, D. Alginate drug delivery systems: Application in context of pharmaceutical and biomedical research. Drug. Dev. Ind. Pharm. 2014, 40, 1576–1584. [Google Scholar] [CrossRef]
- Goh, C.H.; Heng, P.W.S. Alginates as a useful natural polymer for microencapsulation and therapeutic applications. Carbohydr. Polym. 2012, 88, 1–12. [Google Scholar] [CrossRef]
- Cardoso, M.J.; Costa, R.R. Marine Origin Polysaccharides in Drug Delivery Systems. Mar. Drugs 2016, 14, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sosnik, A. Alginate Particles as Platform for Drug Delivery by the Oral Route: State-of-the-Art. ISRN Pharm. 2014, 2014, 926157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwiatek, M.A.; Roman, S. An alginate-antacid formulation (Gaviscon Double Action Liquid) can eliminate or displace the postprandial ‘acid pocket’ in symptomatic GERD patients. Aliment. Pharmacol. Ther. 2011, 34, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Mirtič, J.; Ilaš, J. Influence of different classes of crosslinkers on alginate polyelectrolyte nanoparticle formation, thermodynamics and characteristics. Carbohydr. Polym. 2018, 181, 93–102. [Google Scholar] [CrossRef]
- Cuomo, F.; Cofelice, M. Rheological Characterization of Hydrogels from Alginate-Based Nanodispersion. Polymers 2019, 11, 259. [Google Scholar] [CrossRef] [Green Version]
- Morris, E.R.; Nishinari, K. Gelation of gellan—A review. Food Hydrocoll. 2012, 28, 373–411. [Google Scholar] [CrossRef]
- Masakuni, T.; Teruya, T. Molecular origin for rheological characteristics of native gellan gum. Colloid. Polym. Sci. 2009, 287, 1445–1454. [Google Scholar]
- Kasapis, S.; Giannouli, P. Structural aspects and phase behaviour in deacylated and high acyl gellan systems. Carbohydr. Polym. 1999, 38, 145–154. [Google Scholar] [CrossRef]
- Osmałek, T.Z.; Froelich, A. Rheological investigation of high-acyl gellan gum hydrogel and its mixtures with simulated body fluids. J. Biomater. Appl. 2018, 32, 1435–1449. [Google Scholar] [CrossRef]
- Hägerström, H.; Paulsson, M. Evaluation of mucoadhesion for two polyelectrolyte gels in simulated physiological conditions using a rheological method. Eur. J. Pharm. Sci. 2000, 9, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Osmałek, T.; Froelich, A. Application of gellan gum in pharmacy and medicine. Int. J. Pharm. 2014, 466, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Chandel, V.; Biswas, D. Current Advancements in Pectin: Extraction, Properties and Multifunctional Applications. Foods 2022, 11, 2683. [Google Scholar] [CrossRef]
- Tran Vo, T.M.; Kobayashi, T. Viscoelastic Analysis of Pectin Hydrogels Regenerated from Citrus Pomelo Waste by Gelling Effects of Calcium Ion Crosslinking at Different pHs. Gels 2022, 8, 814. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Khalil, A.A. Sources, Extraction and Biomedical Properties of Polysaccharides. Foods 2019, 8, 304. [Google Scholar] [CrossRef] [Green Version]
- Chan, S.Y.; Choo, W.S. Pectin as a rheology modifier: Origin, structure, commercial production and rheology. Carbohydr. Polym. 2017, 161, 118–139. [Google Scholar] [CrossRef]
- Ishwarya, S.P.; Sandhya, R.; Nisha, P. Advances and prospects in the food applications of pectin hydrogels. Crit. Rev. Food Sci. Nutr. 2022, 62, 4393–4417. [Google Scholar] [CrossRef]
- Seshadri, R.; Weiss, J. Ultrasonic processing influences rheological and optical properties of high-methoxyl pectin dispersions. Food Hydrocoll. 2003, 17, 191–197. [Google Scholar] [CrossRef]
- Ventura, I.; Jammal, J. Insights into the nanostructure of low-methoxyl pectin-calcium gels. Carbohydr. Polym. 2013, 97, 650–658. [Google Scholar] [CrossRef]
- Basak, R.; Bandyopadhyay, R. Formation and rupture of Ca2+ induced pectin biopolymer gels. Soft Matter 2014, 10, 7225–7233. [Google Scholar] [CrossRef] [Green Version]
- Ngouémazong, D.E.; Jolie, R.P. Stiffness of Ca2+-pectin gels: Combined effects of degree and pattern of methylesterification for various Ca2+ concentrations. Carbohydr. Res. 2012, 348, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Mierczyńska, J.; Cybulska, J. Effect of Ca2+, Fe2+ and Mg2+ on rheological properties of new food matrix made of modified cell wall polysaccharides from apple. Carbohydr. Polym. 2015, 133, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Löfgren, C.; Guillotin, S. Microstructure and kinetic rheological behavior of amidated and nonamidated LM pectin gels. Biomacromolecules 2006, 7, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Capel, F.; Nicolai, T. Calcium and acid induced gelation of (amidated) low methoxyl pectin. Food Hydrocoll. 2006, 20, 901–907. [Google Scholar] [CrossRef]
- Vithanage, C.; Grimson, M. Rheological and structural properties of high-methoxyl esterified, low-methoxyl esterified and low-methoxyl amidated pectin gels. J. Texture Stud. 2010, 41, 899–927. [Google Scholar] [CrossRef]
- Moslemi, M. Reviewing the recent advances in application of pectin for technical and health promotion purposes: From laboratory to market. Carbohydr. Polym. 2021, 254, 117324. [Google Scholar] [CrossRef] [PubMed]
- Karavasili, C.; Fatouros, D.G. Smart materials: In situ gel-forming systems for nasal delivery. Drug. Discov. Today 2016, 21, 157–166. [Google Scholar] [CrossRef]
- Hamedi, H.; Moradi, S. Chitosan based hydrogels and their applications for drug delivery in wound dressings: A review. Carbohydr. Polym. 2018, 199, 445–460. [Google Scholar] [CrossRef]
- Maliki, S.; Sharma, G. Chitosan as a Tool for Sustainable Development: A Mini Review. Polymers 2022, 14, 1475. [Google Scholar] [CrossRef]
- Thirupathi, K.; Raorane, C.J. Update on Chitosan-Based Hydrogels: Preparation, Characterization, and Its Antimicrobial and Antibiofilm Applications. Gels 2022, 9, 35. [Google Scholar] [CrossRef]
- Elhefian, E.; Yahaya, A. Rheological study of chitosan and its blends: An overview. Maejo Int. J. Sci. Technol. 2010, 4, 210–220. [Google Scholar]
- Cheung, R.C.; Ng, T.B. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Do Amaral Sobral, P.J.; Gebremariam, G. Rheological and Viscoelastic Properties of Chitosan Solutions Prepared with Different Chitosan or Acetic Acid Concentrations. Foods 2022, 11, 2692. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.R. pH mediated rheological modulation of chitosan hydrogels. Int. J. Biol. Macromol. 2020, 156, 591–597. [Google Scholar] [CrossRef]
- Tsaih, M.L.; Chen, R.H. Effects of ionic strength and pH on the diffusion coefficients and conformation of chitosans molecule in solution. J. Appl. Polym. Sci. 1999, 73, 2041–2050. [Google Scholar] [CrossRef]
- Schatz, C.; Viton, C. Typical physicochemical behaviors of chitosan in aqueous solution. Biomacromolecules 2003, 4, 641–648. [Google Scholar] [CrossRef]
- Chattopadhyay, D.P.; Inamdar, M.S. Aqueous Behaviour of Chitosan. Int. J. Polym. Sci. 2010, 2010, 939536. [Google Scholar] [CrossRef]
- Wang, W.; Xu, D. Viscosity and flow properties of concentrated solutions of chitosan with different degrees of deacetylation. Int. J. Biol. Macromol. 1994, 16, 149–152. [Google Scholar] [CrossRef]
- Kasaai, M.R. Intrinsic viscosity–molecular weight relationship and hydrodynamic volume for pullulan. J. Appl. Polym. Sci. 2006, 100, 4325–4332. [Google Scholar] [CrossRef]
- Martínez-Ruvalcaba, A.; Chornet, E. Dynamic Rheological Properties of Concentrated Chitosan Soltions. Appl. Rheol. 2004, 14, 140–147. [Google Scholar] [CrossRef]
- Montembault, A.; Viton, C. Rheometric study of the gelation of chitosan in a hydroalcoholic medium. Biomaterials 2005, 26, 1633–1643. [Google Scholar] [CrossRef] [PubMed]
- Khunawattanakul, W.; Puttipipatkhachorn, S. Chitosan-magnesium aluminum silicate composite dispersions: Characterization of rheology, flocculate size and zeta potential. Int. J. Pharm. 2008, 351, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, N.; Galbis, E. Reversible pH-Sensitive Chitosan-Based Hydrogels. Influence of Dispersion Composition on Rheological Properties and Sustained Drug Delivery. Polymers 2018, 10, 392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benoso, P.; Bittante, A.M.Q.B. Rheological and viscoelastic properties of colloidal solutions based on gelatins and chitosan as affected by pH. Int. J. Food Sci. Technol. 2022, 57, 2365–2375. [Google Scholar] [CrossRef]
- Bertolo, M.R.V.; Martins, V.C.A. Rheological and antioxidant properties of chitosan/gelatin-based materials functionalized by pomegranate peel extract. Carbohydr. Polym. 2020, 228, 115386. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Cid, P.; Jiménez-Rosado, M. Applied Rheology as Tool for the Assessment of Chitosan Hydrogels for Regenerative Medicine. Polymers 2021, 13, 2189. [Google Scholar] [CrossRef]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Zafar, M.S. Prosthodontic Applications of Polymethyl Methacrylate (PMMA): An Update. Polymers 2020, 12, 2299. [Google Scholar] [CrossRef]
- Rickman, L.J.; Padipatvuthikul, P. Contemporary denture base resins: Part 1. Dent. Update 2012, 39, 25–28, 30. [Google Scholar] [CrossRef] [Green Version]
- Cheng, D.; Chen, T. Study on the Viscoelastic Behaviors of PMMA. J. Phys. Conf. Ser. 2020, 1637, 012120. [Google Scholar] [CrossRef]
- Said, N.S.; Sarbon, N.M. Physical and Mechanical Characteristics of Gelatin-Based Films as a Potential Food Packaging Material: A Review. Membranes 2022, 12, 442. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Guillén, M.C.; Giménez, B. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef] [Green Version]
- Yan, C.; Pochan, D.J. Rheological properties of peptide-based hydrogels for biomedical and other applications. Chem. Soc. Rev. 2010, 39, 3528–3540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreazza, R.; Morales, A. Gelatin-Based Hydrogels: Potential Biomaterials for Remediation. Polymers 2023, 15, 1026. [Google Scholar] [CrossRef]












| Parameter | Unit | Description |
|---|---|---|
| shear strain (γ) | % | Shear strain triggers the deformation of the rheological body. |
| Elastic or storage modulus (G′) | Pa | Storage modulus expresses the elastic feature of materials. |
| Viscous or loss modulus (G″) | Pa | Loss modulus expresses the viscous feature of materials. |
| frequency (f) | s−1 | Oscillation frequency of the upper geometry. |
| phase angle (δ) | ° (0–90°) | The difference in sinusoid oscillational curves, which is a measure of the deformation rate. |
| complex viscosity (η*) | Pa·s | The resistance against flow under oscillational experiments. |
| complex modulus (G*) | Pa | The normalized value of storage and loss moduli. |
| loss factor (tan δ) | - | G″/G′; the ratio of the loss (G″) and storage modulus (G′) is the loss tangent (loss factor). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budai, L.; Budai, M.; Fülöpné Pápay, Z.E.; Vilimi, Z.; Antal, I. Rheological Considerations of Pharmaceutical Formulations: Focus on Viscoelasticity. Gels 2023, 9, 469. https://doi.org/10.3390/gels9060469
Budai L, Budai M, Fülöpné Pápay ZE, Vilimi Z, Antal I. Rheological Considerations of Pharmaceutical Formulations: Focus on Viscoelasticity. Gels. 2023; 9(6):469. https://doi.org/10.3390/gels9060469
Chicago/Turabian StyleBudai, Lívia, Marianna Budai, Zsófia Edit Fülöpné Pápay, Zsófia Vilimi, and István Antal. 2023. "Rheological Considerations of Pharmaceutical Formulations: Focus on Viscoelasticity" Gels 9, no. 6: 469. https://doi.org/10.3390/gels9060469