Nanomaterials in Scaffolds for Periodontal Tissue Engineering: Frontiers and Prospects
Abstract
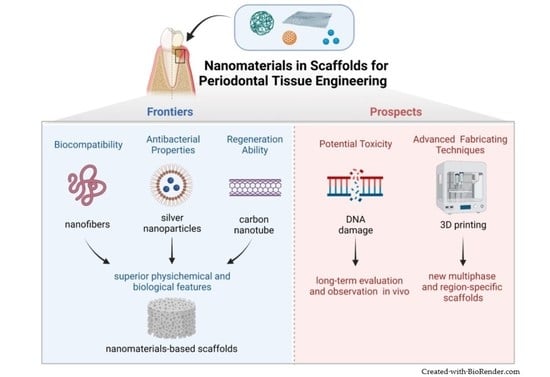
:1. Introduction
2. Tissue Engineering and Scaffolds
3. Properties of Nanomaterials
3.1. Superior Biocompatibility
3.2. Superior Antibacterial Properties
3.3. Superior Regeneration Ability
4. Nanomaterials Applied in Periodontal Tissue Engineering
4.1. Nanofibers
4.1.1. Nanomaterials Incorporated into Nanofibers
4.1.2. Advanced Techniques Fabricated Nanofibers
4.2. Antibacterial Nanomaterials
4.3. Nanomaterials for Regeneration
4.4. Nanomaterials for Drug Delivery Systems and Other Potential Applications
4.4.1. Nanomaterials for Drug Delivery Systems
4.4.2. Nanomaterials for Other Potential Applications
5. Synthesis Methods of Nanomaterials
6. Potential Toxicity of Nanomaterials
7. Conclusions & Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kwon, T.; Lamster, I.B.; Levin, L. Current Concepts in the Management of Periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef] [PubMed]
- Van Dyke, T.E.; Bartold, P.M.; Reynolds, E.C. The Nexus Between Periodontal Inflammation and Dysbiosis. Front. Immunol. 2020, 11, 511. [Google Scholar] [CrossRef] [PubMed]
- Caffesse, R.G.; Echeverria, J.J. Treatment trends in periodontics. Periodontology 2000 2019, 79, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H. Biomaterial-Based Approaches for Regeneration of Periodontal Ligament and Cementum Using 3D Platforms. Int. J. Mol. Sci. 2019, 20, 4364. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Li, X.; Wang, J.; He, X.T.; Sun, H.H.; Chen, F.M. Concise Review: Periodontal Tissue Regeneration Using Stem Cells: Strategies and Translational Considerations. Stem Cells Transl. Med. 2019, 8, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Vaquette, C.; Saifzadeh, S.; Farag, A.; Hutmacher, D.W.; Ivanovski, S. Periodontal Tissue Engineering with a Multiphasic Construct and Cell Sheets. J. Dent. Res. 2019, 98, 673–681. [Google Scholar] [CrossRef]
- Eftekhari, A.; Maleki Dizaj, S.; Sharifi, S.; Salatin, S.; Rahbar Saadat, Y.; Zununi Vahed, S.; Samiei, M.; Ardalan, M.; Rameshrad, M.; Ahmadian, E.; et al. The Use of Nanomaterials in Tissue Engineering for Cartilage Regeneration; Current Approaches and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 536. [Google Scholar] [CrossRef]
- Ni, C.; Zhou, J.; Kong, N.; Bian, T.; Zhang, Y.; Huang, X.; Xiao, Y.; Yang, W.; Yan, F. Gold nanoparticles modulate the crosstalk between macrophages and periodontal ligament cells for periodontitis treatment. Biomaterials 2019, 206, 115–132. [Google Scholar] [CrossRef]
- Shalumon, K.T.; Sowmya, S.; Sathish, D.; Chennazhi, K.P.; Nair, S.V.; Jayakumar, R. Effect of incorporation of nanoscale bioactive glass and hydroxyapatite in PCL/chitosan nanofibers for bone and periodontal tissue engineering. J. Biomed. Nanotechnol. 2013, 9, 430–440. [Google Scholar] [CrossRef]
- Peranidze, K.; Safronova, T.V.; Kildeeva, N.R. Fibrous Polymer-Based Composites Obtained by Electrospinning for Bone Tissue Engineering. Polymers 2021, 14, 96. [Google Scholar] [CrossRef]
- Ruiz-Alonso, S.; Lafuente-Merchan, M.; Ciriza, J.; Saenz-Del-Burgo, L.; Pedraz, J.L. Tendon tissue engineering: Cells, growth factors, scaffolds and production techniques. J. Control Release 2021, 333, 448–486. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, P.; Sharma, R.; Bhatt, V.D.; Dhot, P.S. Tissue Engineering; Current Status & Futuristic Scope. J. Med. Life 2019, 12, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Lin, K.; Yu, H. Advance of Nano-Composite Electrospun Fibers in Periodontal Regeneration. Front. Chem. 2019, 7, 495. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; King, M.W. Biodegradable Polymers as the Pivotal Player in the Design of Tissue Engineering Scaffolds. Adv. Healthc. Mater. 2020, 9, e1901358. [Google Scholar] [CrossRef]
- Ahmad Ruzaidi, D.A.; Mahat, M.M.; Shafiee, S.A.; Mohamed Sofian, Z.; Mohmad Sabere, A.S.; Ramli, R.; Osman, H.; Hamzah, H.H.; Zainal Ariffin, Z.; Sadasivuni, K.K. Advocating Electrically Conductive Scaffolds with Low Immunogenicity for Biomedical Applications: A Review. Polymers 2021, 13, 3395. [Google Scholar] [CrossRef]
- Saeedi Garakani, S.; Khanmohammadi, M.; Atoufi, Z.; Kamrava, S.K.; Setayeshmehr, M.; Alizadeh, R.; Faghihi, F.; Bagher, Z.; Davachi, S.M.; Abbaspourrad, A. Fabrication of chitosan/agarose scaffolds containing extracellular matrix for tissue engineering applications. Int. J. Biol. Macromol. 2020, 143, 533–545. [Google Scholar] [CrossRef]
- Zamani, Y.; Amoabediny, G.; Mohammadi, J.; Seddiqi, H.; Helder, M.N.; Zandieh-Doulabi, B.; Klein-Nulend, J.; Koolstra, J.H. 3D-printed poly(Ɛ-caprolactone) scaffold with gradient mechanical properties according to force distribution in the mandible for mandibular bone tissue engineering. J. Mech. Behav. Biomed. Mater. 2020, 104, 103638. [Google Scholar] [CrossRef]
- Schipani, R.; Nolan, D.R.; Lally, C.; Kelly, D.J. Integrating finite element modelling and 3D printing to engineer biomimetic polymeric scaffolds for tissue engineering. Connect. Tissue Res. 2020, 61, 174–189. [Google Scholar] [CrossRef]
- Maleki Dizaj, S.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Ciprofloxacin HCl-loaded calcium carbonate nanoparticles: Preparation, solid state characterization, and evaluation of antimicrobial effect against Staphylococcus aureus. Artif. Cells Nanomed. Biotechnol. 2017, 45, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Laurencin, C.T.; Ashe, K.M.; Henry, N.; Kan, H.M.; Lo, K.W. Delivery of small molecules for bone regenerative engineering: Preclinical studies and potential clinical applications. Drug. Discov. Today 2014, 19, 794–800. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Xu, Z.; Liu, G.; Wu, J. Polyphenols as a versatile component in tissue engineering. Acta Biomater. 2021, 119, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Safari, B.; Aghanejad, A.; Roshangar, L.; Davaran, S. Osteogenic effects of the bioactive small molecules and minerals in the scaffold-based bone tissue engineering. Colloids Surf. B Biointerfaces 2021, 198, 111462. [Google Scholar] [CrossRef] [PubMed]
- Laird, N.Z.; Acri, T.M.; Chakka, J.L.; Quarterman, J.C.; Malkawi, W.I.; Elangovan, S.; Salem, A.K. Applications of nanotechnology in 3D printed tissue engineering scaffolds. Eur. J. Pharm. Biopharm. 2021, 161, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.U.; Oh, J.H.; Cho, Y.D.; Chung, S.H.; Lee, G.; Baek, J.H.; Ryoo, H.M.; Woo, K.M. Fibrous Topography-Potentiated Canonical Wnt Signaling Directs the Odontoblastic Differentiation of Dental Pulp-Derived Stem Cells. ACS Appl. Mater. Interfaces 2018, 10, 17526–17541. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Acri, T.; Geary, S.; Salem, A.K. Biomimetic Mineralization of Biomaterials Using Simulated Body Fluids for Bone Tissue Engineering and Regenerative Medicine. Tissue Eng. Part A 2017, 23, 1169–1180. [Google Scholar] [CrossRef]
- D’Avanzo, N.; Bruno, M.C.; Giudice, A.; Mancuso, A.; Gaetano, F.; Cristiano, M.C.; Paolino, D.; Fresta, M. Influence of Materials Properties on Bio-Physical Features and Effectiveness of 3D-Scaffolds for Periodontal Regeneration. Molecules 2021, 26, 1643. [Google Scholar] [CrossRef]
- Rahman, S.U.; Nagrath, M.; Ponnusamy, S.; Arany, P.R. Nanoscale and Macroscale Scaffolds with Controlled-Release Polymeric Systems for Dental Craniomaxillofacial Tissue Engineering. Materials 2018, 11, 1478. [Google Scholar] [CrossRef]
- Cafferata, E.A.; Alvarez, C.; Diaz, K.T.; Maureira, M.; Monasterio, G.; González, F.E.; Covarrubias, C.; Vernal, R. Multifunctional nanocarriers for the treatment of periodontitis: Immunomodulatory, antimicrobial, and regenerative strategies. Oral Dis. 2019, 25, 1866–1878. [Google Scholar] [CrossRef]
- Fernandez, C.C.; Sokolonski, A.R.; Fonseca, M.S.; Stanisic, D.; Araújo, D.B.; Azevedo, V.; Portela, R.D.; Tasic, L. Applications of Silver Nanoparticles in Dentistry: Advances and Technological Innovation. Int. J. Mol. Sci. 2021, 22, 2485. [Google Scholar] [CrossRef]
- Noronha, V.T.; Paula, A.J.; Durán, G.; Galembeck, A.; Cogo-Müller, K.; Franz-Montan, M.; Durán, N. Silver nanoparticles in dentistry. Dent. Mater. 2017, 33, 1110–1126. [Google Scholar] [CrossRef]
- Tahriri, M.; Del Monico, M.; Moghanian, A.; Tavakkoli Yaraki, M.; Torres, R.; Yadegari, A.; Tayebi, L. Graphene and its derivatives: Opportunities and challenges in dentistry. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 171–185. [Google Scholar] [CrossRef]
- Liu, C.; Tan, D.; Chen, X.; Liao, J.; Wu, L. Research on Graphene and Its Derivatives in Oral Disease Treatment. Int. J. Mol. Sci. 2022, 23, 4737. [Google Scholar] [CrossRef]
- Yassin, M.A.; Mustafa, K.; Xing, Z.; Sun, Y.; Fasmer, K.E.; Waag, T.; Krueger, A.; Steinmüller-Nethl, D.; Finne-Wistrand, A.; Leknes, K.N. A Copolymer Scaffold Functionalized with Nanodiamond Particles Enhances Osteogenic Metabolic Activity and Bone Regeneration. Macromol. Biosci. 2017, 17, 1600427. [Google Scholar] [CrossRef]
- Feng, C.; Xue, J.; Yu, X.; Zhai, D.; Lin, R.; Zhang, M.; Xia, L.; Wang, X.; Yao, Q.; Chang, J.; et al. Co-inspired hydroxyapatite-based scaffolds for vascularized bone regeneration. Acta Biomater. 2021, 119, 419–431. [Google Scholar] [CrossRef]
- Shang, L.; Liu, Z.; Ma, B.; Shao, J.; Wang, B.; Ma, C.; Ge, S. Dimethyloxallyl glycine/nanosilicates-loaded osteogenic/angiogenic difunctional fibrous structure for functional periodontal tissue regeneration. Bioact. Mater. 2021, 6, 1175–1188. [Google Scholar] [CrossRef]
- Li, G.; Zhou, T.; Lin, S.; Shi, S.; Lin, Y. Nanomaterials for Craniofacial and Dental Tissue Engineering. J. Dent. Res. 2017, 96, 725–732. [Google Scholar] [CrossRef]
- Shen, R.; Xu, W.; Xue, Y.; Chen, L.; Ye, H.; Zhong, E.; Ye, Z.; Gao, J.; Yan, Y. The use of chitosan/PLA nano-fibers by emulsion eletrospinning for periodontal tissue engineering. Artif. Cells Nanomed. Biotechnol. 2018, 46, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Varoni, E.M.; Vijayakumar, S.; Canciani, E.; Cochis, A.; De Nardo, L.; Lodi, G.; Rimondini, L.; Cerruti, M. Chitosan-Based Trilayer Scaffold for Multitissue Periodontal Regeneration. J. Dent. Res. 2018, 97, 303–311. [Google Scholar] [CrossRef]
- De Oliveira Barud, H.G.; Da Silva, R.R.; Borges, M.A.C.; Castro, G.R.; Ribeiro, S.J.L.; Da Silva Barud, H. Bacterial Nanocellulose in Dentistry: Perspectives and Challenges. Molecules 2020, 26, 49. [Google Scholar] [CrossRef] [PubMed]
- Klinthoopthamrong, N.; Chaikiawkeaw, D.; Phoolcharoen, W.; Rattanapisit, K.; Kaewpungsup, P.; Pavasant, P.; Hoven, V.P. Bacterial cellulose membrane conjugated with plant-derived osteopontin: Preparation and its potential for bone tissue regeneration. Int. J. Biol. Macromol. 2020, 149, 51–59. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Jiang, H.; Wang, R.; Xie, Y.; Zhao, C. Fabrication of metronidazole loaded poly (ε-caprolactone)/zein core/shell nanofiber membranes via coaxial electrospinning for guided tissue regeneration. J. Colloid Interface Sci. 2017, 490, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Shao, W.; Qian, W.; He, J.; Zhou, Y.; Qi, K.; Wang, L.; Cui, S.; Wang, R. Biomineralized poly (l-lactic-co-glycolic acid)-tussah silk fibroin nanofiber fabric with hierarchical architecture as a scaffold for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 84, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Ou, Q.; Miao, Y.; Yang, F.; Lin, X.; Zhang, L.M.; Wang, Y. Zein/gelatin/nanohydroxyapatite nanofibrous scaffolds are biocompatible and promote osteogenic differentiation of human periodontal ligament stem cells. Biomater. Sci. 2019, 7, 1973–1983. [Google Scholar] [CrossRef] [PubMed]
- Dieterle, M.P.; Steinberg, T.; Tomakidi, P.; Nohava, J.; Vach, K.; Schulz, S.D.; Hellwig, E.; Proksch, S. Novel in Situ-Cross-Linked Electrospun Gelatin/Hydroxyapatite Nonwoven Scaffolds Prove Suitable for Periodontal Tissue Engineering. Pharmaceutics 2022, 14, 1286. [Google Scholar] [CrossRef]
- Duruel, T.; Çakmak, A.S.; Akman, A.; Nohutcu, R.M.; Gümüşderelioğlu, M. Sequential IGF-1 and BMP-6 releasing chitosan/alginate/PLGA hybrid scaffolds for periodontal regeneration. Int. J. Biol. Macromol. 2017, 104, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Moshaverinia, A.; Chen, C.; Xu, X.; Akiyama, K.; Ansari, S.; Zadeh, H.H.; Shi, S. Bone regeneration potential of stem cells derived from periodontal ligament or gingival tissue sources encapsulated in RGD-modified alginate scaffold. Tissue Eng. Part A 2014, 20, 611–621. [Google Scholar] [CrossRef]
- Wu, S.; Xiao, Z.; Song, J.; Li, M.; Li, W. Evaluation of BMP-2 Enhances the Osteoblast Differentiation of Human Amnion Mesenchymal Stem Cells Seeded on Nano-Hydroxyapatite/Collagen/Poly(l-Lactide). Int. J. Mol. Sci. 2018, 19, 2171. [Google Scholar] [CrossRef]
- Masoudi Rad, M.; Nouri Khorasani, S.; Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Foroughi, M.R.; Kharaziha, M.; Saadatkish, N.; Ramakrishna, S. Fabrication and characterization of two-layered nanofibrous membrane for guided bone and tissue regeneration application. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 80, 75–87. [Google Scholar] [CrossRef]
- Pedram Rad, Z.; Mokhtari, J.; Abbasi, M. Calendula officinalis extract/PCL/Zein/Gum arabic nanofibrous bio-composite scaffolds via suspension, two-nozzle and multilayer electrospinning for skin tissue engineering. Int. J. Biol. Macromol. 2019, 135, 530–543. [Google Scholar] [CrossRef]
- Shoba, E.; Lakra, R.; Kiran, M.S.; Korrapati, P.S. 3D nano bilayered spatially and functionally graded scaffold impregnated bromelain conjugated magnesium doped hydroxyapatite nanoparticle for periodontal regeneration. J. Mech. Behav. Biomed. Mater. 2020, 109, 103822. [Google Scholar] [CrossRef]
- Sunandhakumari, V.J.; Vidhyadharan, A.K.; Alim, A.; Kumar, D.; Ravindran, J.; Krishna, A.; Prasad, M. Fabrication and In Vitro Characterization of Bioactive Glass/Nano Hydroxyapatite Reinforced Electrospun Poly(ε-Caprolactone) Composite Membranes for Guided Tissue Regeneration. Bioengineering 2018, 5, 54. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Li, J.; Chen, Z.; Fan, M.; Lin, F.; Xie, Y. Electrospun Polysaccharides for Periodontal Tissue Engineering: A Review of Recent Advances and Future Perspectives. Ann. Biomed. Eng. 2022, 50, 769–793. [Google Scholar] [CrossRef] [PubMed]
- Furtos, G.; Rivero, G.; Rapuntean, S.; Abraham, G.A. Amoxicillin-loaded electrospun nanocomposite membranes for dental applications. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 966–976. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar-Mohammadi, M.; Zamani, M.; Prabhakaran, M.P.; Bahrami, S.H.; Ramakrishna, S. Electrospinning of PLGA/gum tragacanth nanofibers containing tetracycline hydrochloride for periodontal regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.-n.; Zhang, X.; Xu, H.-y.; Hua, F.; Hu, X.-g.; Xie, Q.; Wang, W.; Jia, J. Development of a Doxycycline Hydrochloride-Loaded Electrospun Nanofibrous Membrane for GTR/GBR Applications. J. Nanomater. 2016, 2016, 6507459. [Google Scholar] [CrossRef]
- Wang, C.Y.; Chiu, Y.C.; Lee, A.K.; Lin, Y.A.; Lin, P.Y.; Shie, M.Y. Biofabrication of Gingival Fibroblast Cell-Laden Collagen/Strontium-Doped Calcium Silicate 3D-Printed Bi-Layered Scaffold for Osteoporotic Periodontal Regeneration. Biomedicines 2021, 9, 431. [Google Scholar] [CrossRef]
- Naghieh, S.; Foroozmehr, E.; Badrossamay, M.; Kharaziha, M. Combinational processing of 3D printing and electrospinning of hierarchical poly(lactic acid)/gelatin-forsterite scaffolds as a biocomposite: Mechanical and biological assessment. Mater. Des. 2017, 133, 128–135. [Google Scholar] [CrossRef]
- Panáček, A.; Smékalová, M.; Večeřová, R.; Bogdanová, K.; Röderová, M.; Kolář, M.; Kilianová, M.; Hradilová, Š.; Froning, J.P.; Havrdová, M.; et al. Silver nanoparticles strongly enhance and restore bactericidal activity of inactive antibiotics against multiresistant Enterobacteriaceae. Colloids Surf. B Biointerfaces 2016, 142, 392–399. [Google Scholar] [CrossRef]
- He, J.; Zhu, X.; Qi, Z.; Wang, C.; Mao, X.; Zhu, C.; He, Z.; Li, M.; Tang, Z. Killing dental pathogens using antibacterial graphene oxide. ACS Appl. Mater. Interfaces 2015, 7, 5605–5611. [Google Scholar] [CrossRef]
- Shao, J.; Yu, N.; Kolwijck, E.; Wang, B.; Tan, K.W.; Jansen, J.A.; Walboomers, X.F.; Yang, F. Biological evaluation of silver nanoparticles incorporated into chitosan-based membranes. Nanomedicine 2017, 12, 2771–2785. [Google Scholar] [CrossRef]
- Singh, P.; Ahn, S.; Kang, J.P.; Veronika, S.; Huo, Y.; Singh, H.; Chokkaligam, M.; El-Agamy Farh, M.; Aceituno, V.C.; Kim, Y.J.; et al. In vitro anti-inflammatory activity of spherical silver nanoparticles and monodisperse hexagonal gold nanoparticles by fruit extract of Prunus serrulata: A green synthetic approach. Artif. Cells Nanomed. Biotechnol. 2018, 46, 2022–2032. [Google Scholar] [CrossRef]
- Qian, Y.; Zhou, X.; Zhang, F.; Diekwisch, T.G.H.; Luan, X.; Yang, J. Triple PLGA/PCL Scaffold Modification Including Silver Impregnation, Collagen Coating, and Electrospinning Significantly Improve Biocompatibility, Antimicrobial, and Osteogenic Properties for Orofacial Tissue Regeneration. ACS Appl. Mater. Interfaces 2019, 11, 37381–37396. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Huang, X.; Gu, L. Periodontal Bifunctional Biomaterials: Progress and Perspectives. Materials 2021, 14, 7588. [Google Scholar] [CrossRef] [PubMed]
- Prado-Prone, G.; Silva-Bermudez, P.; Bazzar, M.; Focarete, M.L.; Rodil, S.E.; Vidal-Gutiérrez, X.; García-Macedo, J.A.; García-Pérez, V.I.; Velasquillo, C.; Almaguer-Flores, A. Antibacterial composite membranes of polycaprolactone/gelatin loaded with zinc oxide nanoparticles for guided tissue regeneration. Biomed. Mater. 2020, 15, 035006. [Google Scholar] [CrossRef] [PubMed]
- Hickey, D.J.; Ercan, B.; Sun, L.; Webster, T.J. Adding MgO nanoparticles to hydroxyapatite-PLLA nanocomposites for improved bone tissue engineering applications. Acta Biomater. 2015, 14, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.N.; Cho, Y.J.; Tarafder, S.; Lee, C.H. The recent advances in scaffolds for integrated periodontal regeneration. Bioact. Mater. 2021, 6, 3328–3342. [Google Scholar] [CrossRef] [PubMed]
- Augustine, A.; Augustine, R.; Hasan, A.; Raghuveeran, V.; Rouxel, D.; Kalarikkal, N.; Thomas, S. Development of titanium dioxide nanowire incorporated poly(vinylidene fluoride-trifluoroethylene) scaffolds for bone tissue engineering applications. J. Mater. Sci. Mater. Med. 2019, 30, 96. [Google Scholar] [CrossRef]
- Lu, M.; Chen, H.; Yuan, B.; Zhou, Y.; Min, L.; Xiao, Z.; Yang, X.; Zhu, X.; Tu, C.; Zhang, X. The morphological effect of nanostructured hydroxyapatite coatings on the osteoinduction and osteogenic capacity of porous titanium. Nanoscale 2020, 12, 24085–24099. [Google Scholar] [CrossRef]
- Zarei, M.; Karbasi, S.; Sari Aslani, F.; Zare, S.; Koohi-Hosseinabad, O.; Tanideh, N. In Vitro and In Vivo Evaluation of Poly (3-hydroxybutyrate)/Carbon Nanotubes Electrospun Scaffolds for Periodontal Ligament Tissue Engineering. J. Dent. 2020, 21, 18–30. [Google Scholar] [CrossRef]
- Nishida, E.; Miyaji, H.; Kato, A.; Takita, H.; Iwanaga, T.; Momose, T.; Ogawa, K.; Murakami, S.; Sugaya, T.; Kawanami, M. Graphene oxide scaffold accelerates cellular proliferative response and alveolar bone healing of tooth extraction socket. Int. J. Nanomed. 2016, 11, 2265–2277. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Liang, X.; Wang, Y.; Wang, D.; Teng, M.; Xu, H.; Zhao, B.; Han, L. Graphene-Based Nanomaterials for Dental Applications: Principles, Current Advances, and Future Outlook. Front. Bioeng. Biotechnol. 2022, 10, 804201. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Park, S.; Kim, J.E.; Jang, K.J.; Seonwoo, H.; Chung, J.H. Enhanced Osteogenic Differentiation of Periodontal Ligament Stem Cells Using a Graphene Oxide-Coated Poly(ε-caprolactone) Scaffold. Polymers 2021, 13, 797. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, Y.; Li, L.; Fu, H.; Yang, W.; Yan, F. Human β-defensin 3-combined gold nanoparticles for enhancement of osteogenic differentiation of human periodontal ligament cells in inflammatory microenvironments. Int. J. Nanomed. 2018, 13, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Burdușel, A.C.; Gherasim, O.; Andronescu, E.; Grumezescu, A.M.; Ficai, A. Inorganic Nanoparticles in Bone Healing Applications. Pharmaceutics 2022, 14, 770. [Google Scholar] [CrossRef]
- Silva, C.; Bobillier, F.; Canales, D.; Antonella Sepúlveda, F.; Cament, A.; Amigo, N.; Rivas, L.M.; Ulloa, M.T.; Reyes, P.; Ortiz, J.A.; et al. Mechanical and Antimicrobial Polyethylene Composites with CaO Nanoparticles. Polymers 2020, 12, 2132. [Google Scholar] [CrossRef]
- Pan, P.; Yue, Q.; Li, J.; Gao, M.; Yang, X.; Ren, Y.; Cheng, X.; Cui, P.; Deng, Y. Smart Cargo Delivery System based on Mesoporous Nanoparticles for Bone Disease Diagnosis and Treatment. Adv. Sci. 2021, 8, e2004586. [Google Scholar] [CrossRef]
- Tan, Y.; Feng, J.; Xiao, Y.; Bao, C. Grafting resveratrol onto mesoporous silica nanoparticles towards efficient sustainable immunoregulation and insulin resistance alleviation for diabetic periodontitis therapy. J. Mater. Chem. B 2022, 10, 4840–4855. [Google Scholar] [CrossRef]
- Pouroutzidou, G.K.; Lazaridou, M.; Papoulia, C.; Tsamesidis, I.; Chrissafis, K.; Vourlias, G.; Paraskevopoulos, K.M.; Bikiaris, D.; Kontonasaki, E. Electrospun PLGA Membranes with Incorporated Moxifloxacin-Loaded Silica-Based Mesoporous Nanocarriers for Periodontal Regeneration. Nanomaterials 2022, 12, 850. [Google Scholar] [CrossRef]
- Afrasiabi, S.; Chiniforush, N.; Barikani, H.R.; Partoazar, A.; Goudarzi, R. Nanostructures as Targeted Therapeutics for Combating Oral Bacterial Diseases. Biomedicines 2021, 9, 1435. [Google Scholar] [CrossRef]
- Zhang, T.; Ying, D.; Qi, M.; Li, X.; Fu, L.; Sun, X.; Wang, L.; Zhou, Y. Anti-Biofilm Property of Bioactive Upconversion Nanocomposites Containing Chlorin e6 against Periodontal Pathogens. Molecules 2019, 24, 2692. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Cai, W.; Zhao, S.; Shi, L.; Chen, Y.; Li, X.; Sun, X.; Mao, Y.; He, B.; Hou, Y.; et al. Oxidative stress-related biomarkers in saliva and gingival crevicular fluid associated with chronic periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Totu, E.E.; Isildak, I.; Nechifor, A.C.; Cristache, C.M.; Enachescu, M. New sensor based on membranes with magnetic nano-inclusions for early diagnosis in periodontal disease. Biosens. Bioelectron. 2018, 102, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Huston, M.; DeBella, M.; DiBella, M.; Gupta, A. Green Synthesis of Nanomaterials. Nanomaterials 2021, 11, 2130. [Google Scholar] [CrossRef]
- Gour, A.; Jain, N.K. Advances in green synthesis of nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 47, 844–851. [Google Scholar] [CrossRef]
- Zambonino, M.C.; Quizhpe, E.M.; Jaramillo, F.E.; Rahman, A.; Santiago Vispo, N.; Jeffryes, C.; Dahoumane, S.A. Green Synthesis of Selenium and Tellurium Nanoparticles: Current Trends, Biological Properties and Biomedical Applications. Int. J. Mol. Sci. 2021, 22, 989. [Google Scholar] [CrossRef] [PubMed]
- Vimbela, G.V.; Ngo, S.M.; Fraze, C.; Yang, L.; Stout, D.A. Antibacterial properties and toxicity from metallic nanomaterials. Int. J. Nanomed. 2017, 12, 3941–3965. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Zhang, X.; Sun, L.; Wei, Y.; Wei, X. Cellular Toxicity and Immunological Effects of Carbon-based Nanomaterials. Part. Fibre Toxicol. 2019, 16, 18. [Google Scholar] [CrossRef]
- Ye, G.; Bao, F.; Zhang, X.; Song, Z.; Liao, Y.; Fei, Y.; Bunpetch, V.; Heng, B.C.; Shen, W.; Liu, H.; et al. Nanomaterial-based scaffolds for bone tissue engineering and regeneration. Nanomedicine 2020, 15, 1995–2017. [Google Scholar] [CrossRef]
- Najahi-Missaoui, W.; Arnold, R.D.; Cummings, B.S. Safe Nanoparticles: Are We There Yet? Int. J. Mol. Sci. 2020, 22, 385. [Google Scholar] [CrossRef]
| Merits | Limitations | Potential Applications | Ref. | |
|---|---|---|---|---|
| Natural polymers | ||||
| Chitosan (CS) | biodegradable, biocompatible, nontoxic, biologically renewable, bacteriostatic | little solubility in organic solvents and neutral aqueous solutions | Chitosan-based scaffold promoted human gingival fibroblasts and osteoblasts metabolism and mineralization. Chitosan NPs promoted the osteogenic differentiation of 1 BMSCs. | [37,38] |
| Bacterial cellulose (BC) | biocompatibility, low cost, ease of processing, ideal mechanical properties like high tensile strength | handicap in quality control related to contaminations | The non-resorbable BC membrane helped the closure of the class II furcation lesions in humans. The commercial BC membrane led to sufficient 2 GTR outcomes in human periodontal defects. | [39,40] |
| Structure protein | great biological properties like biocompatibility, resorbability, enhancing cell adhesion | immunoreactivity associated with its bovine source and allogeneic species | 3 TSF enhanced the mesenchymal stem cell differentiation toward osteoblasts. Core-shell nanofibers utilizing zein prolonged metronidazole release. | [41,42] |
| Gelatin (GEL) | ideal biocompatibility, low immunogenicity | dissolubility in organic solution | GEL possessed bio-signal groups to enhance the proliferation of 4 hPDLSCs. The incorporation of GEL into nanofibrous membranes increased the osteogenic capability of preosteoblasts. | [43,44] |
| Alginates | biocompatible, hydrophilic, non-immunogenic, cost-effective | poor cell adhesion, low mechanical strength, low degradability | Alginates particles in hybrid scaffolds provided an early release of IGF-1 and BMP-6. 5 RGD-modified alginate scaffold enhanced MSC viability and osteogenic differentiation. | [45,46] |
| Synthetic Polymers | ||||
| Polylactic acid(PLA) | high mechanical strength | hydrophobicity, cause inflammation | The nHA/Collagen/PLA scaffolds promoted 6 hAMSCs seeding, proliferation, and osteogenic differentiation. Pure PLA nanofibers scaffolds facilitated BMSCs proliferation. | [37,47] |
| Poly (lactic acid-co-glycolic acid) (PLGA) | biocompatible, biodegradable | weak hydrophilicity, cell adhesion, acidic degradation products | The PLGA particles in hybrid scaffolds provided a lasting release of IGF-1 and BMP-6. The 7 DMOG/nSi-PLGA fibrous compounds enhanced and orchestrated osteogenesis-angiogenesis. | [35,45] |
| Poly-caprolactone (PCL) | enhanced mechanical properties, proper degradation kinetics with morphological characteristics | poor hydrophilicity, cause inflammation | The hybrid PCL scaffolds promoted PDLC differentiation and periostin expression. The electrospun PCL membranes presented a controlled release profile of the active compounds induced fibroblast formation. | [48,49] |
| Bioceramics | ||||
| Hydroxyapatite (HA) | Bioactive, biocompatible, excellent mechanical properties | poor degradation rates, drug release properties | HA-based coil scaffolds promoted angiogenesis and osteogenesis in rat and rabbit critical-sized bone defection. The magnesium-doped and the bromelain-functionalized HA-based scaffold regenerated periodontal tissue in vivo in a Wistar rat model. | [34,50] |
| Bioactive glass (BG) | facilitate growth factor production, gene expression, the proliferation of osteoblasts, and reconstruction of bone tissue | commercial BGs only show bone formation, without cementum or PDL | The nBG in the PCL composite scaffold enhanced the adhesion, and proliferation of hPDLCs. The fish collagen/bioactive glass/chitosan nano-composite scaffold promoted the formation of new bone and light inflammation occurred in the beagle dog’s periodontal defect model. | [51,52] |
| Materials | Potential Applications | References | |
|---|---|---|---|
| Ag | chitosan-AgNPs | inhibited the growth of Porphyromonas gingivalis and Fusobacterium nucleatum related to dose | [60] |
| AgNPs synthesized with an appropriated capping agent | promoted gram-negative bacterial inhibition | [58] | |
| AgNPs | possessed an anti-inflammatory effect by modulating inflammatory cytokines and regenerating growth factors | [61] | |
| the 1 PP-pDA-COL-Ag scaffold | promoted alveolar bone regeneration and accelerated periodontitis treatment in a mouse periodontitis model | [62] | |
| ZnO | chitin hydrogel-ZnO | exhibited osteogenesis promotion both in vitro and rat periodontal defect model in vivo | [63] |
| PCL/GEL-ZnO | decreased the number of planktonic and the formation of the Staphylococcus aureus biofilm | [64] | |
| MgO | HA/2 PLLA-nMgO | enhanced osteoblast adhesion and proliferation | [65] |
| 3 PLA/gelatin-nMgO | guided periodontal tissue regeneration in rat periodontal defect models | [66] | |
| TiO2 | 4 P(VDF-TrFE)- TiO2 nanowires | increased fibroblasts and osteoblasts adhesion and proliferation | [67] |
| Materials | Potential Applications | References | |
|---|---|---|---|
| graphene-based nanomaterials | 1 PHB/1%CNTs scaffolds | enhanced attachment and proliferation of the 2 PDLSCs | [69] |
| 3 GO scaffolds | promoted cellular ingrowth behavior and the formation of dog bone defect | [70] | |
| 4 PGO/HA-AG scaffolds | induced alveolar bone regeneration in bone defects of diabetic rat periodontitis models | [71] | |
| 5 PCL -GO composites | promoted the proliferation of 6 hPDLSCs moderately, favored the differentiation of osteogenic | [72] | |
| Metallic NPs | 7 poly(LLA-co-CL)/nDPs scaffolds | enhanced seeding efficiency of 8 BMSCs | [33] |
| Human β-defensin 3 9 AuNPs | promoted the osteogenic differentiation of 10 hPDLCs in inflammatory microenvironments | [73] | |
| L/D-cysteine anchored AuNPs | facilitated osteogenic differentiation into hPDLCs and regenerating alveolar bones and periodontal ligaments in rat periodontal-defect models | [74] | |
| PCL or PCL/gelatin-nCaO matrices | increased the viability and osteogenic differentiation of osteoprecursor cell | [75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Huang, X. Nanomaterials in Scaffolds for Periodontal Tissue Engineering: Frontiers and Prospects. Bioengineering 2022, 9, 431. https://doi.org/10.3390/bioengineering9090431
Chen S, Huang X. Nanomaterials in Scaffolds for Periodontal Tissue Engineering: Frontiers and Prospects. Bioengineering. 2022; 9(9):431. https://doi.org/10.3390/bioengineering9090431
Chicago/Turabian StyleChen, Siyang, and Xin Huang. 2022. "Nanomaterials in Scaffolds for Periodontal Tissue Engineering: Frontiers and Prospects" Bioengineering 9, no. 9: 431. https://doi.org/10.3390/bioengineering9090431