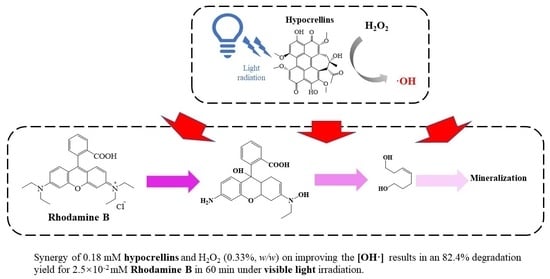
Rapid Degradation of Rhodamine B through Visible-Photocatalytic Advanced Oxidation Using Self-Degradable Natural Perylene Quinone Derivatives—Hypocrellins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of the HYP Aqueous Solution
2.3. Degradation of RhB in the HYPs/H2O2 PAOP
2.4. Determination of the Yield of Hydroxyl Radicals in the PAOP System
2.5. LC–MS Analysis
2.6. Determination of the Self-Degradation of HYPs
3. Results and Discussion
3.1. Stability of the HYP Aqueous Solution
3.2. Improved Degradation of RhB in the HYPs/H2O2 PAOP
| Photocatalyst | Light Sources 1 | Organic Pollutants | Degradation Yield (%), Initial Concentration (M) | Reaction Time (min) | References |
|---|---|---|---|---|---|
| HA | Vis | RhB | 82%, 2.5 × 10−5 | 60 | This study |
| TiO2 | UV | RhB | 96%, 2.1 × 10−5 | 180 | [15] |
| TiO2 film | UV | RhB | 75% 2, 1.0 × 10−5 | 300 | [16] |
| Silica-TiO2 | UV/Solar | Acephate | 100%, 1.0 × 10−4 | 105 | [17] |
| Dimethoate | 100%, 1.0 × 10−4 | 60 | |||
| ZnO | UV | Reactive black 5 | 72%, 1.0 × 10−5 | 780 | [62] |
| g-C3N4/BiVO4 | Vis | Diclofenac Sodium | 65% 3, 3.1 × 10−5 | 180 | [20] |
| Fe2O3/Cu2O(SO4) | UV | Acid orange 2 | 99%, 1.4 × 10−4 | 30 | [63] |
| CuO/Cu2O | UV | Methyl orange | >90%, 2.0 × 10−5 | 30 | [64] |
| WO3 | UV | RhB | 76%, 2.1 × 10−6 | 180 | [65] |
| BaTiO2/GO | UV | Methylene blue | >80%, 1.6 × 10−5 | 120 | [66] |
| Bi2WO6 | UV | RhB | 63%, 1.0 × 10−5 | 180 | [19] |
| (RGO)-Ag | UV | RhB | 70%, 2.0 × 10−6 | 60 | [18] |
| Ag/Bi2WO6 | UV | RhB | 80%, 1.0 × 10−5 | 60 | [58] |
3.3. Possible Pathway for the Degradation of RhB in the HYPs/H2O2 PAOP
3.4. The pH-Dependent Self-Degradation of HYPs
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhatia, D.; Sharma, N.R.; Singh, J.; Kanwar, R.S. Biological methods for textile dye removal from wastewater: A review. Crit. Rev. Env. Sci. Tec. 2017, 47, 1836–1876. [Google Scholar] [CrossRef]
- Deng, D.; Lamssali, M.; Aryal, N.; Ofori-Boadu, A.; Jha, M.K.; Samuel, R.E. Textiles wastewater treatment technology: A review. Water Environ. Res. 2020, 92, 1805–1810. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Tian, F.; Zhao, Y.; Tang, M.; Liu, W.; Liu, C.; Xue, S.; Kong, W.; Sun, Y.; Wang, S. Aerobic decolorization and detoxification of Acid Scarlet GR by a newly isolated salt-tolerant yeast strain Galactomyces geotrichum GG. Int. Biodeter. Biodegr. 2019, 145, 104818. [Google Scholar] [CrossRef]
- Leaper, S.; Abdel-Karim, A.; Gad-Allah, T.A.; Gorgojo, P. Air-gap membrane distillation as a one-step process for textile wastewater treatment. Chem. Eng. J. 2019, 360, 1330–1340. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Lou, Z.; Yang, K.; Wang, Z.; Zhou, C.; Li, Y.; Cao, Z.; Xu, X. Coagulation removal of Sb(V) from textile wastewater matrix with enhanced strategy: Comparison study and mechanism analysis. Chemosphere 2019, 237, 124494. [Google Scholar] [CrossRef]
- Molla Mahmoudi, M.; Nadali, A.; Soheil Arezoomand, H.R.; Mahvi, A.H. Adsorption of cationic dye textile wastewater using Clinoptilolite: Isotherm and kinetic study. J. Text. Inst. 2019, 110, 74–80. [Google Scholar] [CrossRef]
- Srinivasan, S.; Sadasivam, S.K.; Gunalan, S.; Shanmugam, G.; Kothandan, G. Application of docking and active site analysis for enzyme linked biodegradation of textile dyes. Environ. Pollut. 2019, 248, 599–608. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, J.; Zhao, Z.; Liu, J. Degradation of haloacetonitriles with UV/peroxymonosulfate process: Degradation pathway and the role of hydroxyl radicals. Chem. Eng. J. 2019, 364, 1–10. [Google Scholar] [CrossRef]
- Oturan, M.A.; Aaron, J. Advanced Oxidation Processes in Water/Wastewater Treatment: Principles and Applications. A Review. Crit. Rev. Env. Sci. Tec. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Dai, F.; Fan, X.; Stratton, G.R.; Bellona, C.L.; Holsen, T.M.; Crimmins, B.S.; Xia, X.; Mededovic Thagard, S. Experimental and density functional theoretical study of the effects of Fenton’s reaction on the degradation of Bisphenol A in a high voltage plasma reactor. J. Hazard. Mater. 2016, 308, 419–429. [Google Scholar] [CrossRef]
- Xiao, R.; Gao, L.; Wei, Z.; Spinney, R.; Luo, S.; Wang, D.; Dionysiou, D.D.; Tang, C.J.; Yang, W. Mechanistic insight into degradation of endocrine disrupting chemical by hydroxyl radical: An experimental and theoretical approach. Environ. Pollut. 2017, 231, 1446–1452. [Google Scholar] [CrossRef]
- Yang, Z.; Su, R.; Luo, S.; Spinney, R.; Cai, M.; Xiao, R.; Wei, Z. Comparison of the reactivity of ibuprofen with sulfate and hydroxyl radicals: An experimental and theoretical study. Sci. Total. Environ. 2017, 590–591, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, B. Hydroxyl Radical and Its Scavengers in Health and Disease. Oxid. Med. Cell. Longev. 2011, 2011, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, D.; Zhou, H.; Zhang, H.; Zhou, P.; You, J.; Yao, G.; Pan, Z.; Liu, Y.; Lai, B. Heterogeneous photocatalyst-driven persulfate activation process under visible light irradiation: From basic catalyst design principles to novel enhancement strategies. Chem. Eng. J. 2022, 428, 131166. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Thomas, M.; Natarajan, K.; Bajaj, H.C.; Tayade, R.J. Study on UV-LED/TiO2 process for degradation of Rhodamine B dye. Chem. Eng. J. 2011, 169, 126–134. [Google Scholar] [CrossRef]
- Zhuang, J.; Dai, W.; Tian, Q.; Li, Z.; Xie, L.; Wang, J.; Liu, P.; Shi, X.; Wang, D. Photocatalytic Degradation of RhB over TiO2 Bilayer Films: Effect of Defects and Their Location. Langmuir 2010, 26, 9686–9694. [Google Scholar] [CrossRef]
- Echavia, G.R.M.; Matzusawa, F.; Negishi, N. Photocatalytic degradation of organophosphate and phosphonoglycine pesticides using TiO2 immobilized on silica gel. Chemosphere 2009, 76, 595–600. [Google Scholar] [CrossRef]
- Divya, K.S.; Chandran, A.; Reethu, V.N.; Mathew, S. Enhanced photocatalytic performance of RGO/Ag nanocomposites produced via a facile microwave irradiation for the degradation of Rhodamine B in aqueous solution. Appl. Surf. Sci. 2018, 444, 811–818. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, Z.; Lv, H.; Guang, J.; Li, S.; Jiang, J. Hydrothermal synthesis of hierarchical flower-like Bi2WO6 microspheres with enhanced visible-light photoactivity. Mater. Lett. 2015, 157, 158–162. [Google Scholar] [CrossRef]
- Sun, J.; Guo, Y.; Wang, Y.; Cao, D.; Tian, S.; Xiao, K.; Mao, R.; Zhao, X. H2O2 assisted photoelectrocatalytic degradation of diclofenac sodium at g-C3N4/BiVO4 photoanode under visible light irradiation. Chem. Eng. J. 2018, 332, 312–320. [Google Scholar] [CrossRef]
- Fendrich, M.; Quaranta, A.; Orlandi, M.; Bettonte, M.; Miotello, A. Solar Concentration for Wastewaters Remediation: A Review of Materials and Technologies. Appl. Sci. 2019, 9, 118. [Google Scholar] [CrossRef] [Green Version]
- Mecha, A.C.; Chollom, M.N. Photocatalytic ozonation of wastewater: A review. Environ. Chem. Lett. 2020, 18, 1491–1507. [Google Scholar] [CrossRef]
- Qian, Y.; Zhang, F.; Pang, H. A Review of MOFs and Their Composites-Based Photocatalysts: Synthesis and Applications. Adv. Funct. Mater. 2021, 31, 2104231. [Google Scholar] [CrossRef]
- Dao, X.; Sun, W. Single- and mixed-metal-organic framework photocatalysts for carbon dioxide reduction. Inorg. Chem. Front. 2021, 8, 3178–3324. [Google Scholar] [CrossRef]
- Yan, C.; Dong, J.; Chen, Y.; Zhou, W.; Peng, Y.; Zhang, Y.; Wang, L. Organic photocatalysts: From molecular to aggregate level. Nano. Res. 2022, 15, 3835–3858. [Google Scholar] [CrossRef]
- Bobo, M.V.; Kuchta, J.R.; Vannucci, A.K. Recent advancements in the development of molecular organic photocatalysts. Org. Biomol. Chem. 2021, 19, 4816–4834. [Google Scholar] [CrossRef]
- Meng, A.; Chaihu, L.; Chen, H.; Gu, Z. Ultrahigh adsorption and singlet-oxygen mediated degradation for efficient synergetic removal of bisphenol A by a stable zirconium-porphyrin metal-organic framework. Sci. Rep. Sci. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Jiang, L.; He, Y. Photophysics, photochemistry and photobiology of hypocrellin photosensitizers. Chin. Sci. Bull. 2001, 46, 6–16. [Google Scholar] [CrossRef]
- Liang, X.; Cai, Y.; Liao, X.; Wu, K.; Wang, L.; Zhang, D.; Meng, Q. Isolation and identification of a new hypocrellin A-producing strain Shiraia sp. SUPER-H168. Microbiol. Res. 2009, 164, 9–17. [Google Scholar] [CrossRef]
- Kubrak, T.P.; Kołodziej, P.; Sawicki, J.; Mazur, A.; Koziorowska, K.; Aebisher, D. Some Natural Photosensitizers and Their Medicinal Properties for Use in Photodynamic Therapy. Molecules 2022, 27, 1192. [Google Scholar] [CrossRef]
- Lee, H.; Zhou, Z.; Chen, S.; Zhang, M.; Shen, T. New long-wavelength ethanolamino-substituted hypocrellin: Photodynamic activity and toxicity to MGC803 cancer cell. Dye. Pigment. 2006, 68, 1–10. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Q.; Zeng, Z.; Qiao, R.; Wang, X.; Zhang, B. Photodynamic Properties of a Bispyrrolecarboxamide-Modified Hypocrellin B: The Role of Affinity and Ascorbic Acid. J. Phys. Chem. B 2008, 112, 9959–9965. [Google Scholar] [CrossRef] [PubMed]
- Ou, Z.; Jin, H.; Gao, Y.; Li, S.; Li, H.; Li, Y.; Wang, X.; Yang, G. Synthesis and Photophysical Properties of a Supramolecular Host–Guest Assembly Constructed by Fullerenes and Tryptamine Modified Hypocrellin. J. Phys. Chem. B 2012, 116, 2048–2058. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, X.; Chen, S.; Zhang, M.; Shen, T. EPR studies of the photodynamic properties of a novel potential photodynamic therapeutic agent: Photogeneration of semiquinone radical anion and active oxygen species (O2−, OH−, H2O2 and 1O2). Photochem. Photobiol. Sci. 2003, 2, 871–876. [Google Scholar] [CrossRef]
- Zeng, Z.; Qiao, R.; Zhou, J.; Xia, S.; Zhang, Y.; Liu, Y.; Chen, J.; Wang, X.; Zhang, B. Photodynamic Properties of Dipeptide-Modified Hypocrellin B Derivatives: The Role of Tyrosine and Tryptophan Groups. J. Phys. Chem. B 2007, 111, 3742–3749. [Google Scholar] [CrossRef]
- Chio-Srichan, S.; Oudrhiri, N.; Bennaceur-Griscelli, A.; Turhan, A.G.; Dumas, P.; Refregiers, M. Toxicity and phototoxicity of Hypocrellin A on malignant human cell lines, evidence of a synergistic action of photodynamic therapy with Imatinib mesylate. J. Photochem. Photobiol. B Biol. 2010, 99, 100–104. [Google Scholar] [CrossRef]
- Croce, A.C.; Fasani, E.; Bottone, M.G.; De Simone, U.; Santin, G.; Pellicciari, C.; Bottiroli, G. Hypocrellin-B acetate as a fluorogenic substrate for enzyme-assisted cell photosensitization. Photochem. Photobiol. Sci. 2011, 10, 1783–1790. [Google Scholar] [CrossRef]
- Jiang, Y.; Leung, A.W.; Wang, X.; Zhang, H.; Xu, C. Effect of photodynamic therapy with hypocrellin B on apoptosis, adhesion, and migration of cancer cells. Int. J. Radiat. Biol. 2014, 90, 575–579. [Google Scholar] [CrossRef]
- Qi, S.; Guo, L.; Yan, S.; Lee, R.J.; Yu, S.; Chen, S. Hypocrellin A-based photodynamic action induces apoptosis in A549 cells through ROS-mediated mitochondrial signaling pathway. Acta. Pharm. Sin. B 2019, 9, 279–293. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhou, J.; Zhang, Y.; Qiao, R.; Xia, S.; Chen, J.; Wang, X.; Zhang, B. Photodynamic Properties of Hypocrellin A, Complexes with Rare Earth Trivalent Ions: Role of the Excited State Energies of the Metal Ions. J. Phys. Chem. B 2007, 111, 2688–2696. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, J.; Xia, S.; Wang, X.; Zhang, B. Effect of Chelation to Lanthanum Ions on the Photodynamic Properties of Hypocrellin, A. J. Phys. Chem. B 2005, 109, 19529–19535. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Khan, S.I.; Jacob, M.R.; Tekwani, B.L.; Zuqiang, L.; Pasco, D.S.; Walker, L.A.K.I. Antimicrobial and Antileishmanial Activities of Hypocrellins A and B. Antimicrob. Agents. Chemother. 2004, 48, 4450–4452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otieno, W.; Liu, C.; Deng, H.; Li, J.; Zeng, X.; Ji, Y. Hypocrellin B-Mediated Photodynamic Inactivation of Gram-Positive Antibiotic-Resistant Bacteria: AnIn Vitro Study. Photobiomodul. Photomed. Laser Surg. 2020, 38, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.X.; Ma, Y.J.; Wang, J.W. Improved hypocrellin A production in Shiraia bambusicola by light-dark shift. J. Photochem. Photobiol. B Biol. 2018, 182, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wu, X.; Gu, X.; Zhou, L.; Song, K.; Wei, S.; Feng, Y.; Shen, J. Spectroscopic studies on the interaction of hypocrellin A and hemoglobin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 72, 151–155. [Google Scholar] [CrossRef]
- Mehrjouei, M.; Müller, S.; Möller, D. A review on photocatalytic ozonation used for the treatment of water and wastewater. Chem. Eng. J. 2015, 263, 209–219. [Google Scholar] [CrossRef]
- Yan, X.; Wen, Y.; Hu, M.; Wu, Z.; Tian, X. Promotion of the Hypocrellin Yield by a Co-Culture of Shiraia bambusicola (GDMCC 60438) with Arthrinium sp. AF-5 Fungus. Fermentation 2021, 7, 316. [Google Scholar] [CrossRef]
- Torabi Momen, M.; Piri, F.; Karimian, R. Photocatalytic degradation of rhodamine B and methylene blue by electrochemically prepared nano titanium dioxide/reduced graphene oxide/poly (methyl methacrylate) nanocomposite. React. Kinet. Mech. Catal. 2020, 129, 1145–1157. [Google Scholar] [CrossRef]
- Gazi, S.; Ananthakrishnan, R. Semi-Quantitative Determination of Hydroxyl Radicals by Benzoic Acid Hydroxylation: An Analytical Methodology for Photo-Fenton Systems. Curr. Anal. Chem. 2012, 8, 143–149. [Google Scholar] [CrossRef]
- Deng, H.; Li, T.; Xie, J.; Huang, N.; Gu, Y.; Zhao, J. Synthesis and bio-evaluation of novel hypocrellin derivatives: Potential photosensitizers for photodynamic therapy of age-related macular degeneration. Dyes. Pigments 2013, 99, 930–939. [Google Scholar] [CrossRef]
- Deng, H.; Liu, X.; Xie, J.; Yin, R.; Huang, N.; Gu, Y.; Zhao, J. Quantitative and Site-Directed Chemical Modification of Hypocrellins toward Direct Drug Delivery and Effective Photodynamic Activity. J. Med. Chem. 2012, 55, 1910–1919. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Huang, H.; Ge, X.; Yang, X.; Yang, C.; Han, L.; Zhou, J.; Zhou, L. Complexation of Hypocrellin A with Al3+ in water solution and the photodynamic therapy study. Bioorg. Med. Chem. Lett. 2013, 23, 1689–1692. [Google Scholar] [CrossRef] [PubMed]
- Niu, T.; Tian, Y.; Shi, Y.; Guo, G.; Tong, Y.; Wang, G. Antifibrotic effects of Hypocrellin A combined with LED red light irradiation on keloid fibroblasts by counteracting the TGF-β/Smad/autophagy/apoptosis signalling pathway. Photodiagn Photodyn. Ther. 2021, 34, 102202. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Hou, X.; Deng, H.; Zhao, J.; Huang, N.; Zeng, J.; Chen, H.; Gu, Y. Liposomal hypocrellin B as a potential photosensitizer for age-related macular degeneration: Pharmacokinetics, photodynamic efficacy, and skin phototoxicity in vivo. Photochchem. Photobio. Sci. 2015, 14, 972–981. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, J.; Liu, W.; Zheng, X.; Wang, P. Natural-Origin Hypocrellin-HSA Assembly for Highly Efficient NIR Light-Responsive Phototheranostics against Hypoxic Tumors. ACS Appl. Mater. Inter. 2019, 11, 44989–44998. [Google Scholar] [CrossRef]
- Blanksby, S.J.; Bierbaum, V.M.; Ellison, G.B.; Kato, S. Superoxide Does React with Peroxides: Direct Observation of the Haber–Weiss Reaction in the Gas Phase. Angew. Chem. Int. Ed. 2007, 46, 4948–4950. [Google Scholar] [CrossRef] [Green Version]
- Kehrer, J.P. The Haber–Weiss reaction and mechanisms of toxicity. Toxicology 2000, 149, 43–50. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Maneechote, A.; Dumrongrojthanath, P.; Ekthammathat, N.; Thongtem, S.; Thongtem, T. Visible-light driven photocatalytic degradation of rhodamine B by Ag/Bi2WO6 heterostructures. Mater. Lett. 2015, 159, 289–292. [Google Scholar] [CrossRef]
- Gu, J.; Luo, C.; Zhou, W.; Tong, Z.; Zhang, H.; Zhang, P.; Ren, X. Degradation of Rhodamine B in aqueous solution by laser cavitation. Ultrason. Sonochem. 2020, 68, 105181. [Google Scholar] [CrossRef]
- Hernandez, R.; Zappi, M.; Colucci, J.; Jones, R. Comparing the performance of various advanced oxidation processes for treatment of acetone contaminated water. J. Hazard. Mater. 2002, 92, 33–50. [Google Scholar] [CrossRef]
- Xu, B.; Gao, N.; Sun, X.; Xia, S.; Rui, M.; Simonnot, M.; Causserand, C.; Zhao, J. Photochemical degradation of diethyl phthalate with UV/H2O2. J. Hazard. Mater. 2007, 139, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.B.; Santos, J.J.; Corrêa, C.C.; Corio, P.; Andrade, G.F.S. Plasmonic photodegradation of textile dye Reactive Black 5 under visible light: A vibrational and electronic study. J. Photochem. Photobiol. A Chem. 2019, 371, 159–165. [Google Scholar] [CrossRef]
- Guo, X.; Xu, Y.; Zha, F.; Tang, X.; Tian, H. α-Fe2O3/Cu2O(SO4) composite as a novel and efficient heterogeneous catalyst for photo-Fenton removal of Orange II. Appl. Surf. Sci. 2020, 530, 147144. [Google Scholar] [CrossRef]
- Jiang, D.; Xue, J.; Wu, L.; Zhou, W.; Zhang, Y.; Li, X. Photocatalytic performance enhancement of CuO/Cu2O heterostructures for photodegradation of organic dyes: Effects of CuO morphology. Appl. Catal. B Environ. 2017, 211, 199–204. [Google Scholar] [CrossRef]
- Tahir, M.B.; Ashraf, M.; Rafique, M.; Ijaz, M.; Firman, S.; Mubeen, I. Activated carbon doped WO3 for photocatalytic degradation of rhodamine-B. Appl. Nanosci. 2020, 10, 869–877. [Google Scholar] [CrossRef]
- Mengting, Z.; Kurniawan, T.A.; Fei, S.; Ouyang, T.; Othman, M.H.D.; Rezakazemi, M.; Shirazian, S. Applicability of BaTiO3/graphene oxide (GO) composite for enhanced photodegradation of methylene blue (MB) in synthetic wastewater under UV–vis irradiation. Environ. Pollut. 2019, 255, 113182. [Google Scholar] [CrossRef]
- Xie, J.; Lü, X.; Liu, J.; Shu, H. Brookite titania photocatalytic nanomaterials: Synthesis, properties, and applications. Pure Appl. Chem. 2009, 81, 2407–2415. [Google Scholar] [CrossRef]







| Reaction Mixture | Relative Yield of the OH· (%) |
|---|---|
| H2O2 PAOP | 100 |
| HYP PAOP | 98.3 |
| HYP/H2O2 PAOP | 201.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z.; Zhang, F.; Tang, Y.; Wen, Y.; Wu, Z.; Fang, Z.; Tian, X. Rapid Degradation of Rhodamine B through Visible-Photocatalytic Advanced Oxidation Using Self-Degradable Natural Perylene Quinone Derivatives—Hypocrellins. Bioengineering 2022, 9, 307. https://doi.org/10.3390/bioengineering9070307
Huang Z, Zhang F, Tang Y, Wen Y, Wu Z, Fang Z, Tian X. Rapid Degradation of Rhodamine B through Visible-Photocatalytic Advanced Oxidation Using Self-Degradable Natural Perylene Quinone Derivatives—Hypocrellins. Bioengineering. 2022; 9(7):307. https://doi.org/10.3390/bioengineering9070307
Chicago/Turabian StyleHuang, Zhixian, Fan Zhang, Yanbo Tang, Yongdi Wen, Zhenqiang Wu, Zhen Fang, and Xiaofei Tian. 2022. "Rapid Degradation of Rhodamine B through Visible-Photocatalytic Advanced Oxidation Using Self-Degradable Natural Perylene Quinone Derivatives—Hypocrellins" Bioengineering 9, no. 7: 307. https://doi.org/10.3390/bioengineering9070307