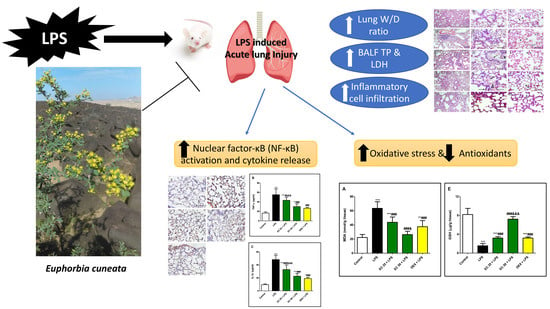
Euphorbia cuneata Represses LPS-Induced Acute Lung Injury in Mice via Its Antioxidative and Anti-Inflammatory Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Extraction Procedures for Pharmacological Study
2.4. Extraction Procedures of Plant Material for High Performance Liquid Chromatography Diode-Array Detection
2.5. HPLC Photodiode Array Determination of Flavonoid Content in TEC
2.6. Calibration Curve of the Isolated Compounds
2.7. Biological Study
2.7.1. Animals and Experimental Model
2.7.2. Lung Wet/Dry Weight (W/D) Ratio
2.7.3. Protein Content
2.7.4. LDH Activity
2.7.5. Total and Differential Cell Counts
2.7.6. Lung Histology
2.7.7. Immunohistopathology
2.7.8. Oxidative Stress and Antioxidants
2.7.9. NF-κB and Inflammatory Cytokines
2.7.10. Statistical Analysis
3. Results
3.1. Effect of EC on LPS-Induced Lung Edema
3.2. Effect of EC on LPS-Induced Increase in the Total and Differential Inflammatory Cell Counts in BALF
3.3. Effect of EC on LPS-Induced Lung Damage
3.4. Effect of EC on LPS-Induced Lipid Peroxidation and Antioxidants in Lung
3.5. Effect of EC on LPS-Induced Inflammatory Response in Lung
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- El-Agamy, D.S.; Mohamed, G.A.; Ahmed, N.; Elkablawy, M.A.; Elfaky, M.A.; Elsaed, W.M.; Mohamed, S.G.A.; Ibrahim, S.R.M. Protective anti-inflammatory activity of tovophyllin A against acute lung injury and its potential cytotoxicity to epithelial lung and breast carcinomas. Inflammopharmacology 2019, 28, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.M.; Ahmed, N.; Almalki, S.; Alharbi, N.; El-Agamy, D.S.; Alahmadi, L.A.; Saubr, M.K.; Elkablawy, M.A.; Elshafie, R.M.; Mohamed, G.A.; et al. Vitex agnus-castus safeguards the lung against lipopolysaccharide-induced toxicity in mice. J. Food Biochem. 2018, 43, e12750. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.-L.; Chen, M.-F.; Tsai, M.-J.; Hsu, Y.-H.; Chen, C.-P.; Lee, T.J.F. Oroxylin-A rescues LPS-induced acute lung injury via regulation of NF-κB signaling pathway in rodents. PLOS ONE 2012, 7, e47403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaaban, A.A.; El-Kashef, D.H.; Hamed, M.F.; El-Agamy, D.S. Protective effect of pristimerin against LPS-induced acute lung injury in mice. Int. Immunopharmacol. 2018, 59, 31–39. [Google Scholar] [CrossRef] [PubMed]
- El-Agamy, D.S. Nilotinib ameliorates lipopolysaccharide-induced acute lung injury in rats. Toxicol. Appl. Pharmacol. 2011, 253, 153–160. [Google Scholar] [CrossRef]
- Guo, S.; Jiang, K.; Wu, H.; Yang, C.; Yang, Y.; Yang, J.; Zhao, G.; Deng, G. Magnoflorine ameliorates lipopolysaccharide-induced acute lung injury via suppressing NF-κB and MAPK activation. Front. Pharmacol. 2018, 9, 982. [Google Scholar] [CrossRef]
- Zhu, T.; Wang, D.-X.; Zhang, W.; Liao, X.-Q.; Guan, X.; Bo, H.; Sun, J.-Y.; Huang, N.-W.; He, J.; Zhang, Y.-K.; et al. Andrographolide protects against LPS-induced acute lung injury by inactivation of NF-κB. PLOS ONE 2013, 8, e56407. [Google Scholar] [CrossRef] [Green Version]
- Lu, R.; Wu, Y.; Guo, H.; Huang, X. Salidroside protects lipopolysaccharide-induced acute lung injury in mice. Dose-Response 2016, 14, 1559325816678492. [Google Scholar] [CrossRef]
- Ahmed, N.; Aljuhani, N.; Salamah, S.; Surrati, H.; El-Agamy, D.S.; Elkablawy, M.A.; Ibrahim, S.R.M.; Mohamed, G.A. Pulicaria petiolaris effectively attenuates lipopolysaccharide (LPS)-induced acute lung injury in mice. Arch. Biol. Sci. 2018, 70, 699–706. [Google Scholar]
- Wu, H.; Yang, Y.; Guo, S.; Yang, J.; Jiang, K.; Zhao, G.; Qiu, C.; Deng, G. Nuciferine ameliorates inflammatory responses by inhibiting the TLR4-mediated pathway in lipopolysaccharide-induced acute lung injury. Front. Pharmacol. 2017, 8, 939. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Chen, S.; Zeng, M.; Lin, D.; Wang, Y.; Wen, X.; Xu, C.; Yang, L.; Fan, X.; Gong, Y.; et al. Apelin-13 administration protects against LPS-induced acute lung injury by inhibiting NF-κB pathway and NLRP3 inflammasome activation. Cell. Physiol. Biochem. 2018, 49, 1918–1932. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.A.; Al-Abd, A.M.; El-Halawany, A.M.; Abdallah, H.M.; Ibrahim, S.R.M. New xanthones and cytotoxic constituents from Garcinia mangostana fruit hulls against human hepatocellular, breast, and colorectal cancer cell lines. J. Ethnopharmacol. 2017, 198, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Vasas, A.; Hohmann, J. Euphorbia diterpenes: Isolation, structure, biological activity, and synthesis (2008–2012). Chem. Rev. 2014, 114, 8579–8612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, A.; Akter, M. Taxonomy and medicinal uses of Euphorbiaceae (Spurge) family of Rajshahi, Bangladesh. Res. Plant Sci. 2013, 1, 74–80. [Google Scholar]
- Awaad, A.S.; Al-Jaber, N.A.; Moses, J.E.; El-Meligy, R.M.; E Zain, M. Antiulcerogenic activities of the extracts and isolated flavonoids of Euphorbia cuneata Vahl. Phytother. Res. 2012, 27, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Awaad, S.A.; Alothman, M.R.; Zain, Y.M.; Alqasoumi, S.I.; Alothman, E.A. Quantitative and qualitative analysis for standardization of Euphorbia cuneata Vahl. Saudi Pharm. J. 2017, 25, 1175–1178. [Google Scholar] [CrossRef]
- Das, B.; Alam, S.; Bhattacharjee, R.; Das, B.K. Analgesic and anti-inflammatory activity of Euphorbia antiquorum Linn. Am. J. Pharmacol. Toxicol. 2015, 10, 46–55. [Google Scholar] [CrossRef]
- Al-Fatimi, M. Ethnobotanical survey of medicinal plants in central Abyan governorate, Yemen. J. Ethnopharmacol. 2019, 241, 111973. [Google Scholar] [CrossRef]
- Zain, M.E.; Awaad, A.S.; Al-Outhman, M.R.; El-Meligy, R.M. Antimicrobial activities of Saudi Arabian desert plants. Phytopharmacology 2012, 2, 106–113. [Google Scholar]
- Elghamdi, A.A.; Abdallah, H.M.; Shehata, I.A.; Mohamed, G.A.; Shati, A.A.; Alfaifi, M.Y.; Elbehairi, S.E.I.; Koshak, A.E.; Ibrahim, S.R.M. Cyclocuneatol and cuneatannin, new cycloartane triterpenoid and ellagitannin glycoside from Euphorbia cuneata. ChemistrySelect 2019, 4, 12375–12379. [Google Scholar] [CrossRef]
- Ghazanfar, S.A. Handbook of Arabian Medicinal Plants; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA, 1994. [Google Scholar]
- Collenette, S. Wildflowers of Saudi Arabia; National Commission for Wildlife Conservation and Development; NCWCD: Riyadh, Saudi Arabia, 1999; p. 167. [Google Scholar]
- Al-Harbi, N.O.; Imam, F.; Al-Harbi, M.M.; Ansari, M.A.; Zoheir, K.M.A.; Korashy, H.M.; Sayed-Ahmed, M.M.; Attia, S.M.; Shabanah, O.A.; Ahmad, A.M. Dexamethasone attenuates LPS-induced acute lung injury through inhibition of NF-κB, COX-2, and pro-inflammatory mediators. Immunol. Investig. 2016, 45, 349–369. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Du, J. Anti-inflammatory and protective effects of D-carvone on lipopolysaccharide (LPS)-induced acute lung injury in mice. J. King Saud Univ. Sci. 2020, 32, 1592–1596. [Google Scholar] [CrossRef]
- El-Kholy, A.A.; Elkablawy, M.A.; El-Agamy, D.S. Lutein mitigates cyclophosphamide induced lung and liver injury via NF-κB/MAPK dependent mechanism. Biomed. Pharmacother. 2017, 92, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Xiu, Y.; Zhou, P.; Qiu, Y.; Li, Y.-H. A New Use for an Old Drug: Carmofur attenuates lipopolysaccharide (LPS)-induced acute lung injury via inhibition of FAAH and NAAA activities. Front. Pharmacol. 2019, 10, 818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Yu, X.; Yu, S.; Kou, J. Molecular mechanisms in lipopolysaccharide-induced pulmonary endothelial barrier dysfunction. Int. Immunopharmacol. 2015, 29, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ci, X.; Wen, Z.; Peng, L. Diosmetin alleviates lipopolysaccharide-induced acute lung injury through activating the Nrf2 pathway and inhibiting the NLRP3 inflammasome. Biomol. Ther. 2018, 26, 157–166. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Li, S.; Wang, L.; Du, F.; Zhou, X.; Song, Q.; Zhao, J.; Fang, R. Ginsenoside Rg3 attenuates lipopolysaccharide-induced acute lung injury via MerTK-dependent activation of the PI3K/AKT/mTOR pathway. Front. Pharmacol. 2018, 9, 850. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Xu, D.; Liu, J.; Gu, L. Protective effect of sophocarpine on lipopolysaccharide-induced acute lung injury in mice. Int. Immunopharmacol. 2019, 70, 180–186. [Google Scholar] [CrossRef]
- Yang, H.; Li, Y.; Huo, P.; Li, X.-O.; Kong, D.; Mu, W.; Fang, W.; Li, L.; Liu, N.; Fang, L.; et al. Protective effect of Jolkinolide B on LPS-induced mouse acute lung injury. Int. Immunopharmacol. 2015, 26, 119–124. [Google Scholar] [CrossRef]
- Uto, T.; Qin, G.-W.; Morinaga, O.; Shoyama, Y. 17-Hydroxy-jolkinolide B, a diterpenoid from Euphorbia fischeriana, inhibits inflammatory mediators but activates heme oxygenase-1 expression in lipopolysaccharide-stimulated murine macrophages. Int. Immunopharmacol. 2012, 12, 101–109. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, S.; Cheng, X.; Lu, C.; Tao, W.; Zhang, Y.; William, B.C.; Cao, X.; Yi, S.; Liu, Y.; et al. Euphorbia factor L2 alleviates lipopolysaccharide-induced acute lung injury and inflammation in mice through the suppression of NF-κB activation. Biochem. Pharmacol. 2018, 155, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, inflammation and immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Ngo, C.; Willcox, J.C.; Lappas, M. Anti-Diabetic, anti-Inflammatory, and anti-Oxidant effects of naringenin in an in vitro human model and an in vivo murine model of gestational diabetes mellitus. Mol. Nutr. Food Res. 2019, 63, e1900224. [Google Scholar] [CrossRef] [PubMed]
- Calderon-Montano, J.M.; Burgos-Moron, E.; Perez-Guerrero, C.; Lopez-Lazaro, M. A Review on the dietary flavonoid kaempferol. Mini Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdallah, H.M.; El-Agamy, D.S.; Ibrahim, S.R.M.; Mohamed, G.A.; Elsaed, W.M.; Elghamdi, A.A.; Safo, M.K.; Malebari, A.M. Euphorbia cuneata Represses LPS-Induced Acute Lung Injury in Mice via Its Antioxidative and Anti-Inflammatory Activities. Plants 2020, 9, 1620. https://doi.org/10.3390/plants9111620
Abdallah HM, El-Agamy DS, Ibrahim SRM, Mohamed GA, Elsaed WM, Elghamdi AA, Safo MK, Malebari AM. Euphorbia cuneata Represses LPS-Induced Acute Lung Injury in Mice via Its Antioxidative and Anti-Inflammatory Activities. Plants. 2020; 9(11):1620. https://doi.org/10.3390/plants9111620
Chicago/Turabian StyleAbdallah, Hossam M., Dina S. El-Agamy, Sabrin R. M. Ibrahim, Gamal A. Mohamed, Wael M. Elsaed, Amjad A. Elghamdi, Martin K. Safo, and Azizah M. Malebari. 2020. "Euphorbia cuneata Represses LPS-Induced Acute Lung Injury in Mice via Its Antioxidative and Anti-Inflammatory Activities" Plants 9, no. 11: 1620. https://doi.org/10.3390/plants9111620