Advancements in Spinal Cord Injury Repair: Insights from Dental-Derived Stem Cells
Abstract
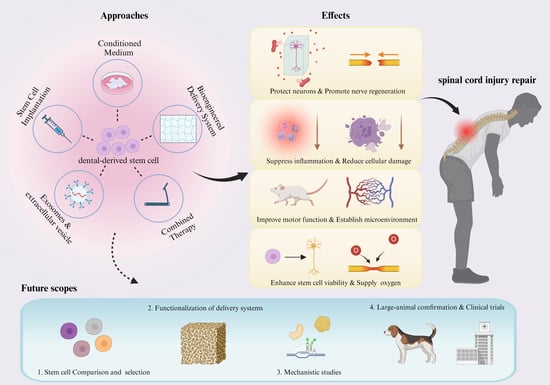
:1. Introduction
2. Phases and Pathophysiology of SCI
3. The Neurodegenerative Potential of Dental Stem Cells
3.1. DPSCs
3.2. SHED
3.3. SCAP
3.4. DFSCs
4. Treatment Approaches
4.1. Stem Cell Implantation
4.2. Condition Medium Injection
4.3. Bioengineered Delivery System Approaches
4.3.1. Hydrogel
4.3.2. Chitosan
4.3.3. PLGA
4.3.4. Microcapsules and Microspheres
4.4. Exosomes and Extracellular Vesicles
4.5. Combined Therapies
5. Discussion
5.1. Limitations of Treatment Methods
5.2. Future Research Direction
5.3. Exploration of Critical Questions
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pajer, K.; Bellák, T.; Nógrádi, A. Stem Cell Secretome for Spinal Cord Repair: Is It More than Just a Random Baseline Set of Factors? Cells 2021, 10, 3214. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Xu, W.; Ren, Y.; Wang, Z.; He, X.; Huang, R.; Ma, B.; Zhao, J.; Zhu, R.; Cheng, L. Spinal cord injury: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 245. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, M.; Zhang, T.; Ma, Y.; Chen, X.; Zhou, P.; Zhao, X.; Pang, F.; Liang, W. Spinal cord regeneration using dental stem cell-based therapies. Acta Neurobiol. Exp. 2019. [Google Scholar]
- Anjum, A.; Yazid, M.D.; Fauzi Daud, M.; Idris, J.; Ng, A.M.H.; Selvi Naicker, A.; Ismail, O.H.R.; Athi Kumar, R.K.; Lokanathan, Y. Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. Int. J. Mol. Sci. 2020, 21, 7533. [Google Scholar] [CrossRef] [PubMed]
- Khorasanizadeh, M.; Yousefifard, M.; Eskian, M.; Lu, Y.; Chalangari, M.; Harrop, J.S.; Jazayeri, S.B.; Seyedpour, S.; Khodaei, B.; Hosseini, M.; et al. Neurological recovery following traumatic spinal cord injury: A systematic review and meta-analysis. J. Neurosurg. Spine 2019, 30, 683–699. [Google Scholar] [CrossRef]
- Mackiewicz-Milewska, M.; Newland, P. Spinal Cord Injury (SCI) 2016 Facts and Figures at a Glance. J. Spinal Cord. Med. 2016, 39, 493–494. [Google Scholar] [CrossRef]
- Venkatesh, K.; Ghosh, S.K.; Mullick, M.; Manivasagam, G.; Sen, D. Spinal cord injury: Pathophysiology, treatment strategies, associated challenges, and future implications. Cell Tissue Res. 2019, 377, 125–151. [Google Scholar] [CrossRef]
- Gao, L.; Peng, Y.; Xu, W.; He, P.; Li, T.; Lu, X.; Chen, G. Progress in Stem Cell Therapy for Spinal Cord Injury. Stem Cells Int. 2020, 2020, 2853650. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wu, Q.; Wang, P.; Jing, Y.; Yao, H.; Tang, Y.; Li, Z.; Zhang, H.; Xiu, R. Exosomes Derived from Pericytes Improve Microcirculation and Protect Blood-Spinal Cord Barrier After Spinal Cord Injury in Mice. Front. Neurosci. 2019, 13, 319. [Google Scholar] [CrossRef] [PubMed]
- Kirshblum, S.C.; Burns, S.P.; Biering-Sorensen, F.; Donovan, W.; Graves, D.E.; Jha, A.; Johansen, M.; Jones, L.; Krassioukov, A.; Mulcahey, M.J.; et al. International standards for neurological classification of spinal cord injury (Revised 2011). J. Spinal Cord Med. 2011, 34, 535–546. [Google Scholar] [CrossRef]
- Roberts, T.T.; Leonard, G.R.; Cepela, D.J. Classifications in Brief: American Spinal Injury Association (ASIA) Impairment Scale. Clin. Orthop. Relat. Res. 2017, 475, 1499–1504. [Google Scholar] [CrossRef]
- Dietz, V.; Colombo, G.; Jensen, L. Locomotor activity in spinal man. Lancet 1994, 344, 1260–1263. [Google Scholar] [CrossRef]
- Piira, A.; Lannem, A.M.; Sørensen, M.; Glott, T.; Knutsen, R.; Jørgensen, L.; Gjesdal, K.; Hjeltnes, N.; Knutsen, S.F. Manually assisted body-weight supported locomotor training does not re-establish walking in non-walking subjects with chronic incomplete spinal cord injury: A randomized clinical trial. J. Rehabil. Med. 2019, 51, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Stillman, M.D. SCI Facts and Figures. J. Spinal Cord. Med. 2017, 40, 126–127. [Google Scholar] [CrossRef]
- Lima, R.; Monteiro, A.; Salgado, A.J.; Monteiro, S.; Silva, N.A. Pathophysiology and Therapeutic Approaches for Spinal Cord Injury. Int. J. Mol. Sci. 2022, 23, 13833. [Google Scholar] [CrossRef]
- Katoh, H.; Yokota, K.; Fehlings, M.G. Regeneration of Spinal Cord Connectivity Through Stem Cell Transplantation and Biomaterial Scaffolds. Front. Cell Neurosci. 2019, 13, 248. [Google Scholar] [CrossRef] [PubMed]
- Couillard-Despres, S.; Bieler, L.; Vogl, M. Pathophysiology of Traumatic Spinal Cord Injury. In Neurological Aspects of Spinal Cord Injury; Weidner, N., Rupp, R., Tansey, K.E., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 503–528. [Google Scholar] [CrossRef]
- Hachem, L.D.; Fehlings, M.G. Pathophysiology of Spinal Cord Injury. Neurosurg. Clin. N. Am. 2021, 32, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Tran, A.P.; Warren, P.M.; Silver, J. New insights into glial scar formation after spinal cord injury. Cell Tissue Res. 2022, 387, 319–336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Al Mamun, A.; Yuan, Y.; Lu, Q.; Xiong, J.; Yang, S.; Wu, C.; Wu, Y.; Wang, J. Acute spinal cord injury: Pathophysiology and pharmacological intervention (Review). Mol. Med. Rep. 2021, 23, 417. [Google Scholar] [CrossRef]
- Tran, A.P.; Warren, P.M.; Silver, J. The Biology of Regeneration Failure and Success After Spinal Cord Injury. Physiol. Rev. 2018, 98, 881–917. [Google Scholar] [CrossRef]
- Chay, W.; Kirshblum, S. Predicting Outcomes After Spinal Cord Injury. Phys. Med. Rehabil. Clin. N. Am. 2020, 31, 331–343. [Google Scholar] [CrossRef]
- Bozzo, A.; Marcoux, J.; Radhakrishna, M.; Pelletier, J.; Goulet, B. The Role of Magnetic Resonance Imaging in the Management of Acute Spinal Cord Injury. J. Neurotrauma 2011, 28, 1401–1411. [Google Scholar] [CrossRef] [PubMed]
- Kakulas, B.A. The clinical neuropathology of spinal cord injury a guide to the future. Spinal Cord. 1987, 25, 212–216. [Google Scholar] [CrossRef]
- Siegenthaler, M.M.; Tu, M.K.; Keirstead, H.S. The Extent of Myelin Pathology Differs following Contusion and Transection Spinal Cord Injury. J. Neurotrauma 2007, 24, 1631–1646. [Google Scholar] [CrossRef]
- Choo, A.M.; Liu, J.; Dvorak, M.; Tetzlaff, W.; Oxland, T.R. Secondary pathology following contusion, dislocation, and distraction spinal cord injuries. Exp. Neurol. 2008, 212, 490–506. [Google Scholar] [CrossRef]
- Ropper, A.E.; Ropper, A.H. Acute Spinal Cord Compression. N. Engl. J. Med. 2017, 376, 1358–1369. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.W.; Schiff, D. Metastatic Epidural Spinal Cord Compression. Semin. Neurol. 2010, 30, 245–253. [Google Scholar] [CrossRef]
- Chen, K.; Liu, J.; Assinck, P.; Bhatnagar, T.; Streijger, F.; Zhu, Q.; Dvorak, M.F.; Kwon, B.K.; Tetzlaff, W.; Oxland, T.R. Differential Histopathological and Behavioral Outcomes Eight Weeks after Rat Spinal Cord Injury by Contusion, Dislocation, and Distraction Mechanisms. J. Neurotrauma 2016, 33, 1667–1684. [Google Scholar] [CrossRef]
- Choo, A.M.-T. Clinically Relevant Mechanisms of Spinal Cord Injury: Contusion, Dislocation, and Distraction. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2007. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, J.; Tang, H.; Li, X.; Jiang, S.; Lv, Z.; Liu, S.; Chen, S.; Liu, J.; Hong, Y. Characteristics and rehabilitation for patients with spinal cord stab injury. J. Phys. Ther. Sci. 2015, 27, 3671–3673. [Google Scholar] [CrossRef]
- Cheriyan, T.; Ryan, D.J.; Weinreb, J.H.; Cheriyan, J.; Paul, J.C.; Lafage, V.; Kirsch, T.; Errico, T.J. Spinal cord injury models: A review. Spinal Cord. 2014, 52, 588–595. [Google Scholar] [CrossRef]
- Zipser, C.M.; Cragg, J.J.; Guest, J.D.; Fehlings, M.G.; Jutzeler, C.R.; Anderson, A.J.; Curt, A. Cell-based and stem-cell-based treatments for spinal cord injury: Evidence from clinical trials. Lancet Neurol. 2022, 21, 659–670. [Google Scholar] [CrossRef]
- Sybil, D.; Jain, V.; Mohanty, S.; Husain, S.A. Oral stem cells in intraoral bone formation. J. Oral Biosci. 2020, 62, 36–43. [Google Scholar] [CrossRef]
- Bianco, P. “Mesenchymal” Stem Cells. Annu. Rev. Cell Dev. Biol. 2014, 30, 677–704. [Google Scholar] [CrossRef]
- Liu, Q.; Telezhkin, V.; Jiang, W.; Gu, Y.; Wang, Y.; Hong, W.; Tian, W.; Yarova, P.; Zhang, G.; Lee, S.M.; et al. Electric field stimulation boosts neuronal differentiation of neural stem cells for spinal cord injury treatment via PI3K/Akt/GSK-3β/β-catenin activation. Cell Biosci. 2023, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Levy, O.; Kuai, R.; Siren, E.M.J.; Bhere, D.; Milton, Y.; Nissar, N.; De Biasio, M.; Heinelt, M.; Reeve, B.; Abdi, R.; et al. Shattering barriers toward clinically meaningful MSC therapies. Sci. Adv. 2020, 6, eaba6884. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.-J.; Jung, G.N.; Park, W.-T.; Seo, M.-S.; Lee, G.W. Therapeutic potential of small extracellular vesicles derived from mesenchymal stem cells for spinal cord and nerve injury. Front. Cell Dev. Biol. 2023, 11, 1151357. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Scholtemeijer, M.; Shah, K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol. Sci. 2020, 41, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Tang, S.; Li, M.; Wang, D.; Chen, C.; Qiu, Y.; Fang, Z.; Zhang, H.; Gao, H.; Weng, H.; et al. Single-Cell and Spatial Transcriptomics Decodes Wharton’s Jelly-Derived Mesenchymal Stem Cells Heterogeneity and a Subpopulation with Wound Repair Signatures. Adv. Sci. 2023, 10, e2204786. [Google Scholar] [CrossRef] [PubMed]
- Forte, D.; García-Fernández, M.; Sánchez-Aguilera, A.; Stavropoulou, V.; Fielding, C.; Martín-Pérez, D.; López, J.A.; Costa, A.S.H.; Tronci, L.; Nikitopoulou, E.; et al. Bone Marrow Mesenchymal Stem Cells Support Acute Myeloid Leukemia Bioenergetics and Enhance Antioxidant Defense and Escape from Chemotherapy. Cell Metab. 2020, 32, 829–843.e9. [Google Scholar] [CrossRef] [PubMed]
- Mazini, L.; Rochette, L.; Admou, B.; Amal, S.; Malka, G. Hopes and Limits of Adipose-Derived Stem Cells (ADSCs) and Mesenchymal Stem Cells (MSCs) in Wound Healing. Int. J. Mol. Sci. 2020, 21, 1306. [Google Scholar] [CrossRef] [PubMed]
- Diotallevi, F.; Di Vincenzo, M.; Martina, E.; Radi, G.; Lariccia, V.; Offidani, A.; Orciani, M.; Campanati, A. Mesenchymal Stem Cells and Psoriasis: Systematic Review. Int. J. Mol. Sci. 2022, 23, 15080. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Wang, D.; Alraies, A.; Liu, Q.; Zhu, B.; Sloan, A.J.; Ni, L.; Song, B. Wnt-GSK3 β/β -Catenin Regulates the Differentiation of Dental Pulp Stem Cells into Bladder Smooth Muscle Cells. Stem Cells Int. 2019, 2019, 1–13. [Google Scholar] [CrossRef]
- Stanko, P.; Altanerova, U.; Jakubechova, J.; Repiska, V.; Altaner, C. Dental Mesenchymal Stem/Stromal Cells and Their Exosomes. Stem Cells Int. 2018, 2018, 8973613. [Google Scholar] [CrossRef] [PubMed]
- Mattei, V.; Martellucci, S.; Pulcini, F.; Santilli, F.; Sorice, M.; Delle Monache, S. Regenerative Potential of DPSCs and Revascularization: Direct, Paracrine or Autocrine Effect? Stem Cell Rev. Rep. 2021, 17, 1635–1646. [Google Scholar] [CrossRef]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, Y.; He, J. Stem Cells from the Apical Papilla (SCAPs): Past, Present, Prospects, and Challenges. Biomedicines 2023, 11, 2047. [Google Scholar] [CrossRef]
- Zhang, J.; Ding, H.; Liu, X.; Sheng, Y.; Liu, X.; Jiang, C. Dental Follicle Stem Cells: Tissue Engineering and Immunomodulation. Stem Cells Dev. 2019, 28, 986–994. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef]
- Fu, J.; Li, X.; Jin, F.; Dong, Y.; Zhou, H.; Alhaskawi, A.; Wang, Z.; Lai, J.; Yao, C.; Ezzi, S.H.A.; et al. The potential roles of dental pulp stem cells in peripheral nerve regeneration. Front. Neurol. 2023, 13, 1098857. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Xu, X.; Yao, L.; Xue, B.; Xi, H.; Cao, X.; Piao, G.; Lin, S.; Wang, X. VEGFA-modified DPSCs combined with LC-YE-PLGA NGCs promote facial nerve injury repair in rats. Heliyon 2023, 9, e14626. [Google Scholar] [CrossRef]
- Mu, X.; Liu, H.; Yang, S.; Li, Y.; Xiang, L.; Hu, M.; Wang, X. Chitosan Tubes Inoculated with Dental Pulp Stem Cells and Stem Cell Factor Enhance Facial Nerve-Vascularized Regeneration in Rabbits. ACS Omega 2022, 7, 18509–18520. [Google Scholar] [CrossRef] [PubMed]
- Takaoka, S.; Uchida, F.; Ishikawa, H.; Toyomura, J.; Ohyama, A.; Watanabe, M.; Matsumura, H.; Marushima, A.; Iizumi, S.; Fukuzawa, S.; et al. Transplanted neural lineage cells derived from dental pulp stem cells promote peripheral nerve regeneration. Hum. Cell 2022, 35, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Logan, A.; Berry, M.; Leadbeater, W.; Scheven, B.A. Intravitreally transplanted dental pulp stem cells promote neuroprotection and axon regeneration of retinal ganglion cells after optic nerve injury. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7544–7556. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, L.; Zhang, C.; Dissanayaka, W.L. Guiding Lineage Specific Differentiation of SHED for Target Tissue/Organ Regeneration. Curr. Stem Cell Res. Ther. 2021, 16, 518–534. [Google Scholar] [CrossRef] [PubMed]
- Anoop, M.; Datta, I. Stem Cells Derived from Human Exfoliated Deciduous Teeth (SHED) in Neuronal Disorders: A Review. Curr. Stem Cell Res. Ther. 2021, 16, 535–550. [Google Scholar] [CrossRef]
- Fuloria, S.; Jain, A.; Singh, S.; Hazarika, I.; Salile, S.; Fuloria, N.K. Regenerative Potential of Stem Cells Derived from Human Exfoliated Deciduous (SHED) Teeth during Engineering of Human Body Tissues. Curr. Stem Cell Res. Ther. 2021, 16, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Sugimura-Wakayama, Y.; Katagiri, W.; Osugi, M.; Kawai, T.; Ogata, K.; Sakaguchi, K.; Hibi, H. Peripheral Nerve Regeneration by Secretomes of Stem Cells from Human Exfoliated Deciduous Teeth. Stem Cells Dev. 2015, 24, 2687–2699. [Google Scholar] [CrossRef]
- Sonoyama, W.; Liu, Y.; Fang, D.; Yamaza, T.; Seo, B.-M.; Zhang, C.; Liu, H.; Gronthos, S.; Wang, C.-Y.; Shi, S.; et al. Mesenchymal Stem Cell-Mediated Functional Tooth Regeneration in Swine. PLoS ONE 2006, 1, e79. [Google Scholar] [CrossRef]
- Nagata, M.; Ono, N.; Ono, W. Unveiling diversity of stem cells in dental pulp and apical papilla using mouse genetic models: A literature review. Cell Tissue Res. 2021, 383, 603–616. [Google Scholar] [CrossRef]
- Calabrese, E.J. Hormesis and dental apical papilla stem cells. Chem.-Biol. Interact. 2022, 357, 109887. [Google Scholar] [CrossRef]
- Eskander, M.A.; Takimoto, K.; Diogenes, A. Evaluation of mesenchymal stem cell modulation of trigeminal neuronal responses to cold. Neuroscience 2017, 360, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.M.; Liu, Y.; Yu, S.; Jiang, L.M.; Song, B.; Chen, X. Potential immunomodulatory effects of stem cells from the apical papilla on Treg conversion in tissue regeneration for regenerative endodontic treatment. Int. Endod. J. 2019, 52, 1758–1767. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, W.; Wu, Y.; Sun, W.; Dörfer, C.E.; Fawzy El-Sayed, K.M. Oral Mesenchymal Stem/Progenitor Cells: The Immunomodulatory Masters. Stem Cells Int. 2020, 2020, 1327405. [Google Scholar] [CrossRef]
- Morsczeck, C.; Götz, W.; Schierholz, J.; Zeilhofer, F.; Kühn, U.; Möhl, C.; Sippel, C.; Hoffmann, K.H. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005, 24, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Wise, G.E.; Lin, F.; Fan, W. Culture and characterization of dental follicle cells from rat molars. Cell Tissue Res. 1992, 267, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Du, X.-Y.; Luo, W. Clinical application prospects and transformation value of dental follicle stem cells in oral and neurological diseases. World J. Stem Cells 2023, 15, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Morsczeck, C.; Völlner, F.; Saugspier, M.; Brandl, C.; Reichert, T.E.; Driemel, O.; Schmalz, G. Comparison of human dental follicle cells (DFCs) and stem cells from human exfoliated deciduous teeth (SHED) after neural differentiation in vitro. Clin. Oral Investig. 2010, 14, 433–440. [Google Scholar] [CrossRef]
- Bi, R.; Lyu, P.; Song, Y.; Li, P.; Song, D.; Cui, C.; Fan, Y. Function of Dental Follicle Progenitor/Stem Cells and Their Potential in Regenerative Medicine: From Mechanisms to Applications. Biomolecules 2021, 11, 997. [Google Scholar] [CrossRef]
- Mead, B.; Logan, A.; Berry, M.; Leadbeater, W.; Scheven, B.A. Concise Review: Dental Pulp Stem Cells: A Novel Cell Therapy for Retinal and Central Nervous System Repair. Stem Cells 2017, 35, 61–67. [Google Scholar] [CrossRef]
- Bonaventura, G.; Incontro, S.; Iemmolo, R.; La Cognata, V.; Barbagallo, I.; Costanzo, E.; Barcellona, M.L.; Pellitteri, R.; Cavallaro, S. Dental mesenchymal stem cells and neuro-regeneration: A focus on spinal cord injury. Cell Tissue Res. 2020, 379, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Mohebichamkhorami, F.; Niknam, Z.; Zali, H.; Mostafavi, E. Therapeutic Potential of Oral-Derived Mesenchymal Stem Cells in Retinal Repair. Stem Cell Rev. Rep. 2023, 19, 2709–2723. [Google Scholar] [CrossRef]
- Koutsoumparis, A.E.; Patsiarika, A.; Tsingotjidou, A.; Pappas, I.; Tsiftsoglou, A.S. Neural Differentiation of Human Dental Mesenchymal Stem Cells Induced by ATRA and UDP-4: A Comparative Study. Biomolecules 2022, 12, 218. [Google Scholar] [CrossRef] [PubMed]
- Mukhamedshina, Y.; Shulman, I.; Ogurcov, S.; Kostennikov, A.; Zakirova, E.; Akhmetzyanova, E.; Rogozhin, A.; Masgutova, G.; James, V.; Masgutov, R.; et al. Mesenchymal Stem Cell Therapy for Spinal Cord Contusion: A Comparative Study on Small and Large Animal Models. Biomolecules 2019, 9, 811. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Yamamoto, A.; Matsubara, K.; Nakamura, S.; Naruse, M.; Yamagata, M.; Sakamoto, K.; Tauchi, R.; Wakao, N.; Imagama, S.; et al. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J. Clin. Investig. 2012, 122, 80–90. [Google Scholar] [CrossRef]
- Zhu, S.; Ying, Y.; He, Y.; Zhong, X.; Ye, J.; Huang, Z.; Chen, M.; Wu, Q.; Zhang, Y.; Xiang, Z.; et al. Hypoxia response element-directed expression of bFGF in dental pulp stem cells improve the hypoxic environment by targeting pericytes in SCI rats. Bioact. Mater. 2021, 6, 2452–2466. [Google Scholar] [CrossRef]
- do Couto Nicola, F.; Marques, M.R.; Odorcyk, F.; Arcego, D.M.; Petenuzzo, L.; Aristimunha, D.; Vizuete, A.; Sanches, E.F.; Pereira, D.P.; Maurmann, N.; et al. Neuroprotector effect of stem cells from human exfoliated deciduous teeth transplanted after traumatic spinal cord injury involves inhibition of early neuronal apoptosis. Brain Res. 2017, 1663, 95–105. [Google Scholar] [CrossRef]
- Nicola, F.; Marques, M.R.; Odorcyk, F.; Petenuzzo, L.; Aristimunha, D.; Vizuete, A.; Sanches, E.F.; Pereira, D.P.; Maurmann, N.; Gonçalves, C.-A.; et al. Stem Cells from Human Exfoliated Deciduous Teeth Modulate Early Astrocyte Response after Spinal Cord Contusion. Mol. Neurobiol. 2019, 56, 748–760. [Google Scholar] [CrossRef]
- Taghipour, Z.; Karbalaie, K.; Kiani, A.; Niapour, A.; Bahramian, H.; Nasr-Esfahani, M.H.; Baharvand, H. Transplantation of undifferentiated and induced human exfoliated deciduous teeth-derived stem cells promote functional recovery of rat spinal cord contusion injury model. Stem Cells Dev. 2012, 21, 1794–1802. [Google Scholar] [CrossRef]
- Yang, C.; Li, X.; Sun, L.; Guo, W.; Tian, W. Potential of human dental stem cells in repairing the complete transection of rat spinal cord. J. Neural Eng. 2017, 14, 026005. [Google Scholar] [CrossRef]
- Matsubara, K.; Matsushita, Y.; Sakai, K.; Kano, F.; Kondo, M.; Noda, M.; Hashimoto, N.; Imagama, S.; Ishiguro, N.; Suzumura, A.; et al. Secreted Ectodomain of Sialic Acid-Binding Ig-Like Lectin-9 and Monocyte Chemoattractant Protein-1 Promote Recovery after Rat Spinal Cord Injury by Altering Macrophage Polarity. J. Neurosci. 2015, 35, 2452–2464. [Google Scholar] [CrossRef]
- Asadi-Golshan, R.; Razban, V.; Mirzaei, E.; Rahmanian, A.; Khajeh, S.; Mostafavi-Pour, Z.; Dehghani, F. Sensory and Motor Behavior Evidences Supporting the Usefulness of Conditioned Medium from Dental Pulp-Derived Stem Cells in Spinal Cord Injury in Rats. Asian Spine J. 2018, 12, 785–793. [Google Scholar] [CrossRef]
- Asadi-Golshan, R.; Razban, V.; Mirzaei, E.; Rahmanian, A.; Khajeh, S.; Mostafavi-Pour, Z.; Dehghani, F. Efficacy of dental pulp-derived stem cells conditioned medium loaded in collagen hydrogel in spinal cord injury in rats: Stereological evidence. J. Chem. Neuroanat. 2021, 116, 101978. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Liu, Y.; Han, F.; Guo, M.; Hou, X.; Ye, K.; Deng, S.; Shen, Y.; Zhao, Y.; Wei, H.; et al. An Intelligent Neural Stem Cell Delivery System for Neurodegenerative Diseases Treatment. Adv. Healthc. Mater. 2018, 7, 1800080. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xiao, B.; Mu, J.; Zhang, Y.; Zhang, C.; Cao, H.; Chen, R.; Patra, H.K.; Yang, B.; Feng, S.; et al. A MnO2 Nanoparticle-Dotted Hydrogel Promotes Spinal Cord Repair via Regulating Reactive Oxygen Species Microenvironment and Synergizing with Mesenchymal Stem Cells. ACS Nano 2019, 13, 14283–14293. [Google Scholar] [CrossRef]
- Ying, Y.; Huang, Z.; Tu, Y.; Wu, Q.; Li, Z.; Zhang, Y.; Yu, H.; Zeng, A.; Huang, H.; Ye, J.; et al. A shear-thinning, ROS-scavenging hydrogel combined with dental pulp stem cells promotes spinal cord repair by inhibiting ferroptosis. Bioact. Mater. 2023, 22, 274–290. [Google Scholar] [CrossRef]
- Zhou, H.; Jing, S.; Xiong, W.; Zhu, Y.; Duan, X.; Li, R.; Peng, Y.; Kumeria, T.; He, Y.; Ye, Q. Metal-organic framework materials promote neural differentiation of dental pulp stem cells in spinal cord injury. J. Nanobiotechnol. 2023, 21, 316. [Google Scholar] [CrossRef]
- Cortiella, J.; Nichols, J.E.; Kojima, K.; Bonassar, L.J.; Dargon, P.; Roy, A.K.; Vacant, M.P.; Niles, J.A.; Vacanti, C.A. Tissue-engineered lung: An in vivo and in vitro comparison of polyglycolic acid and pluronic F-127 hydrogel/somatic lung progenitor cell constructs to support tissue growth. Tissue Eng. 2006, 12, 1213–1225. [Google Scholar] [CrossRef]
- Luo, L.; Albashari, A.A.; Wang, X.; Jin, L.; Zhang, Y.; Zheng, L.; Xia, J.; Xu, H.; Zhao, Y.; Xiao, J.; et al. Effects of Transplanted Heparin-Poloxamer Hydrogel Combining Dental Pulp Stem Cells and bFGF on Spinal Cord Injury Repair. Stem Cells Int. 2018, 2018, 2398521. [Google Scholar] [CrossRef] [PubMed]
- Albashari, A.; He, Y.; Zhang, Y.; Ali, J.; Lin, F.; Zheng, Z.; Zhang, K.; Cao, Y.; Xu, C.; Luo, L.; et al. Thermosensitive bFGF-Modified Hydrogel with Dental Pulp Stem Cells on Neuroinflammation of Spinal Cord Injury. ACS Omega 2020, 5, 16064–16075. [Google Scholar] [CrossRef]
- Zhu, S.; Ying, Y.; Wu, Q.; Ni, Z.; Huang, Z.; Cai, P.; Tu, Y.; Ying, W.; Ye, J.; Zhang, R.; et al. Alginate self-adhesive hydrogel combined with dental pulp stem cells and FGF21 repairs hemisection spinal cord injury via apoptosis and autophagy mechanisms. Chem. Eng. J. 2021, 426, 130827. [Google Scholar] [CrossRef]
- De Berdt, P.; Vanvarenberg, K.; Ucakar, B.; Bouzin, C.; Paquot, A.; Gratpain, V.; Loriot, A.; Payen, V.; Bearzatto, B.; Muccioli, G.G.; et al. The human dental apical papilla promotes spinal cord repair through a paracrine mechanism. Cell Mol. Life Sci. 2022, 79, 252. [Google Scholar] [CrossRef] [PubMed]
- De Berdt, P.; Vanacker, J.; Ucakar, B.; Elens, L.; Diogenes, A.; Leprince, J.G.; Deumens, R.; des Rieux, A. Dental Apical Papilla as Therapy for Spinal Cord Injury. J. Dent. Res. 2015, 94, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, X.; Feng, G.; Gu, Z.; Sun, Y.; Bao, G.; Xu, G.; Lu, Y.; Chen, J.; Xu, L.; et al. Chitosan scaffolds induce human dental pulp stem cells to neural differentiation: Potential roles for spinal cord injury therapy. Cell Tissue Res. 2016, 366, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Feng, G.; Zhang, J.; Xing, J.; Huang, D.; Lian, M.; Zhang, W.; Wu, W.; Hu, Y.; Lu, X.; et al. Basic fibroblast growth factor promotes human dental pulp stem cells cultured in 3D porous chitosan scaffolds to neural differentiation. Int. J. Neurosci. 2021, 131, 625–633. [Google Scholar] [CrossRef]
- Guo, S.; Redenski, I.; Landau, S.; Szklanny, A.; Merdler, U.; Levenberg, S. Prevascularized Scaffolds Bearing Human Dental Pulp Stem Cells for Treating Complete Spinal Cord Injury. Adv. Healthc. Mater. 2020, 9, 2000974. [Google Scholar] [CrossRef]
- Li, X.; Yang, C.; Li, L.; Xiong, J.; Xie, L.; Yang, B.; Yu, M.; Feng, L.; Jiang, Z.; Guo, W.; et al. A therapeutic strategy for spinal cord defect: Human dental follicle cells combined with aligned PCL/PLGA electrospun material. Biomed. Res. Int. 2015, 2015, 197183. [Google Scholar] [CrossRef]
- San Jose, L.H.; Stephens, P.; Song, B.; Barrow, D. Microfluidic Encapsulation Supports Stem Cell Viability, Proliferation, and Neuronal Differentiation. Tissue Eng. Part. C-Methods 2018, 24, 158–170. [Google Scholar] [CrossRef]
- Kandalam, S.; De Berdt, P.; Ucakar, B.; Vanvarenberg, K.; Bouzin, C.; Gratpain, V.; Diogenes, A.; Montero-Menei, C.N.; des Rieux, A. Human dental stem cells of the apical papilla associated to BDNF-loaded pharmacologically active microcarriers (PAMs) enhance locomotor function after spinal cord injury. Int. J. Pharm. 2020, 587, 119685. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Fayazi, N.; Sheykhhasan, M.; Soleimani Asl, S.; Najafi, R. Stem Cell-Derived Exosomes: A New Strategy of Neurodegenerative Disease Treatment. Mol. Neurobiol. 2021, 58, 3494–3514. [Google Scholar] [CrossRef]
- Liu, C.; Hu, F.; Jiao, G.; Guo, Y.; Zhou, P.; Zhang, Y.; Yi, J.; You, Y.; Li, Z.; Wang, H.; et al. Dental pulp stem cell-derived exosomes suppress M1 macrophage polarization through the ROS-MAPK-NFκB P65 signaling pathway after spinal cord injury. J. Nanobiotechnol. 2022, 20, 65. [Google Scholar] [CrossRef]
- Feitosa, M.L.T.; Sarmento, C.A.P.; Bocabello, R.Z.; Beltrão-Braga, P.C.B.; Pignatari, G.C.; Giglio, R.F.; Miglino, M.A.; Orlandin, J.R.; Ambrósio, C.E. Transplantation of human immature dental pulp stem cell in dogs with chronic spinal cord injury. Acta Cir. Bras. 2017, 32, 540–549. [Google Scholar] [CrossRef]
- Prado, C.; Fratini, P.; de Sá Schiavo Matias, G.; Bocabello, R.Z.; Monteiro, J.; dos Santos, C.J.; Joaquim, J.G.F.; Giglio, R.F.; Possebon, F.S.; Sakata, S.H.; et al. Combination of stem cells from deciduous teeth and electroacupuncture for therapy in dogs with chronic spinal cord injury: A pilot study. Res. Vet. Sci. 2019, 123, 247–251. [Google Scholar] [CrossRef]
- Nicola, F.C.; Rodrigues, L.P.; Crestani, T.; Quintiliano, K.; Sanches, E.F.; Willborn, S.; Aristimunha, D.; Boisserand, L.; Pranke, P.; Netto, C.A. Human dental pulp stem cells transplantation combined with treadmill training in rats after traumatic spinal cord injury. Braz. J. Med. Biol. Res. 2016, 49, e5319. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Rong, Y.; Wang, J.; Zhou, Z.; Ge, X.; Ji, C.; Jiang, D.; Gong, F.; Li, L.; Chen, J.; et al. Exosome-shuttled miR-216a-5p from hypoxic preconditioned mesenchymal stem cells repair traumatic spinal cord injury by shifting microglial M1/M2 polarization. J. Neuroinflamm. 2020, 17, 47. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-L.; Rao, J.; Lin, F.-B.; Liang, Z.-Y.; Xu, X.-J.; Lin, Y.-K.; Chen, X.-Y.; Wang, C.-H.; Chen, C.-M. The Role of Exosomes and Exosomal Noncoding RNAs From Different Cell Sources in Spinal Cord Injury. Front. Cell Neurosci. 2022, 16, 882306. [Google Scholar] [CrossRef] [PubMed]
- Kabatas, S.; Demir, C.S.; Civelek, E.; Yilmaz, I.; Kircelli, A.; Yilmaz, C.; Akyuva, Y.; Karaoz, E. Neuronal regeneration in injured rat spinal cord after human dental pulp derived neural crest stem cell transplantation. Bratisl. Lek. Listy 2018, 119, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Arul, K.; Mesfin, A. Spinal Cord Injury from Spinal Tumors: Prevalence, Management, and Outcomes. World Neurosurg. 2019, 122, e1551–e1556. [Google Scholar] [CrossRef] [PubMed]
- Sahni, V.; Kessler, J.A. Stem cell therapies for spinal cord injury. Nat. Rev. Neurol. 2010, 6, 363–372. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, Y.; Xie, Y.; Zhang, L.; Tang, P. Cell Transplantation for Spinal Cord Injury: Tumorigenicity of Induced Pluripotent Stem Cell-Derived Neural Stem/Progenitor Cells. Stem Cells Int. 2018, 2018, e5653787. [Google Scholar] [CrossRef] [PubMed]



| Models | Characteristics | Refs. |
|---|---|---|
| Contusion | Extensive tissue pathology; White matter apoptosis; Demyelination Incomplete remyelination; Robust macrophage response Early cell apoptosis; Presence of cavities and fibrotic scars Changes extending several millimetres cranial and caudal to the injury epicentre | [25,26,27] |
| Compression | Widespread inflammation; Edema; Bleeding Ischemia and demyelination due to venous congestion Changes occur near the injury epicentre | [28,29] |
| Distraction | No significant vascular damage Membrane damage to neuronal cell bodies and axons extends several vertebral segments rostrally Extracellular space enlargement and white matter structural alterations | [30,31] |
| Dislocation | Intramedullary bleeding; Early cell apoptosis Membrane damage to neuronal cell bodies and axons extends several vertebral segments rostrally Extensive loss of nerve fibres and accumulation of β-amyloid precursor protein; Greatest loss in ventral and dorsal horn neuron | [27,30,31] |
| Transaction | Focal tissue pathology with white matter apoptosis; Demyelination Incomplete remyelination; Robust macrophage response at the injury epicentre Absence of apoptosis and demyelination at a distance from the epicentre Damage to the dural membrane, epidural hematoma, and leakage of cerebrospinal fluid due to knife wound, potentially leading to infection | [26,32] |
| Chemical | Ischemia; Demyelination; Oxidative damage (lipids and proteins) Inflammation; Cellular injury | [33] |
| Approach | Advantages | Disadvantages | The Role of SCI Repair | Refs. |
|---|---|---|---|---|
| Stem Cell Transplantation | Easy isolation, Minimal ethical controversy lower immunogenicity risk | Low stem cell survival Risk of immune rejection Cell dedifferentiation Risk of potential tumour formation | Promoting nerve regeneration. Improving motor function. Reducing spinal cord tissue loss. Protecting motor neurons. Mitigating inflammation. Decreasing neuronal apoptosis. | [77,78,79,80,81,82,108] |
| Conditioned Medium Injection | Contains growth factors for effective tissue repair. Avoid tumorigenesis and immune issues associated with stem cell transplantation. | The rapid diffusion of culture medium may be uncontrollable. | Establishing a favourable repair microenvironment. Promoting nerve regeneration. | [83,84,85] |
| Delivery System Approaches | Provide a suitable environment for stem cell survival, growth, and differentiation. Protects existing cells from apoptosis/necrosis. Allows controlled release of growth factors. | Material instability and potential quick degradation. Risk of cytotoxicity. | Enhancing stem cell viability and neural differentiation. Promoting neuronal regeneration. Inhibiting inflammation. Improving oxygen supply to damaged areas. Reducing gelatinous scarring to aid axonal and vascular regeneration. | [85,88,89,91,92,93,94,95,96,97,98,99,100,101] |
| Exosomes and Extracellular Vesicle | Exosomes exhibit most of the biological properties of stem cells. Exosomes are small and less likely to block microvessels. Exosomes have a low tumour risk. | Limited exosome production. Sensitivity to microenvironment pH. | Reducing inflammation and nerve damage. Improving spinal cord neuron survival. Enhancing motor function. | [104,109] |
| Combined Therapies | Electroacupuncture, treadmill training, and physiotherapy have shown some potential for improving functional recovery after SCI. | The timing and intensity of training can affect recovery outcomes. | No significant improvement in combination with treatment. | [105,106,107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, X.; Jiang, W.; Li, X.; Liu, Q.; Kang, Y.; Song, B. Advancements in Spinal Cord Injury Repair: Insights from Dental-Derived Stem Cells. Biomedicines 2024, 12, 683. https://doi.org/10.3390/biomedicines12030683
Wen X, Jiang W, Li X, Liu Q, Kang Y, Song B. Advancements in Spinal Cord Injury Repair: Insights from Dental-Derived Stem Cells. Biomedicines. 2024; 12(3):683. https://doi.org/10.3390/biomedicines12030683
Chicago/Turabian StyleWen, Xueying, Wenkai Jiang, Xiaolin Li, Qian Liu, Yuanyuan Kang, and Bing Song. 2024. "Advancements in Spinal Cord Injury Repair: Insights from Dental-Derived Stem Cells" Biomedicines 12, no. 3: 683. https://doi.org/10.3390/biomedicines12030683