Bioconversion of Plastic Waste Based on Mass Full Carbon Backbone Polymeric Materials to Value-Added Polyhydroxyalkanoates (PHAs)
Abstract
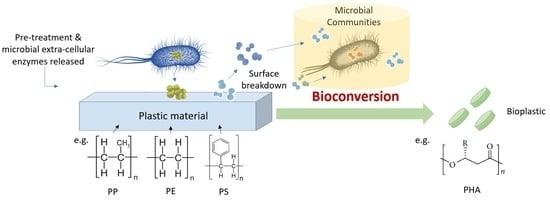
:1. Introduction
2. Microbes of Interest
3. Target Plastics
3.1. Polypropylene
3.2. Polystyrene
3.3. Polyethylene
3.4. Tetra Pak
3.5. Poly(ethylene Terephthalate)
4. Value-Added Bioplastic Synthesis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Somleva, M.N.; Peoples, O.P.; Snell, K.D. PHA Bioplastics, Biochemicals, and Energy from Crops. Plant Biotechnol. J. 2013, 11, 233–252. [Google Scholar] [CrossRef] [PubMed]
- Johnston, B.; Jiang, G.; Hill, D.; Adamus, G.; Kwiecien, I.; Zieba, M.; Sikorska, W.; Green, M.; Kowalczuk, M.; Radecka, I. The Molecular Level Characterization of Biodegradable Polymers Originated from Polyethylene Using Non-Oxygenated Polyethylene Wax as a Carbon Source for Polyhydroxyalkanoate Production. Bioengineering 2017, 4, 73. [Google Scholar] [CrossRef] [PubMed]
- Johnston, B.; Radecka, I.; Hill, D.; Chiellini, E.; Ilieva, V.I.; Sikorska, W.; Musioł, M.; Zieba, M.; Marek, A.A.; Keddie, D.; et al. The Microbial Production of Polyhydroxyalkanoates from Waste Polystyrene Fragments Attained Using Oxidative Degradation. Polymers 2018, 10, 957. [Google Scholar] [CrossRef]
- Johnston, B.; Radecka, I.; Hill, D.; Chiellini, E.; Sikorska, W.; Musioł, M.; Zieba, M.; Marek, A.A.; Mendrek, B.; Ekere, I.; et al. Mass Spectrometry Reveals Molecular Structure of Polyhydroxyalkanoates Derived from Waste Polypropylene Attained Using Oxidative Degradation. Polymers 2019, 11, 1580. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Mukherjee, A. A new wave of industrialization of PHA biopolyesters. Bioengineering 2022, 9, 74. [Google Scholar] [CrossRef]
- Da Cruz Pradella, J.G. Economics and Industrial Aspects of PHA Production. In The Handbook of Polyhydroxyalkanoates; Koller, M., Ed.; CRC Press: Boca Raton, FL, USA, 2020; Volume 3, pp. 389–404. [Google Scholar]
- Geyer, R.; Jambeck, J.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Peng, R.T.; Xia, M.L.; Ru, J.K.; Huo, Y.X.; Yang, Y. Microbial degradation of polyurethane plastics. Chin. J. Biotechnol. 2018, 34, 1398–1409. [Google Scholar]
- Ru, J.; Huo, Y.; Yang, Y. Microbial Degradation and Valorization of Plastic Wastes. Front. Microbiol. 2020, 11, 442. [Google Scholar] [CrossRef]
- Blank, L.M.; Narancic, T.; Mampel, J.; Tiso, T.; O’Connor, K.E. Biotechnological upcycling of plastic waste and other non-conventional feedstocks in a circular economy. Curr. Opin. Biotechnol. 2020, 62, 212–219. [Google Scholar] [CrossRef]
- Garcia, J.M.; Robertson, M.L. The future of plastics recycling. Science 2017, 358, 870–872. [Google Scholar] [CrossRef]
- Peng, Y.; Wu, P.; Schartup, A.T.; Zhang, Y. Plastic waste release caused by COVID-19 and its fate in the global ocean. Proc. Natl. Acad. Sci. USA 2021, 118, 47. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, S.; Tittarelli, F.; Sabbatini, S.; Cespi, M.; Bonacucina, G.; Eusebi, A.L.; Fatone, F.; Stipa, P. Effects of different pre-treatments on the properties of polyhydroxyalkanoates extracted from sidestreams of a municipal wastewater treatment plant. Sci. Total Environ. 2021, 801, 149633. [Google Scholar] [CrossRef] [PubMed]
- Rabnawaz, M.; Wyman, I.; Auras, R.; Cheng, S. A roadmap towards green packaging: The current status and future outlook for polyesters in the packaging industry. Green Chem. 2017, 19, 4737–4753. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Chyc, M.; Ryszka, P.; Latowski, D. Achromobacter xylosoxidans as a new microorganism strain colonizing high-density polyethylene as a key step to its biodegradation. Environ. Sci. Pollut. Res. Int. 2016, 23, 11349–11356. [Google Scholar] [CrossRef] [PubMed]
- Delacuvellerie, A.; Cyriaque, V.; Gobert, S.; Benali, S.; Wattiez, R. The plastisphere in marine ecosystem hosts potential specific microbial degraders including Alcanivorax borkumensis as a key player for the low-density polyethylene degradation. J. Hazard. Mater. 2019, 380, 120899. [Google Scholar] [CrossRef]
- Skariyachan, S.; Patil, A.A.; Shankar, A.; Manjunath, M.; Bachappanavar, N.; Kiran, S. Enhanced polymer degradation of polyethylene and polypropylene by novel thermophilic consortia of Brevibacillus sps. and Aneurinibacillus sp. screened from waste management landfills and sewage treatment plants. Polym. Degrad. Stab. 2018, 149, 52–68. [Google Scholar] [CrossRef]
- Auta, H.S.; Emenike, C.U.; Jayanthi, B.; Fauziah, S.H. Growth kinetics and biodeterioration of polypropylene microplastics by Bacillus sp. and Rhodococcus sp. isolated from mangrove sediment. Mar. Pollut. Bull. 2018, 127, 15–21. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Y.; Wu, W.M.; Zhao, J.; Jiang, L. Evidence of polyethylene biodegradation by bacterial strains from the guts of plastic-eating waxworms. Environ. Sci. Technol. 2014, 48, 13776–13784. [Google Scholar] [CrossRef]
- Harshvardhan, K.; Jha, B. Biodegradation of low–density polyethylene by marine bacteria from pelagic waters, Arabian Sea, India. Mar. Pollut. Bull. 2013, 77, 100–106. [Google Scholar] [CrossRef]
- Radecka, I.; Irorere, V.; Jiang, G.; Hill, D.; Williams, C.; Adamus, G.; Kwiecień, M.; Marek, A.A.; Zawadiak, J.; Johnston, B.; et al. Oxidized Polyethylene Wax as a Potential Carbon Source for PHA Production. Materials 2016, 9, 367. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, J.; Wu, W.M.; Zhao, J.; Song, Y.; Gao, L. Biodegradation and mineralization of polystyrene by plastic-eating mealworms. Role of gut microorganisms. Environ. Sci. Technol. 2015, 49, 12087–12093. [Google Scholar] [CrossRef] [PubMed]
- Atiq, N.; Garba, A.; Ali, M.I.; Andleeb, S.; Khan, N.A.; Robson, G.D. Isolation and identification of polystyrene biodegrading bacteria from soil. Afr. J. Microbiol. Res. 2010, 4, 1537–1541. [Google Scholar]
- Tribedi, P.; Sil, A.K. Low-density polyethylene degradation by Pseudomonas sp. AKS2 biofilm. Environ. Sci. Pollut. R 2013, 20, 4146–4153. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.G.; Jeon, H.J.; Kim, M.N. Biodegradation of polyethylene by a soil bacterium and AlkB cloned recombinant cell. J. Bioremediat. Biodegrad. 2012, 3, 145. [Google Scholar]
- Mor, R.; Sivan, A. Biofilm formation and partial biodegradation of polystyrene by the actinomycete Rhodococcus ruber. Biodegradation 2008, 19, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Azeko, S.T.; Etuk-Udo, G.A.; Odusanya, O.S.; Malatesta, K.; Anuku, N.; Soboyejo, W.O. Biodegradation of linear low density polyethylene by Serratia marcescens sub sp. marcescens and its cell free extracts. Waste Biomass Valori. 2015, 6, 1047–1057. [Google Scholar] [CrossRef]
- Eisaku, O.; Linn, K.T. Isolation and characterization of polystyrene degrading microorganisms for zero emission treatment of expanded polystyrene. Environ. Eng. Res. 2003, 40, 373–379. [Google Scholar]
- Jeon, H.J.; Kim, M.N. Isolation of mesophilic bacterium for biodegradation of polypropylene. Int. Biodeter. Biodegr. 2016, 115, 244–249. [Google Scholar] [CrossRef]
- Jeyakumar, D.; Chirsteen, J.; Doble, M. Synergistic effects of pretreatment and blending on fungi mediated biodegradation of polypropylenes. Bioresour. Technol. 2013, 148, 78–85. [Google Scholar] [CrossRef]
- Paço, A.; Duarte, K.; da Costa, J.P.; Santos, P.S.; Pereira, R.; Pereira, M.E.; Freitas, A.C.; Duarte, A.C.; Rocha-Santos, T.A.P. Biodegradation of polyethylene microplastics by the marine fungus Zalerion maritimum. Sci. Total Environ. 2017, 586, 10–15. [Google Scholar] [CrossRef]
- Priya, A.; Dutta, K.; Daverey, A. A comprehensive biotechnological and molecular insight into plastic degradation by microbial community. J. Chem. Technol. Biotechnol. 2022, 97, 381–390. [Google Scholar] [CrossRef]
- Cacciari, I.; Quatrini, P.; Zirletta, G.; Mincione, E.; Vinciguerra, V.; Lupattelli, P.; Sermanni, G.G. Isotactic polypropylene biodegradation by a microbial community: Physicochemical characterization of metabolites produced. Appl. Environ. Microbiol. 1993, 59, 3695–3700. [Google Scholar] [CrossRef] [PubMed]
- Collinet, P.; Belot, F.; Debodinance, P.; Duc, E.H.; Lucot, J.-P.; Cosson, M. Transvaginal mesh technique for pelvic organ prolapse repair: Mesh exposure management and risk factors. Int. Urogynecology J. 2006, 17, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Arutchelvi, J.; Sudhakar, M.; Arkatkar, A.; Doble, M.; Bhaduri, S.; Uppara, P.V. Biodegradation of polyethylene and polypropylene. Indian J. Biotechnol. 2008, 725, 9–22. [Google Scholar]
- Danso, D.; Chow, J.; Streit, W.R. Plastics: Environmental and biotechnological perspectives on microbial degradation. Appl. Environ. Microbiol. 2019, 85, e01095-19. [Google Scholar] [CrossRef] [Green Version]
- Karger-Kocsis, J.; Bárány, T. Polypropylene Handbook; Springer Nature: Basel, Switzerland, 2019. [Google Scholar]
- Iakovlev, V.V.; Guelcher, S.A.; Bendavid, R. Degradation of polypropylene in vivo: A microscopic analysis of meshes explanted from patients. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 237–248. [Google Scholar] [CrossRef]
- Jain, K.; Bhunia, H.; Sudhakara Reddy, M. Degradation of polypropylene–poly-L-lactide blend by bacteria isolated from compost. Bioremediat. J. 2018, 22, 73–90. [Google Scholar] [CrossRef]
- Adamus, G.; Sikorska, W.; Kowalczuk, M.; Noda, I.; Satkowski, M.M. Electrospray Ion-Trap Multistage Mass Spectrometry for characterization of co-monomer compositional distribution of bacterial poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) at the molecular level. Rapid Commun. Mass Spectrom. 2003, 17, 2260. [Google Scholar] [CrossRef]
- Ho, B.T.; Roberts, T.K.; Lucas, S. An overview on biodegradation of polystyrene and modified polystyrene: The microbial approach. Crit. Rev. Biotechnol. 2018, 38, 308–320. [Google Scholar] [CrossRef]
- Oelschlägel, M.; Zimmerling, J.; Tischler, D. A Review: The styrene metabolizing cascade of side-chain oxygenation as biotechnological basis to gain various valuable compounds. Front. Microbiol. 2018, 9, 490. [Google Scholar] [CrossRef]
- Tischler, D.; Eulberg, D.; Lakner, S.; Kaschabek, S.R.; van Berkel, W.J.H.; Schlömann, M. Identification of a novel self-sufficient styrene monooxygenase from Rhodococcus opacus 1CP. J. Bacteriol. 2009, 191, 4996–5009. [Google Scholar] [CrossRef] [PubMed]
- Veethahavya, K.S.; Rajath, B.S.; Noobia, S.; Kumar, B.M. Biodegradation of low density polyethylene in aqueous media. Procedia Environ. Sci. 2016, 35, 709–713. [Google Scholar] [CrossRef]
- Yilgor, N.; Köse, C.; Terzi, E.; Figen, A.K.; Ibach, R.; Kartal, S.N.; Pişkin, S. Degradation behavior and accelerated weathering of composite boards produced from waste Tetra Pak® packaging materials. BioResources 2014, 9, 4784–4807. [Google Scholar] [CrossRef]
- Ma, Y. Changing Tetra Pak: From waste to resource. Sci. Prog. 2018, 101, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Blair, E.M.; Dickson, K.L.; O’Malley, M.A. Microbial communities and their enzymes facilitate degradation of recalcitrant polymers in anaerobic digestion. Curr. Opin. Microbiol. 2021, 64, 100–108. [Google Scholar] [CrossRef]
- Li, D.; Song, L.; Fang, H.; Li, P.; Teng, Y.; Li, Y.Y.; Liu, R.; Niu, Q. Accelerated bio-methane production rate in thermophilic digestion of cardboard with appropriate biochar: Dose-response kinetic assays, hybrid synergistic mechanism, and microbial networks analysis. Bioresour. Technol. 2019, 290, 121782. [Google Scholar] [CrossRef]
- Li, D.; Song, L.; Fang, H.; Shi, Y.; Li, Y.Y.; Liu, R.; Niu, Q. Effect of temperature on the anaerobic digestion of cardboard with waste yeast added: Dose-response kinetic assays, temperature coefficient and microbial co-metabolism. J. Clean. Prod. 2020, 275, 122949. [Google Scholar] [CrossRef]
- Ferreira, I.; Pinho, O.; Vieira, E.; Tavarela, J.G. Brewer’s Saccharomyces yeast biomass: Characteristics and potential applications. Trends Food Sci. Technol. 2010, 21, 77–84. [Google Scholar] [CrossRef]
- Arora, P.; Singh, G.; Tiwari, A. Effect of Microbial inoculation in combating the aluminium toxicity effect on growth of Zea mays. Cell. Mol. Biol. 2017, 63, 79–82. [Google Scholar] [CrossRef]
- Solisio, C.; Lodi, A. Bioleaching of zinc and aluminium from industrial waste sludges by means of Thiobacillus ferrooxidans. Waste Manag. 2002, 22, 667–675. [Google Scholar] [CrossRef]
- Ekere, I.; Johnston, B.; Tchuenbou-Magaia, F.; Townrow, D.; Wojciechowski, S.; Marek, A.; Zawadiak, J.; Duale, K.; Zieba, M.; Sikorska, W.; et al. Bioconversion Process of Polyethylene from Waste Tetra Pak® Packaging to Polyhydroxyalkanoates. Polymers 2022, 14, 2840. [Google Scholar] [CrossRef] [PubMed]
- Ghatge, S.; Yang, Y.; Ahn, J.H.; Hur, H.G. Biodegradation of polyethylene: A brief review. Appl. Biol. Chem. 2020, 63, 27. [Google Scholar] [CrossRef]
- Ekere, A.I.; Johnston, B.; Zięba, M.; Chaber, P.; Adamus, G.; Tchuenbou-Magaia, F.; Barsi, D.; Amaro, L.P.; Chiellini, E.; Radecka, I.; et al. Environmental cleaning mission. Chim. Oggi Chem. Today 2019, 37, 36–39. [Google Scholar]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Austin, H.P.; Allen, M.D.; Donohoe, B.S.; Rorrer, N.A.; Kearns, F.L.; Silveira, R.L.; Pollard, B.C.; Dominick, G.; Duman, R.; El Omari, K.; et al. Characterization and engineering of a plastic degrading aromatic polyesterase. Proc. Natl. Acad. Sci. USA 2018, 115, E4350–E4357. [Google Scholar] [CrossRef] [PubMed]
- Raza, Z.A.; Abid, S.; Banat, I.M. Polyhydroxyalkanoates: Characteristics, production, recent developments and applications. Int. Biodeterior. Biodegrad 2018, 126, 45–56. [Google Scholar] [CrossRef]
- Dietrich, K.; Dumont, M.-J.; Del Rio, L.F.; Orsat, V. Producing PHAs in the bioeconomy—Towards a sustainable bioplastic. Sustain. Prod. Consum. 2017, 9, 5870. [Google Scholar] [CrossRef]
- Rodríguez-Contreras, A.; Koller, M.; Braunegg, G.; Marqués-Calvo, M.S. Poly[(R)-3-hydroxybutyrate] production under different salinity conditions by a novel Bacillus megaterium strain. New Biotechnol. 2016, 33, 73–77. [Google Scholar] [CrossRef]
- Valappil, S.; Boccaccini, A.R.; Bucke, C.; Roy, I. Polyhydroxyalkanoates in Gram-positive bacteria: Insights from the genera Bacillus and Streptomyces. Antonie Leeuwenhoek 2007, 91, 1–17. [Google Scholar] [CrossRef]
- Pan, W.; Perrotta, J.A.; Stipanovic, A.J.; Nomura, C.T.; Nakas, J.P. Production of polyhydroxyalkanoates by Burkholderia cepacia ATCC 17759 using a detoxified sugar maple hemicellulosic hydrolysate. J. Ind. Microbiol. Biotechnol. 2012, 39, 459–469. [Google Scholar] [CrossRef]
- Jendrossek, D.; Selchow, O.; Hoppert, M. Poly(3-Hydroxybutyrate) Granules at the Early Stages of Formation Are Localized Close to the Cytoplasmic Membrane in Caryophanon latum. Appl. Environ. Microbiol. 2007, 73, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Flores-Sánchez, A.; López-Cuellar, M.R.; Pérez-Guevara, F.; López, U.F.; Martín-Bufájer, J.M.; Vergara-Porras, B. Synthesis of Poly-(R-hydroxyalkanoates) by Cupriavidus necator ATCC 17699 Using Mexican Avocado (Persea americana) Oil as a Carbon Source. Int. J. Polym. Sci. 2017, 2017, 6942950. [Google Scholar] [CrossRef]
- Sharma, P.K.; Fu, J.; Spicer, V.; Krokhin, O.V.; Cicek, N.; Sparling, R.; Levin, D.B. Global changes in the proteome of Cupriavidus necator H16 during poly-(3-hydroxybutyrate) synthesis from various biodiesel by-product substrates. AMB Express 2016, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Sheu, D.S.; Chen, W.M.; Yang, J.Y.; Chang, R.C. Thermophilic bacterium Caldimonas taiwanensis produces poly(3-hydroxybutyrate-co-3-hydroxyvalerate) from starch and valerate as carbon sources. Enzyme Microb. Technol. 2009, 44, 289–294. [Google Scholar] [CrossRef]
- Tan, G.Y.A.; Chen, C.L.; Ge, L.; Li, L.; Tan, S.N.; Wang, J.Y. Bioconversion of styrene to poly(hydroxyalkanoate) (PHA) by the new bacterial strain Pseudomonas putida NBUS12. Microbes Environ. 2015, 30, 76–85. [Google Scholar] [CrossRef]
- Cui, Y.W.; Gong, X.Y.; Shia, Y.P.; Wang, Z. Salinity effect on production of PHA and EPS by Haloferax mediterranei. RSC Adv. 2017, 7, 53587–53595. [Google Scholar] [CrossRef] [Green Version]
- Tiso, T.; Narancic, T.; Wei, R.; Pollet, E.; Beagan, N.; Schröder, K.; Honak, A.; Jiang, M.; Kenny, S.T.; Wierckx, N.; et al. Towards bio-upcycling of polyethylene terephthalate. Metab. Eng. 2021, 66, 167–178. [Google Scholar] [CrossRef]
- Muller, E.E.L.; Narayanasamy, S.; Zeimes, M.; Laczny, C.C.; Lebrun, L.A.; Herold, M.; Hicks, N.D.; Gillece, J.D.; Schupp, J.M.; Keim, P.; et al. First draft genome sequence of a strain belonging to the Zoogloea genus and its gene expression in situ. Stand. Genom. Sci. 2017, 12, 64. [Google Scholar] [CrossRef]
- Emadian, S.M.; Onay, T.T.; Demirel, B. Biodegradation of bioplastics in natural environments. Waste Manag. 2017, 59, 526–536. [Google Scholar] [CrossRef]
- Passanha, P.; Esteves, S.R.; Kedia, G.; Dinsdale, R.M.; Guwy, A.J. Increasing polyhydroxyalkanoate (PHA) yields from Cupriavidus necator by using filtered digestate liquors. Bioresour. Technol. 2013, 147, 345–352. [Google Scholar] [CrossRef]
- Faccin, D.J.L.; Rech, R.; Secchi, A.R.; Cardozo, N.S.M.; Ayub, M.A.Z. Influence of oxygen transfer rate on the accumulation of poly(3-hydroxybutyrate) by Bacillus megaterium. Process. Biochem. 2013, 48, 420–425. [Google Scholar] [CrossRef]
- Hermann-Krauss, C.; Koller, M.; Muhr, A.; Fasl, H.; Stelzer, F.; Braunegg, G. Archaeal production of polyhydroxyalkanoate (PHA) Co- and terpolyesters from biodiesel industry-derived by-products. Archaea 2013, 2013, 129268. [Google Scholar] [CrossRef] [PubMed]
- Tsuge, T. Metabolic Improvements and Use of Inexpensive Carbon Sources in Microbial Production of Polyhydroxyalkanoates. J. Biosci. Bioeng. 2002, 94, 579–584. [Google Scholar] [CrossRef]
- Palmeiro-Sánchez, T.; O’Flaherty, V.; Lens, P.N. Polyhydroxyalkanoate bio-production and its rise as biomaterial of the future. J. Biotechnol. 2022, 348, 10–25. [Google Scholar] [CrossRef]
- Aldor, I.S.; Keasling, J.D. Process design for microbial plastic factories: Metabolic engineering of polyhydroxyalkanoates. Curr. Opin. Biotechnol. 2003, 14, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Steinbüchel, A.; Lütke-Eversloh, T. Metabolic engineering and pathway construction for biotechnological production of relevant polyhydroxyalkanoates in microorganisms. Biochem. Eng. J. 2003, 16, 81–96. [Google Scholar] [CrossRef]
- Rosenboom, J.G.; Langer, R.; Traverso, G. Bioplastics for a circular economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef]






| Strain | Isolated Source | Plastic Substrate | Days Incubation | References |
|---|---|---|---|---|
| Achromobacter xylosoxidans | Soil | HDPE | 150 | [15] |
| Alcanivorax borkumensis | Mediterranean Sea | LDPE | 7 | [16] |
| Aneurinibacillus aneurinilyticus | Landfill or sewage sites | PP | 140 | [17] |
| Bacillus sp. strain 27 | Mangrove environments | PP microplastics | 40 | [18] |
| Bacillus sp. YP1 | Waxworm guts | LDPE film | 60 | [19] |
| Bacillus subtilis H1584 | Marine water | LDPE film | 30 | [20] |
| Brevibacilus argi; Brevibacilus brevis; Brevibacilus sp. | Sewage | PP | 140 | [17] |
| Cupriavidus necator | Soil | LDPE, PP, PS | 2 | [3,4,21] |
| Exiguobacterium sp. YT2 | Mealworm guts | PS film | 60 | [22] |
| Microbacterium sp. NA23 | Soil | PS film | 56 | [23] |
| Paenibacillus urinalis NA26 | Soil | PS film | 56 | [23] |
| Pseudomonas sp. AKS2 | Soil | LDPE | 45 | [24] |
| Pseudomonas sp. E4 | Soil | LMWPE | 80 | [25] |
| Rhodococcus ruber C208 | Soil | PS film | 56 | [26] |
| Rhodococcus sp. strain 36 | Mangrove environments | PP | 40 | [18] |
| Serratia marcescens | Soil | LLDPE film | 70 | [27] |
| Sphingobacterium sp. | Field soil | PS film | 8 | [28] |
| Stenotrophomonas panacihumi | Soil | PP film | 90 | [29] |
| Xanthomonas sp. | Field soil | PS film | 8 | [28] |
| Strain | Carbon Source | Polymer Synthesised | References |
|---|---|---|---|
| Bacillus megaterium (+) | Glucose salt medium | PHB | [61] |
| Bacillus spp. (+) | Soy molasses, nutrient broth, glucose, butyrate, valerate, hexanoate, octanoate, decanoate, 4-hydroxybutanoate, e-caprolactone | PHB, PHBV, copolymers | [61] |
| Burkholderia cepacia (−) | Palm olein, palm stearin, crude palm oil, palm kernel oil, oleic acid, xylose, levulinic acid, sugarbeet molasses, sugar maple hemicellulosic hydrolysate | PHB, PHBV | [62] |
| Caryophanon latum (+) | Nutrient broth | PHA | [63] |
| Cupriavidus necator (−) | Glucose, soybean oil, waste PE, PP, PS, plastics, biodiesel by-product substrates | PHB, PHBV, PHBH, PHBHx, copolymers | [2,3,4,21,54,56,64,65] |
| Caldimonas taiwanensis (−) | Potatoe and wheat starch | PHBV | [66] |
| Bacillus odysseyi SUK3 (+) | PS plastic | PHB | [67] |
| Haloferax mediterranei (−) | Molasses and wastewater | PHBV | [68] |
| Pseudomonas umsongensis GO16 (−) | Ethylene glycol | PHA, * Bio-PU | [69] |
| Zoogloea spp. (−) | Nutrient broth (activated sludge/wastewater) | PHA | [70] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnston, B.; Adamus, G.; Ekere, A.I.; Kowalczuk, M.; Tchuenbou-Magaia, F.; Radecka, I. Bioconversion of Plastic Waste Based on Mass Full Carbon Backbone Polymeric Materials to Value-Added Polyhydroxyalkanoates (PHAs). Bioengineering 2022, 9, 432. https://doi.org/10.3390/bioengineering9090432
Johnston B, Adamus G, Ekere AI, Kowalczuk M, Tchuenbou-Magaia F, Radecka I. Bioconversion of Plastic Waste Based on Mass Full Carbon Backbone Polymeric Materials to Value-Added Polyhydroxyalkanoates (PHAs). Bioengineering. 2022; 9(9):432. https://doi.org/10.3390/bioengineering9090432
Chicago/Turabian StyleJohnston, Brian, Grazyna Adamus, Anabel Itohowo Ekere, Marek Kowalczuk, Fideline Tchuenbou-Magaia, and Iza Radecka. 2022. "Bioconversion of Plastic Waste Based on Mass Full Carbon Backbone Polymeric Materials to Value-Added Polyhydroxyalkanoates (PHAs)" Bioengineering 9, no. 9: 432. https://doi.org/10.3390/bioengineering9090432