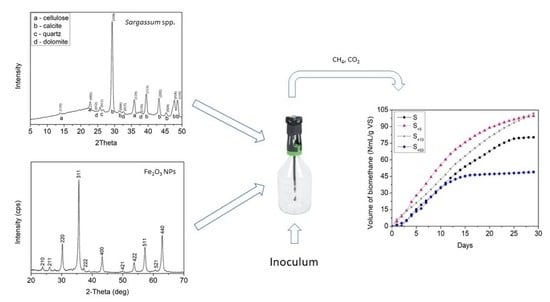
Influence of Fe2O3 Nanoparticles on the Anaerobic Digestion of Macroalgae Sargassum spp.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sargassum spp. Characterization
2.1.1. Physico-Chemical Characterization
2.1.2. Thermogravimetric Analysis and Differential Scanning Calorimetry
2.1.3. X-ray Diffractometry
2.1.4. FT-IR Analysis
2.2. Fe2O3 NPs Characterization
2.3. Sargassum + NPs Samples
2.4. Inoculum
2.5. Long-Term Biochemical Potential Test
2.6. Mathematical Models
3. Results and Discussion
3.1. Sargassum spp. Characterization
3.2. Fe2O3 NPs Characterization
3.3. Inoculum
3.4. Anaerobic Digestion
3.5. Mathematical Models
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Milledge, J.J.; Nielsen, B.V.; Maneein, S.; Harvey, P.J. A Brief Review of Anaerobic Digestion of Algae for BioEnergy. Energies 2019, 12, 1166. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Xu, R.; Xiang, Y.; Lu, Y.; Jia, M.; Huang, J.; Xu, Z.; Cao, J.; Xiong, W.; Yang, Z. Addition of Nanoparticles Increases the Abundance of Mobile Genetic Elements and Changes Microbial Community in the Sludge Anaerobic Digestion System. J. Hazard Mater. 2021, 405, 124206. [Google Scholar] [CrossRef]
- Tsui, T.H.; Zhang, L.; Zhang, J.; Dai, Y.; Tong, Y.W. Methodological Framework for Wastewater Treatment Plants Delivering Expanded Service: Economic Tradeoffs and Technological Decisions. Sci. Total Environ. 2022, 823, 153616. [Google Scholar] [CrossRef] [PubMed]
- Tsui, T.H.; Zhang, L.; Zhang, J.; Dai, Y.; Tong, Y.W. Engineering Interface between Bioenergy Recovery and Biogas Desulfurization: Sustainability Interplays of Biochar Application. Renew. Sustain. Energy Rev. 2022, 157, 112053. [Google Scholar] [CrossRef]
- Jadhava, P.; Muhammad, N.; Bhuyar, P.; Krishnan, S.; Razak, A.S.A.; Zularisam, A.W.; Nasrullah, M. A Review on the Impact of Conductive Nanoparticles (CNPs) in Anaerobic Digestion: Applications and Limitations. Environ. Technol. Innov. 2021, 23, 101526. [Google Scholar] [CrossRef]
- Jadhav, P.; Nasrullah, M.; Zularisam, A.W.; Bhuyar, P.; Krishnan, S.; Mishra, P. Direct Interspecies Electron Transfer Performance through Nanoparticles (NPs) for Biogas Production in the Anaerobic Digestion Process. Int. J. Environ. Sci. Technol. 2022, 19, 10427–10439. [Google Scholar] [CrossRef]
- Kumar, S.S.; Ghosh, P.; Kataria, N.; Kumar, D.; Thakur, S.; Pathania, D.; Kumar, V.; Nasrullah, M.; Singh, L. The Role of Conductive Nanoparticles in Anaerobic Digestion: Mechanism, Current Status and Future Perspectives. Chemosphere 2021, 280, 130601. [Google Scholar] [CrossRef]
- Dehhaghi, M.; Tabatabaei, M.; Aghbashlo, M.; Kazemi Shariat Panahi, H.; Nizami, A.S. A State-of-the-Art Review on the Application of Nanomaterials for Enhancing Biogas Production. J. Environ. Manag. 2019, 251, 109597. [Google Scholar] [CrossRef]
- Ossinga, C.G. Application of Iron Oxide Nanoparticles for Biogas Yield Optimization from Winery Solid Waste and Sorghum Stover. Master’s Thesis, Chemical Engineering, Cape Peninsula University of Technology, Bellville, South Africa, 2020. [Google Scholar]
- Ugwu, S.N.; Enweremadu, C.C. Enhancement of Biogas Production Process from Biomass Wastes Using Iron-Based Additives: Types, Impacts, and Implications. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 44, 4458–4480. [Google Scholar] [CrossRef]
- Abdelwahab, T.A.M.; Fodah, A.E.M. Utilization of Nanoparticles for Biogas Production Focusing on Process Stability and Effluent Quality. SN Appl. Sci. 2022, 4, 332. [Google Scholar] [CrossRef]
- Jadhav, P.; Khalid, Z.B.; Zularisam, A.W.; Krishnan, S.; Nasrullah, M. The Role of Iron-Based Nanoparticles (Fe-NPs) on Methanogenesis in Anaerobic Digestion (AD) Performance. Environ. Res 2022, 204, 112043. [Google Scholar] [CrossRef]
- Bharathi, P.; Dayana, R.; Rangaraju, M.; Varsha, V.; Subathra, M.; Gayathri; Sundramurthy, V.P. Biogas Production from Food Waste Using Nanocatalyst. J. Nanomater. 2022, 2022, 7529036. [Google Scholar] [CrossRef]
- Tetteh, E.K.; Rathilal, S. Application of Biomagnetic Nanoparticles for Biostimulation of Biogas Production from Wastewater Treatment. Mater. Today Proc. 2021, 45, 5214–5220. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, D.; Dai, L.; Chen, Y.; Dai, X. Effects of Metal Nanoparticles on Methane Production from Waste-Activated Sludge and Microorganism Community Shift in Anaerobic Granular Sludge. Sci. Rep. 2016, 6, 25857. [Google Scholar] [CrossRef] [PubMed]
- Farghali, M.; Andriamanohiarisoamanana, F.J.; Ahmed, M.M.; Kotb, S.; Yamashiro, T.; Iwasaki, M.; Umetsu, K. Impacts of Iron Oxide and Titanium Dioxide Nanoparticles on Biogas Production: Hydrogen Sulfide Mitigation, Process Stability, and Prospective Challenges. J. Environ. Manag. 2019, 240, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.S.; Bhushan, S.; Prajapati, S.K. New Insights on Improved Growth and Biogas Production Potential of Chlorella Pyrenoidosa through Intermittent Iron Oxide Nanoparticle Supplementation. Sci. Rep. 2020, 10, 14119. [Google Scholar] [CrossRef]
- Kökdemir Ünşar, E.; Perendeci, N.A. What Kind of Effects Do Fe2O3 and Al2O3 Nanoparticles Have on Anaerobic Digestion, Inhibition or Enhancement? Chemosphere 2018, 211, 726–735. [Google Scholar] [CrossRef]
- Lopresto, C.G.; Paletta, R.; Filippelli, P.; Galluccio, L.; de la Rosa, C.; Amaro, E.; Jáuregui-Haza, U.; de Frias, J.A. Sargassum Invasion in the Caribbean: An Opportunity for Coastal Communities to Produce Bioenergy Based on Biorefinery—An Overview. Waste Biomass Valorization 2022, 13, 2769–2793. [Google Scholar] [CrossRef]
- El Nemr, A.; Hassaan, M.A.; Elkatory, M.R.; Ragab, S.; Pantaleo, A. Efficiency of Fe3O4 Nanoparticles with Different Pretreatments for Enhancing Biogas Yield of Macroalgae Ulva Intestinalis Linnaeus. Molecules 2021, 26, 5105. [Google Scholar] [CrossRef]
- Zaidi, A.A.; Khan, S.Z.; Shi, Y. Optimization of Nickel Nanoparticles Concentration for Biogas Enhancement from Green Algae Anaerobic Digestion. Mater. Today Proc. 2019, 39, 1025–1028. [Google Scholar] [CrossRef]
- Zaidi, A.A.; RuiZhe, F.; Shi, Y.; Khan, S.Z.; Mushtaq, K. Nanoparticles Augmentation on Biogas Yield from Microalgal Biomass Anaerobic Digestion. Int. J. Hydrogen Energy 2018, 43, 14202–14213. [Google Scholar] [CrossRef]
- Zaidi, A.A.; Khan, S.Z.; Naseer, M.N.; Almohammadi, H.; Asif, M.; Abdul Wahab, Y.; Islam, M.A.; Johan, M.R.; Hussin, H. Optimization of Cobalt Nanoparticles for Biogas Enhancement from Green Algae Using Response Surface Methodology. Period. Polytech. Chem. Eng. 2023, 67, 116–126. [Google Scholar] [CrossRef]
- Zaidi, A.A.; Feng, R.; Malik, A.; Khan, S.Z.; Shi, Y.; Bhutta, A.J.; Shah, A.H. Combining Microwave Pretreatment with Iron Oxide Nanoparticles Enhanced Biogas and Hydrogen Yield from Green Algae. Processes 2019, 7, 24. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Huang, K.; Feng, R.; Wang, R.; Liu, G.; Zaidi, A.A.; Zhang, K. Combined MgO Nanoparticle and Microwave Pre-Treatment on Biogas Increase from Enteromorpha during Anaerobic Digestion. IOP Conf. Ser. Earth Environ. Sci. 2020, 450, 012025. [Google Scholar] [CrossRef]
- Zaidi, A.A.; RuiZhe, F.; Malik, A.; Khan, S.Z.; Bhutta, A.J.; Shi, Y.; Mushtaq, K. Conjoint Effect of Microwave Irradiation and Metal Nanoparticles on Biogas Augmentation from Anaerobic Digestion of Green Algae. Int. J. Hydrogen Energy 2019, 44, 14661–14670. [Google Scholar] [CrossRef]
- Zaidi, A.A.; Khan, S.Z.; Almohamadi, H.; Mahmoud, E.R.I.; Naseer, M.N. Nanoparticles Synergistic Effect with Various Substrate Pretreatment and Their Comparison on Biogas Production from Algae Waste. Bull. Chem. React. Eng. Catal. 2021, 16, 374–382. [Google Scholar] [CrossRef]
- Ajay, C.M.; Mohan, S.; Dinesha, P.; Rosen, M.A. Review of Impact of Nanoparticle Additives on Anaerobic Digestion and Methane Generation. Fuel 2020, 277, 118234. [Google Scholar] [CrossRef]
- Liu, M.; Wei, Y.; Leng, X. Improving Biogas Production Using Additives in Anaerobic Digestion: A Review. J. Clean. Prod. 2021, 297, 126666. [Google Scholar] [CrossRef]
- Rosato, M.A. Manuale per Il Gestore Dell’impianto Di Biogas; Editoriale Delfino: Milano, Italy, 2015; ISBN 978-88-97323-41-9. [Google Scholar]
- Syaichurrozi, I.; Budiyono; Sumardiono, S. Predicting Kinetic Model of Biogas Production and Biodegradability Organic Materials: Biogas Production from Vinasse at Variation of COD/N Ratio. Bioresour. Technol. 2013, 149, 390–397. [Google Scholar] [CrossRef]
- Thompson, T.M.; Young, B.R.; Baroutian, S. Efficiency of Hydrothermal Pretreatment on the Anaerobic Digestion of Pelagic Sargassum for Biogas and Fertiliser Recovery. Fuel 2020, 279, 118527. [Google Scholar] [CrossRef]
- Wall, D.M.; Allen, E.; Straccialini, B.; O’Kiely, P.; Murphy, J.D. The Effect of Trace Element Addition to Mono-Digestion of Grass Silage at High Organic Loading Rates. Bioresour. Technol. 2014, 172, 349–355. [Google Scholar] [CrossRef]
- Schmidt, T.; McCabe, B.K.; Harris, P.W.; Lee, S. Effect of Trace Element Addition and Increasing Organic Loading Rates on the Anaerobic Digestion of Cattle Slaughterhouse Wastewater. Bioresour. Technol. 2018, 264, 51–57. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, H.; Huang, H.; Zheng, L.; Li, S.; Hao, H.; Yin, M.; Cao, Y. Process Analysis of Anaerobic Fermentation Exposure to Metal Mixtures. Int. J. Environ. Res. Public Health 2019, 16, 2458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alzate-Gaviria, L.; Domínguez-Maldonado, J.; Chablé-Villacís, R.; Olguin-Maciel, E.; Leal-Bautista, R.M.; Canché-Escamilla, G.; Caballero-Vázquez, A.; Hernández-Zepeda, C.; Barredo-Pool, F.A.; Tapia-Tussell, R. Presence of Polyphenols Complex Aromatic “Lignin” in Sargassum Spp. From Mexican Caribbean. J. Mar. Sci. Eng. 2021, 9, 6. [Google Scholar] [CrossRef]
- López-Sosa, L.B.; Alvarado-Flores, J.J.; Corral-Huacuz, J.C.; Aguilera-Mandujano, A.; Rodríguez-Martínez, R.E.; Guevara-Martínez, S.J.; Alcaraz-Vera, J.V.; Rutiaga-Quiñones, J.G.; Zárate-Medina, J.; Ávalos-Rodríguez, M.L.; et al. A Prospective Study of the Exploitation of Pelagic Sargassum Spp. As a Solid Biofuel Energy Source. Appl. Sci. 2020, 10, 8706. [Google Scholar] [CrossRef]
- Candamano, S.; Crea, F.; Coppola, L.; de Luca, P.; Coffetti, D. Influence of Acrylic Latex and Pre-Treated Hemp Fibers on Cement Based Mortar Properties. Constr. Build. Mater. 2021, 273, 121720. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Anbalagan, G.; Pandi, S. Raman and Infrared Spectra of Carbonates of Calcite Structure. J. Raman Spectrosc. 2006, 37, 892–899. [Google Scholar] [CrossRef]
- Wang, Z.; Che, Y.; Li, J.; Wu, W.; Yan, B.; Zhang, Y.; Wang, X.; Yu, F.; Chen, G.; Zuo, X.; et al. Effects of Anaerobic Digestion Pretreatment on the Pyrolysis of Sargassum: Investigation by TG-FTIR and Py-GC/MS. Energy Convers. Manag. 2022, 267, 115934. [Google Scholar] [CrossRef]
- Davis, D.; Simister, R.; Campbell, S.; Marston, M.; Bose, S.; McQueen-Mason, S.J.; Gomez, L.D.; Gallimore, W.A.; Tonon, T. Biomass Composition of the Golden Tide Pelagic Seaweeds Sargassum Fluitans and S. Natans (Morphotypes I and VIII) to Inform Valorisation Pathways. Sci. Total Environ. 2021, 762, 143134. [Google Scholar] [CrossRef] [PubMed]
- Alvarado Flores, J.J.; Alcaraz Vera, J.V.; Ávalos Rodríguez, M.L.; Rutiaga Quiñones, J.G.; Valencia, J.E.; Guevara Martínez, S.J.; Ríos, E.T.; Zarraga, R.A. Kinetic, Thermodynamic, FT-IR, and Primary Constitution Analysis of Sargassum Spp from Mexico: Potential for Hydrogen Generation. Int. J. Hydrogen Energy 2022, 47, 30107–30127. [Google Scholar] [CrossRef]
- Kannan, S. FT-IR and EDS Analysis of the SeaweedsSargassum Wightii (Brown Algae) and Gracilaria Corticata (Red Algae). Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 341–351. [Google Scholar]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds; John Wiley and Sons: New York, NY, USA, 1986. [Google Scholar]
- Xyla, A.G.; Koutsoukos, P.G. Quantitative Analysis of Calcium Carbonate Polymorphs by Infrared Spectroscopy. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1989, 85, 3165–3172. [Google Scholar] [CrossRef]
- Shan, C.; Ma, Z.; Tong, M. Efficient Removal of Trace Antimony(III) through Adsorption by Hematite Modified Magnetic Nanoparticles. J. Hazard. Mater 2014, 268, 229–236. [Google Scholar] [CrossRef]
- Shokrollahi, H. A Review of the Magnetic Properties, Synthesis Methods and Applications of Maghemite. J. Magn. Magn. Mater. 2017, 426, 74–81. [Google Scholar] [CrossRef]
- Ali, A.; Mahar, R.B.; Soomro, R.A.; Sherazi, S.T.H. Fe3O4 Nanoparticles Facilitated Anaerobic Digestion of Organic Fraction of Municipal Solid Waste for Enhancement of Methane Production. Energy Sources Part A Recovery Util. Environ. Eff. 2017, 39, 1815–1822. [Google Scholar] [CrossRef]
- Wu, J.; Zhu, G.; Yu, R. Fates and Impacts of Nanomaterial Contaminants in Biological Wastewater Treatment System: A Review. Water Air Soil Pollut. 2018, 229, 9. [Google Scholar] [CrossRef]
- Abdelwahab, T.A.M.; Mohanty, M.K.; Sahoo, P.K.; Behera, D. Application of Nanoparticles for Biogas Production: Current Status and Perspectives. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 1–13. [Google Scholar] [CrossRef]
- Xia, T.; Kovochich, M.; Liong, M.; Mädler, L.; Gilbert, B.; Shi, H.; Yeh, J.I.; Zink, J.I.; Nel, A.E. Comparison of the Mechanism of Toxicity of Zinc Oxide and Cerium Oxide Nanoparticles Based on Dissolution and Oxidative Stress Properties. ACS Nano 2008, 2, 2121–2134. [Google Scholar] [CrossRef] [Green Version]
- Brunner, T.J.; Wick, P.; Manser, P.; Spohn, P.; Grass, R.N.; Limbach, L.K.; Bruinink, A.; Stark, W.J. In Vitro Cytotoxicity of Oxide Nanoparticles: Comparison to Asbestos, Silica, and the Effect of Particle Solubility. Environ. Sci. Technol. 2006, 40, 4374–4381. [Google Scholar] [CrossRef]
- Franklin, N.M.; Rogers, N.J.; Apte, S.C.; Batley, G.E.; Gadd, G.E.; Casey, P.S. Comparative Toxicity of Nanoparticulate ZnO, Bulk ZnO, and ZnCl2 to a Freshwater Microalga (Pseudokirchneriella Subcapitata): The Importance of Particle Solubility. Environ. Sci. Technol. 2007, 41, 8484–8490. [Google Scholar] [CrossRef]
- Li, K.; Liu, R.; Sun, C. Comparison of Anaerobic Digestion Characteristics and Kinetics of Four Livestock Manures with Different Substrate Concentrations. Bioresour. Technol. 2015, 198, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, S.K.; Suja, F.B.; Porhemmat, M.; Pramanik, B.K. Performance and Kinetic Model of a Single-Stage Anaerobic Digestion System Operated at Different Successive Operating Stages for the Treatment of Food Waste. Processes 2019, 7, 600. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Champagne, P.; Anderson, B.C. Evaluating and Modeling Biogas Production from Municipal Fat, Oil, and Grease and Synthetic Kitchen Waste in Anaerobic Co-Digestions. Bioresour. Technol. 2011, 102, 9471–9480. [Google Scholar] [CrossRef] [PubMed]





| Sample | Open-Air Pre-Dried Substrate [mg NPs/g S] | Total Solids of Substrate [mg NPs/g TS] | Volatile Solids of Substrate [mg NPs/g VS] |
|---|---|---|---|
| S+5 | 5 | 6.6 | 14.4 |
| S+10 | 10 | 12.1 | 17.9 |
| S+50 | 50 | 60.6 | 89.4 |
| Compound | Content [%] | |
|---|---|---|
| Carbohydrates | 51.9 | Percentage of open-air pre-dried sample |
| Lipids | 1.65 | |
| Proteins | 0.62 | |
| C | 19 | Percentage of open-air pre-dried sample |
| N | 2.4 | |
| Na | 1.22 | Percentage of total solids after oven drying |
| Mg | 0.76 | |
| Al | 0.013 | |
| K | 3.36 | |
| Ca | 1.93 | |
| Mn | 0.003 | |
| Fe | <10−3 | |
| Ni | 0.001 | |
| Cu | <10−4 | |
| Zn | 0.006 | |
| Ba | <10−3 | |
| Pb | 0.003 | |
| B | 0.002 | |
| P | 0.009 | |
| As | <10−4 |
| Sample | BMP30 (NmL·g−1VS) | Variation Compared to Control |
|---|---|---|
| S | 80.25 ± 3.21 | - |
| S+5 | 99.57 ± 2.76 | +24.07 |
| S+10 | 101.90 ± 2.98 | +26.97 |
| S+50 | 48.97 ± 2.32 | −38.97 |
| S | S+5 | S+10 | S+50 | |
|---|---|---|---|---|
| First-order kinetic | ||||
| A (NmL·g−1VS) | 165.542 | 126.443 | 204.634 | 56.221 |
| k (day−1) | 0.021 | 0.063 | 0.022 | 0.082 |
| R2 | 0.983 | 0.992 | 0.997 | 0.948 |
| Modified Gompertz model | ||||
| A (NmL·g−1VS) | 86.749 | 101.217 | 113.595 | 48.517 |
| u (NmL·g−1VS·day−1) | 4.468 | 6.045 | 4.595 | 4.949 |
| m (day) | 2.289 | 0.768 | 0.829 | 2.440 |
| R2 | 0.996 | 0.997 | 0.998 | 0.998 |
| Logistic function model | ||||
| A (NmL·g−1VS) | 81.111 | 97.483 | 103.412 | 47.792 |
| u (NmL·g−1VS·day−1) | 11.701 | 9.203 | 12.382 | 7.502 |
| m (day) | 0.110 | 0.121 | 0.093 | 0.207 |
| R2 | 0.989 | 0.987 | 0.992 | 0.997 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paletta, R.; Candamano, S.; Filippelli, P.; Lopresto, C.G. Influence of Fe2O3 Nanoparticles on the Anaerobic Digestion of Macroalgae Sargassum spp. Processes 2023, 11, 1016. https://doi.org/10.3390/pr11041016
Paletta R, Candamano S, Filippelli P, Lopresto CG. Influence of Fe2O3 Nanoparticles on the Anaerobic Digestion of Macroalgae Sargassum spp. Processes. 2023; 11(4):1016. https://doi.org/10.3390/pr11041016
Chicago/Turabian StylePaletta, Rosy, Sebastiano Candamano, Pierpaolo Filippelli, and Catia Giovanna Lopresto. 2023. "Influence of Fe2O3 Nanoparticles on the Anaerobic Digestion of Macroalgae Sargassum spp." Processes 11, no. 4: 1016. https://doi.org/10.3390/pr11041016