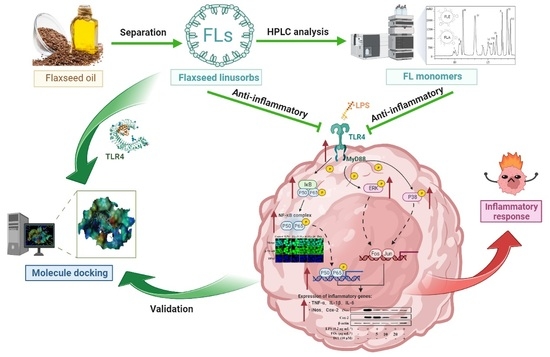
The Anti-Inflammatory Mechanism of Flaxseed Linusorbs on Lipopolysaccharide-Induced RAW 264.7 Macrophages by Modulating TLR4/NF-κB/MAPK Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of FLA and FLE
2.3. Cell Cultures
2.4. Cell Viability Assays
2.5. Determination of NO Secretion
2.6. ELISA Assays for Cytokine Determination
2.7. Quantitative Real-Time PCR
2.8. Western Blot Analysis
2.9. Confocal Assays
2.10. Molecular Docking of FLs with TLR4
2.11. Statistical Analysis
3. Results
3.1. Effect of FLs on Cell Viability and Inhibition of NO
3.2. Effects of FLs on the Inhibiting Secretion of Pro-Inflammatory Mediators and Cytokine
3.3. Effects of FLs on Blocking NF-κB/MAPK Signaling Pathway
3.4. FLs Target TLR4 Protein Directly
3.5. In Silico Affinity of FLs Binding to TLR4
3.6. Anti-Inflammatory Activity of FLA and FLE
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kobayashi, T.; Siegmund, B.; Le Berre, C.; Wei, S.C.; Ferrante, M.; Shen, B.; Bernstein, C.N.; Danese, S.; Peyrin-Biroulet, L.; Hibi, T. Ulcerative colitis. Nat. Rev. Dis. Prim. 2020, 6, 74. [Google Scholar] [CrossRef]
- Boutou, A.K.; Shrikrishna, D.; Tanner, R.J.; Smith, C.; Kelly, J.L.; Ward, S.P.; Polkey, M.I.; Hopkinson, N.S. Lung function indices for predicting mortality in COPD. Eur. Respir. J. 2013, 42, 616–625. [Google Scholar] [CrossRef] [Green Version]
- Figus, F.A.; Piga, M.; Azzolin, I.; McConnell, R.; Iagnocco, A. Rheumatoid arthritis: Extra-articular manifestations and comorbidities. Autoimmun. Rev. 2021, 20, 102776. [Google Scholar] [CrossRef]
- Hajishengallis, G. Interconnection of periodontal disease and comorbidities: Evidence, mechanisms, and implications. Periodontol. 2000 2022, 89, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Fest, J.; Ruiter, R.; Mulder, M.; Groot Koerkamp, B.; Ikram, M.A.; Stricker, B.H.; van Eijck, C.H.J. The systemic immune-inflammation index is associated with an increased risk of incident cancer-A population-based cohort study. Int. J. Cancer 2020, 146, 692–698. [Google Scholar] [CrossRef]
- Margină, D.; Ungurianu, A.; Purdel, C.; Tsoukalas, D.; Sarandi, E.; Thanasoula, M.; Tekos, F.; Mesnage, R.; Kouretas, D.; Tsatsakis, A. Chronic Inflammation in the Context of Everyday Life: Dietary Changes as Mitigating Factors. Int. J. Environ. Res. Public Health 2020, 17, 4135. [Google Scholar] [CrossRef] [PubMed]
- Minihane, A.M.; Vinoy, S.; Russell, W.R.; Baka, A.; Roche, H.M.; Tuohy, K.M.; Teeling, J.L.; Blaak, E.E.; Fenech, M.; Vauzour, D.; et al. Low-grade inflammation, diet composition and health: Current research evidence and its translation. Br. J. Nutr. 2015, 114, 999–1012. [Google Scholar] [CrossRef] [Green Version]
- Nogueira Silva Lima, M.T.; Howsam, M.; Anton, P.M.; Delayre-Orthez, C.; Tessier, F.J. Effect of Advanced Glycation End-Products and Excessive Calorie Intake on Diet-Induced Chronic Low-Grade Inflammation Biomarkers in Murine Models. Nutrients 2021, 13, 3091. [Google Scholar] [CrossRef] [PubMed]
- Adolph, T.E.; Meyer, M.; Schwärzler, J.; Mayr, L.; Grabherr, F.; Tilg, H. The metabolic nature of inflammatory bowel diseases. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 753–767. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Zhao, Y.; Sun, X.; Song, Z.; McClain, C.J.; Zhou, Z. Dietary zinc deficiency exaggerates ethanol-induced liver injury in mice: Involvement of intrahepatic and extrahepatic factors. PLoS ONE 2013, 8, e76522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehman, S.; Jianglin, Z. Micronutrient deficiencies and cardiac health. Front. Nutr. 2022, 9, 1010737. [Google Scholar] [CrossRef]
- Mohammad, M.K.; Zhou, Z.; Cave, M.; Barve, A.; McClain, C.J. Zinc and liver disease. Nutr. Clin. Pract. 2012, 27, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Mylka, V.; Deckers, J.; Ratman, D.; De Cauwer, L.; Thommis, J.; De Rycke, R.; Impens, F.; Libert, C.; Tavernier, J.; Vanden Berghe, W.; et al. The autophagy receptor SQSTM1/p62 mediates anti-inflammatory actions of the selective NR3C1/glucocorticoid receptor modulator compound A (CpdA) in macrophages. Autophagy 2018, 14, 2049–2064. [Google Scholar] [CrossRef] [Green Version]
- Childs, C.E.; Calder, P.C.; Miles, E.A. Diet and Immune Function. Nutrients 2019, 11, 1933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rong, X.; Xu, J.; Jiang, Y.; Li, F.; Chen, Y.; Dou, Q.P.; Li, D. Citrus peel flavonoid nobiletin alleviates lipopolysaccharide-induced inflammation by activating IL-6/STAT3/FOXO3a-mediated autophagy. Food Funct. 2021, 12, 1305–1317. [Google Scholar] [CrossRef] [PubMed]
- Menu, I.M.N.; Karunarathne, W.A.H.M.; Lee, M.H.; Kang, C.H.; Lee, K.T.; Choi, Y.H.; Lee, S.; Kim, G.Y. Acertannin attenuates LPS-induced inflammation by interrupting the binding of LPS to the TLR4/MD2 complex and activating Nrf2-mediated HO-1 activation. Int. Immunopharmacol. 2022, 113, 109344. [Google Scholar] [CrossRef] [PubMed]
- Monmai, C.; Go, S.H.; Shin, I.S.; You, S.; Kim, D.O.; Kang, S.; Park, W.J. Anti-Inflammatory Effect of Asterias amurensis Fatty Acids through NF-κB and MAPK Pathways against LPS-Stimulated RAW264.7 Cells. J. Microbiol. Biotechnol. 2018, 28, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- Shim, Y.Y.; Kim, J.H.; Cho, J.Y.; Reaney, M.J.T. Health benefits of flaxseed and its peptides (linusorbs). Crit. Rev. Food Sci. Nutr. 2022. [Google Scholar] [CrossRef] [PubMed]
- Gui, B.; Shim, Y.Y.; Reaney, M.J. Distribution of cyclolinopeptides in flaxseed fractions and products. J. Agric. Food Chem. 2012, 60, 8580–8589. [Google Scholar] [CrossRef]
- Mueed, A.; Madjirebaye, P.; Shibli, S.; Deng, Z. Flaxseed Peptides and Cyclolinopeptides: A Critical Review on Proteomic Approaches, Biological Activity, and Future Perspectives. J. Agric. Food Chem. 2022, 70, 14600–14612. [Google Scholar] [CrossRef]
- Stefanowicz, P. Detection and sequencing of new cyclic peptides from linseed by electrospray ionization mass spectrometry. Acta Biochim. Pol. 2001, 48, 1125–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, T.; Frank, O.; Lang, R.; Hofmann, T.; Behrens, M. Activation Spectra of Human Bitter Taste Receptors Stimulated with Cyclolinopeptides Corresponding to Fresh and Aged Linseed Oil. J. Agric. Food Chem. 2022, 70, 4382–4390. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cai, Z.Z.; Lee, W.J.; Lu, X.X.; Reaney, M.J.T.; Zhang, J.P.; Li, Y.; Zhang, N.; Wang, Y. A practical and fast isolation of 12 cyclolinopeptides (linusorbs) from flaxseed oil via preparative HPLC with phenyl-hexyl column. Food Chem. 2021, 351, 129318. [Google Scholar] [CrossRef] [PubMed]
- Sung, N.Y.; Jeong, D.; Shim, Y.Y.; Ratan, Z.A.; Jang, Y.J.; Reaney, M.J.T.; Lee, S.; Lee, B.H.; Kim, J.H.; Yi, Y.S.; et al. The Anti-Cancer Effect of Linusorb B3 from Flaxseed Oil through the Promotion of Apoptosis, Inhibition of Actin Polymerization, and Suppression of Src Activity in Glioblastoma Cells. Molecules 2020, 25, 5881. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Li, P.; Li, Z. Antibacterial properties of cyclolinopeptides from flaxseed oil and their application on beef. Food Chem. 2022, 385, 132715. [Google Scholar] [CrossRef]
- Zou, X.G.; Hu, J.N.; Wang, M.; Du, Y.X.; Li, J.; Mai, Q.Y.; Deng, Z.Y. [1–9-NαC]-linusorb B2 and [1–9-NαC]-linusorb B3 isolated from flaxseed induce G1 cell cycle arrest on SGC-7901 cells by modulating the AKT/JNK signaling pathway. J. Funct. Foods 2019, 52, 332–339. [Google Scholar] [CrossRef]
- Kaneda, T.; Nakajima, Y.; Koshikawa, S.; Nugroho, A.E.; Morita, H. Cyclolinopeptide F, a cyclic peptide from flaxseed inhibited RANKL-induced osteoclastogenesis via downergulation of RANK expression. J. Nat. Med. 2019, 73, 504–512. [Google Scholar] [CrossRef]
- Ruchala, P.; Picur, B.; Lisowski, M.; Cierpicki, T.; Wieczorek, Z.; Siemion, I.Z. Synthesis, conformation, and immunosuppressive activity of CLX and its analogues. Biopolymers 2003, 70, 497–511. [Google Scholar] [CrossRef]
- Zou, X.G.; Shim, Y.Y.; Cho, J.Y.; Jeong, D.; Yang, J.; Deng, Z.Y.; Reaney, M.J.T. Flaxseed orbitides, linusorbs, inhibit LPS-induced THP-1 macrophage inflammation. RSC Adv. 2020, 10, 22622–22630. [Google Scholar] [CrossRef]
- Ratan, Z.A.; Jeong, D.; Sung, N.Y.; Shim, Y.Y.; Reaney, M.J.T.; Yi, Y.S.; Cho, J.Y. LOMIX, a Mixture of Flaxseed Linusorbs, Exerts Anti-Inflammatory Effects through Src and Syk in the NF-κB Pathway. Biomolecules 2020, 10, 859. [Google Scholar] [CrossRef] [PubMed]
- Sharav, O.; Shim, Y.Y.; Okinyo-Owiti, D.P.; Sammynaiken, R.; Reaney, M.J. Effect of cyclolinopeptides on the oxidative stability of flaxseed oil. J. Agric. Food Chem. 2014, 62, 88–96. [Google Scholar] [CrossRef]
- Man, M.Q.; Wakefield, J.S.; Mauro, T.M.; Elias, P.M. Regulatory Role of Nitric Oxide in Cutaneous Inflammation. Inflammation 2022, 45, 949–964. [Google Scholar] [CrossRef]
- Ahmad, N.; Ansari, M.Y.; Haqqi, T.M. Role of iNOS in osteoarthritis: Pathological and therapeutic aspects. J. Cell. Physiol. 2020, 235, 6366–6376. [Google Scholar] [CrossRef]
- Desai, S.J.; Prickril, B.; Rasooly, A. Mechanisms of Phytonutrient Modulation of Cyclooxygenase-2 (COX-2) and Inflammation Related to Cancer. Nutr. Cancer 2018, 70, 350–375. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Park, D.H.; Lee, M.J.; Jeon, C.Y.; Kang, K.S.; Choi, Y.K. Beneficial Effect of Paeonol on Antibiotic-Associated Inflammatory Response in Mice with Diarrhea. Biomolecules 2022, 12, 1634. [Google Scholar] [CrossRef]
- Mitchell, J.P.; Carmody, R.J. NF-κB and the Transcriptional Control of Inflammation. Int. Rev. Cell Mol. Biol. 2018, 335, 41–84. [Google Scholar]
- Zhao, L.; Tao, X.; Song, T. Astaxanthin alleviates neuropathic pain by inhibiting the MAPKs and NF-κB pathways. Eur. J. Pharmacol. 2021, 912, 174575. [Google Scholar] [CrossRef]
- Marasinghe, C.K.; Jung, W.K.; Je, J.Y. Anti-inflammatory action of ark shell (Scapharca subcrenata) protein hydrolysate in LPS-stimulated RAW264.7 murine macrophages. J. Food Biochem. 2022, 46, e14493. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Luo, W.; Han, J.; Zhang, Q.; Ji, L.; Samorodov, A.V.; Pavlov, V.N.; Zhuang, Z.; Yang, D.; Yin, L.; et al. Schisandrin B protects against LPS-induced inflammatory lung injury by targeting MyD88. Phytomedicine 2022, 108, 154489. [Google Scholar] [CrossRef]
- Pagadala, N.S.; Syed, K.; Tuszynski, J. Software for molecular docking: A review. Biophys. Rev. 2017, 9, 91–102. [Google Scholar] [PubMed]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the Control of Immunity. Cell 2020, 180, 1044–1066. [Google Scholar]
- Sanz, A.B.; Sanchez-Niño, M.D.; Ramos, A.M.; Moreno, J.A.; Santamaria, B.; Ruiz-Ortega, M.; Egido, J.; Ortiz, A. NF-kappaB in renal inflammation. J. Am. Soc. Nephrol. 2010, 21, 1254–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberini, C.M. Transcription factors in long-term memory and synaptic plasticity. Physiol. Rev. 2009, 89, 121–145. [Google Scholar] [CrossRef]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [PubMed]
- Ma, Z.; Du, B.; Li, J.; Yang, Y.; Zhu, F. An Insight into Anti-Inflammatory Activities and Inflammation Related Diseases of Anthocyanins: A Review of Both In Vivo and In Vitro Investigations. Int. J. Mol. Sci. 2021, 22, 11076. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Wang, Z.Y.; Kim, H.J.; Lee, Y.H.; Kim, H.A. Pear pomace alleviated atopic dermatitis in NC/Nga mice and inhibited LPS-induced inflammation in RAW 264.7 macrophages. Nutr. Res. Pract. 2022, 16, 577–588. [Google Scholar] [CrossRef]
- Jeong, J.; Lim, M.K.; Han, E.H.; Lee, S.H.; Kang, S.; Lee, S. Extract of Aster glehni ameliorates potassium oxonate-induced hyperuricemia by modulating renal urate transporters and renal inflammation by suppressing TLR4/MyD88 signaling. Food Sci. Biotechnol. 2022, 31, 1729–1739. [Google Scholar] [CrossRef] [PubMed]
- Thalhamer, T.; McGrath, M.A.; Harnett, M.M. MAPKs and their relevance to arthritis and inflammation. Rheumatology 2008, 47, 409–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arthur, J.S.; Ley, S.C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 2013, 13, 679–692. [Google Scholar] [CrossRef]
- Kopalli, S.R.; Cha, K.M.; Cho, J.Y.; Kim, S.K.; Koppula, S. Cordycepin from Medicinal Fungi Cordyceps militaris Mitigates Inflammaging-Associated Testicular Damage via Regulating NF-kappa B/MAPKs Signaling in Naturally Aged Rats. Mycobiology 2022, 50, 89–98. [Google Scholar] [CrossRef]
- Ye, L.; Xin, Y.; Wu, Z.Y.; Sun, H.J.; Huang, D.J.; Sun, Z.Q. A Newly Synthesized Flavone from Luteolin Escapes from COMT-Catalyzed Methylation and Inhibits Lipopolysaccharide-Induced Inflammation in RAW264.7 Macrophages via JNK, p38 and NF-Kappa B Signaling Pathways. J. Microbiol. Biotechnol. 2022, 32, 15–26. [Google Scholar] [CrossRef]
- Chen, C.; Liang, H.; Wang, J.Y.; Ren, G.Q.; Li, R.S.; Cui, Z.G.; Zhang, C.F. Heterophyllin B an Active Cyclopeptide Alleviates Dextran Sulfate Sodium-Induced Colitis by Modulating Gut Microbiota and Repairing Intestinal Mucosal Barrier via AMPK Activation. Mol. Nutr. Food Res. 2022, 66, 15. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.H.; Zhou, K.X.; Zhang, L.Q.; Nan, S.J.; Fu, Z.W. Hydrolyzed chicken meat extract boosts the immunoregulatory effect by regulating M1/M2 Macrophage polarization. J. Funct. Foods 2022, 95, 11. [Google Scholar] [CrossRef]
- Abdelsattar, A.S.; Dawoud, A.; Helal, M.A. Interaction of nanoparticles with biological macromolecules: A review of molecular docking studies. Nanotoxicology 2021, 15, 66–95. [Google Scholar] [CrossRef] [PubMed]
- Eze, C.C.; Ezeokonkwo, A.M.; Ugwu, I.D.; Eze, U.F.; Onyeyilim, E.L.; Attah, I.S.; Okonkwo, I.V. Azole-Pyrimidine Hybrid Anticancer Agents: A Review of Molecular Structure, Structure Activity Relationship, and Molecular Docking. Anti-Cancer Agents Med. Chem. 2022, 22, 2822–2851. [Google Scholar]
- Ferreira, L.G.; Dos Santos, R.N.; Oliva, G.; Andricopulo, A.D. Molecular docking and structure-based drug design strategies. Molecules 2015, 20, 13384–13421. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhang, Q.; Chai, Y.; Liu, Y.; Li, F.; Wang, B.; Zhu, c.; Cui, J.; Qu, H.; Zhu, M. 1, 25 (OH) 2 D 3 downregulates the Toll-like receptor 4-mediated inflammatory pathway and ameliorates liver injury in diabetic rats. J. Endocrinol. Investig. 2015, 38, 1083–1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, P.; Yue, L.F.; Yang, H.; Fan, Y.N.; Bai, J.Y.; Li, S.Y.; Yuan, Z.Q.; Yao, C.S.; Lin, M.B.; Hou, Q. Chondroprotective and anti-inflammatory effects of amurensin H by regulating TLR4/Syk/NF-κB signals. J. Cell. Mol. Med. 2020, 24, 1958–1968. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Target | Sequence (5′ to 3′) | |
|---|---|---|
| iNos | Forward | GGAAGAGACACTACTGCTGGT |
| Reverse | GAACTGGAGGTACTGCTGGAGC | |
| Cox-2 | Forward | TTTCTACCAGAAGGGCAGGAT |
| Reverse | TATCACAGGCTTCCATTGACC | |
| IL-1β | Forward | TGCCACCTTTTGACAGTGATG |
| Reverse | AAGGTCCACGGGAAAGACAC | |
| IL-6 | Forward | CCCCAATTTCCAATGCTCTCC |
| Reverse | CGCACTAGGTTTGCCGAGTA | |
| TNF-α | Forward | ATGGCCTCCCTCTCATCAGT |
| Reverse | TTTGCTACGACGTGGGCTAC | |
| GAPDH | Forward | CCAGCTACTCGCGGCTTTA |
| Reverse | GTTCACACCGACCTTCACCA | |
| Compound | Total Score | Crash | Polar | Number of H-Bond | Proportion (%) |
|---|---|---|---|---|---|
| FLH | 11.97 | −7.43 | 4.37 | 5 | 3.84 |
| FLL | 11.46 | −6.52 | 2.10 | 3 | 5.95 |
| FLN | 8.18 | −7.96 | 2.44 | 5 | 2.54 |
| FLG | 8.07 | −2.45 | 2.08 | 3 | 7.44 |
| FLF | 8.01 | −4.51 | 2.22 | 3 | 4.28 |
| FLB | 7.94 | −7.17 | 2.34 | 3 | 2.91 |
| FLM | 7.16 | −3.78 | 2.05 | 2 | 6.39 |
| FLE | 7.13 | −1.87 | 1.08 | 2 | 21.08 |
| FLC | 5.55 | −6.69 | 1.37 | 3 | 11.78 |
| FLA | 5.07 | −4.77 | 2.53 | 4 | 23.56 |
| FLP | 4.92 | −3.68 | 2.84 | 4 | 6.82 |
| FLO | 4.57 | −5.42 | 1.79 | 3 | 3.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Chen, J.; Huang, P.; Cai, Z.; Zhang, N.; Wang, Y.; Li, Y. The Anti-Inflammatory Mechanism of Flaxseed Linusorbs on Lipopolysaccharide-Induced RAW 264.7 Macrophages by Modulating TLR4/NF-κB/MAPK Pathway. Foods 2023, 12, 2398. https://doi.org/10.3390/foods12122398
Li J, Chen J, Huang P, Cai Z, Zhang N, Wang Y, Li Y. The Anti-Inflammatory Mechanism of Flaxseed Linusorbs on Lipopolysaccharide-Induced RAW 264.7 Macrophages by Modulating TLR4/NF-κB/MAPK Pathway. Foods. 2023; 12(12):2398. https://doi.org/10.3390/foods12122398
Chicago/Turabian StyleLi, Jialong, Jing Chen, Ping Huang, Zizhe Cai, Ning Zhang, Yong Wang, and Ying Li. 2023. "The Anti-Inflammatory Mechanism of Flaxseed Linusorbs on Lipopolysaccharide-Induced RAW 264.7 Macrophages by Modulating TLR4/NF-κB/MAPK Pathway" Foods 12, no. 12: 2398. https://doi.org/10.3390/foods12122398